Key Points
Natural IgM antibodies in plasma bind to circulating MVs and modulate coagulation.
A monoclonal natural IgM antibody specific for malondialdehyde epitopes inhibits MV-induced coagulation and thrombosis in mice in vivo.
Abstract
Thrombosis and its associated complications are a major cause of morbidity and mortality worldwide. Microvesicles (MVs), a class of extracellular vesicles, are increasingly recognized as mediators of coagulation and biomarkers of thrombotic risk. Thus, identifying factors targeting MV-driven coagulation may help in the development of novel antithrombotic treatments. We have previously identified a subset of circulating MVs that is characterized by the presence of oxidation-specific epitopes and bound by natural immunoglobulin M (IgM) antibodies targeting these structures. This study investigated whether natural IgM antibodies, which are known to have important anti-inflammatory housekeeping functions, inhibit the procoagulatory properties of MVs. We found that the extent of plasma coagulation is inversely associated with the levels of both free and MV-bound endogenous IgM. Moreover, the oxidation epitope-specific natural IgM antibody LR04, which recognizes malondialdehyde adducts, reduced MV-dependent plasmatic coagulation and whole blood clotting without affecting thrombocyte aggregation. Intravenous injection of LR04 protected mice from MV-induced pulmonary thrombosis. Of note, LR04 competed the binding of coagulation factor X/Xa to MVs, providing a mechanistic explanation for its anticoagulatory effect. Thus, our data identify natural IgM antibodies as hitherto unknown modulators of MV-induced coagulation in vitro and in vivo and their prognostic and therapeutic potential in the management of thrombosis.
Introduction
Cardiovascular diseases remain the major cause of morbidity and mortality worldwide.1 Most cardiovascular events such as stroke, myocardial infarction, and pulmonary embolism are caused by thrombotic vessel occlusion.2,3 Moreover, several chronic conditions, such as diabetes4 and certain autoimmune5 and autoinflammatory6 disorders, as well as many types of cancer,7 are associated with a high thrombotic risk. Current strategies for the prevention and treatment of thrombosis are based on targeting the coagulation cascade and/or platelet reactivity and therefore increase the risk of bleeding.8-12 Further insights into the pathophysiology of thrombosis and the development of novel antithrombotic treatments are thus needed.
Inflammation and the cellular responses involved in it are increasingly recognized as important modulators of thrombosis, mainly by activating the procoagulant pathway.13-15 Increased oxidative stress and the production of reactive oxygen species are a hallmark of inflammatory processes. In turn, oxidative damage of membrane lipids give rise to the formation of oxidation-specific epitopes (OSEs)16 such as malondialdehyde (MDA) and oxidized phosphatidylcholine. OSEs are considered a class of danger-associated molecular patterns that trigger inflammatory responses in both acute and chronic settings.17 For example, oxidized low-density lipoprotein (LDL), which carries different OSEs, represents a key trigger of vascular inflammation, in particular in the context of atherosclerosis.18 Moreover, oxidized LDL has been shown to induce tissue factor (TF) expression in monocytes,19,20 neutrophil extracellular cellular trap formation,21 and a prothrombotic phenotype in endothelial cells.22-24 Thus, innate immune receptors that recognize OSEs can mediate procoagulant and prothrombotic responses.18 However, whether recognition of OSEs could also directly dampen coagulation has not yet been investigated, to the best of our knowledge.
We and others have previously shown that low levels of immunoglobulin M (IgM) antibodies binding OSEs are inversely associated with the risk of atherosclerotic cardiovascular events and venous thrombosis.25-28 Most, if not all, of these immunoglobulins are germline encoded (so-called natural antibodies), produced without prior exposure to pathogens.29 We have previously shown that a large part of IgM antibodies recognize OSEs.16,30 The protective effects of these OSE IgM antibodies have been largely attributed to their ability to neutralize the proinflammatory effects of oxidized lipids and mediate the clearance of apoptotic cells carrying OSEs.30-34
We recently reported that OSE IgM antibodies recognize a subset of circulating microvesicles (MVs),35 a class of extracellular vesicles (EVs) that can be found in all biological fluids.36 Cellular stress, in particular inflammatory activation, increases MV production.37 Accordingly, the levels of circulating MVs are elevated in many pathologic settings and have therefore been suggested as mediators and biomarkers in a variety of diseases,38 including metabolic39 and cardiovascular disease,40 cancer,41 and infections.42 Initially, MVs were discovered as mediators of coagulation and have been studied for their contribution to thrombosis43-45 by presenting negatively charged phospholipids, which enable the assembly of the prothrombinase complex,46 and initiating the extrinsic coagulation pathway by exposing TF.47 Furthermore, MVs may also indirectly contribute to a prothrombotic state by triggering inflammatory responses in vascular cells. MVs are therefore considered important mediators of arterial48 and venous49 thrombosis. The current study investigated whether natural IgM antibodies and OSE IgM antibodies in particular could directly modulate the procoagulatory potential of MVs and thereby limit thrombotic processes.
Methods
IgM antibodies recognizing OSEs
Monoclonal IgM antibodies with specificity for MDA adducts (LR04 and NA17; kind gifts of J.L. Witztum, University of California, San Diego) and for phosphocholine (E06; Avanti Polar Lipids) were used in this study. The cloning and characterization of these antibodies have been described before.30,50,51 A mouse IgM isotype (clone number MM30, 401604; BioLegend) was used as a control. All antibodies were tested to be free of endotoxin contamination.
Thrombin generation assay
Thrombin generation (TG) was performed by using a Ceveron alpha analyzer (Technoclone). Isolated MVs were used to initiate clotting as described in the figure legends. Plasma deficient in factors V (5134004), VII (5144015), VIII (5154004), IX (5164003), XI (5184004), and XII (5194008) and a control plasma (5020020) (all from Technoclone) were used. OSE-specific antibodies (LR04, NA17, and E06) or isotype control were used to inhibit coagulation at concentrations of 25 and 50 µg/mL. For blocking of TF, an anti-CD142 monoclonal antibody (clone number HTF-1, 16-1429-82; eBioscience) was used (10 µg/mL). Antibodies were mixed with MVs in phosphate-buffered saline (125 µL) and incubated for 20 minutes at room temperature before adding to 125 µL of plasma.
Pulmonary thrombosis model
A modified mouse model of pulmonary thrombosis was used.52 Eight- to 12-week-old C57BL6/J mice were anesthetized by intraperitoneal injection of ketamine (AniMedica) (100 µg/g body weight [bw]) and xylazine (Bayer) (10 µg/g bw), diluted in sterile saline (injection volume, 100 µL). The mixture containing epinephrine (60 ng/g bw), HPAF-II–derived MVs (0.1 µg/g bw), or rat tail collagen (Corning) (3 µg/g bw), and LR04 or isotype control IgM (25 µg/mL of estimated blood volume; corresponding to 2.25 µg/g bw) in sterile phosphate-buffered saline (total volume, 120 µL), was injected into the retro-orbital plexus using 29 gauge insulin syringes. The antibody concentrations were chosen according to the effective concentrations (25 µg/mL) observed in plasma (TG) and whole blood (rotational thromboelastometry) coagulation assays. Terminal breath was counted as time of death. Surviving animals were killed by cervical dislocation after 30 minutes. Experiments were performed in a blinded manner.
A detailed description of the methods is provided in the supplemental Methods (available on the Blood Web site).
Statistics
GraphPad Prism 8.3 for Windows (GraphPad Software) software was used for statistical analyses. The Mann-Whitney U test was used for comparing data from 2 unpaired groups, and the Wilcoxon matched-pairs signed-rank test was used for data from 2 paired groups. The log-rank (Mantel-Cox) test was used for the comparison of survival curves. One-way analysis of variance test with subsequent Bonferroni’s multiple comparison tests were used for multiple group data analysis. Data are presented as mean ± SEM.
Ethics
Human blood and plasma samples were collected under the approval by the Ethics Committee of the Medical University of Vienna (EK2051/2013 and EK1845/2015). All in vivo experiments were conducted in accordance with the approval of the animal ethics committee of the Medical University of Vienna (BMBWF-66.009/0017-V/3b/2018).
Results
Endogenous IgM antibodies affect MV-dependent TG
To investigate whether IgM antibodies can influence MV-induced plasma coagulation, we selectively depleted circulating IgM antibodies (Figure 1A; supplemental Figure 1A) from pooled, EV-depleted (supplemental Figure 1B) human plasma. Blood drawing leads to plasma preactivation via the contact pathway53 and therefore does not require TF as a trigger. Gradual depletion of endogenous IgM antibodies led to a concurrent increase in propagation of TG triggered by platelet-derived MVs (TF–), reflected by a peak height increase (Figure 1B; supplemental Figure 1C) and by TF+ MVs (supplemental Figure 1D). Activated partial thromboplastin time (aPTT), which in contrast to TG54 is not sensitive to the presence of MVs,55 was not affected by IgM depletion (supplemental Figure 1E).
Endogenous IgM antibodies decrease MV-dependent TG. (A) Chemiluminescent enzyme-linked immunosorbent assay of plasma IgM levels after depletion of free IgM from pooled EV-free human plasma with increasing amounts of anti–human IgM/agarose beads. (B) TG curves and peak heights of IgM-depleted MV-free plasma triggered by addition of platelet-derived MVs. Plots depict representative experiment of n = 4. Bars represent mean ± SEM of each group. *P < .05, ****P < .001, two-way analysis of variance with Bonferroni’s multiple comparisons test. (C) Percentages of IgM+ MVs within annexin V–positive events isolated from plasma of healthy volunteers (n = 22) measured by using flow cytometry. Groups were divided into MV IgMlow and MV IgMhigh based on the mean percentage (horizontal bar). (D) Comparison of the procoagulatory potential of IgMlow and IgMhigh MV average TG curves and peak heights of pooled MV-depleted plasma reconstituted with isolated IgMlow (n = 9) and IgMhigh (n = 13) MVs. Columns and error bars or solid lines and light-colored areas represent the mean ± SEM of each group. (E) Percentages of IgM+ MVs of WT (n = 15) and Siglec-G−/− (n = 18) mice within annexin V–positive events measured by flow cytometry. Bars represent mean ± SEM. (F) Spontaneous TG of platelet-poor plasma from WT and Siglec-G−/− mice. Average TG curves and peak heights of pooled platelet-poor plasma of WT vs Siglec-G−/− mice. Columns and error bars or solid lines and light-colored areas represent the mean ± SEM of each group. (D-F) **P < .01, ***P < .005, Mann-Whitney U test. RLU, relative light units.
Endogenous IgM antibodies decrease MV-dependent TG. (A) Chemiluminescent enzyme-linked immunosorbent assay of plasma IgM levels after depletion of free IgM from pooled EV-free human plasma with increasing amounts of anti–human IgM/agarose beads. (B) TG curves and peak heights of IgM-depleted MV-free plasma triggered by addition of platelet-derived MVs. Plots depict representative experiment of n = 4. Bars represent mean ± SEM of each group. *P < .05, ****P < .001, two-way analysis of variance with Bonferroni’s multiple comparisons test. (C) Percentages of IgM+ MVs within annexin V–positive events isolated from plasma of healthy volunteers (n = 22) measured by using flow cytometry. Groups were divided into MV IgMlow and MV IgMhigh based on the mean percentage (horizontal bar). (D) Comparison of the procoagulatory potential of IgMlow and IgMhigh MV average TG curves and peak heights of pooled MV-depleted plasma reconstituted with isolated IgMlow (n = 9) and IgMhigh (n = 13) MVs. Columns and error bars or solid lines and light-colored areas represent the mean ± SEM of each group. (E) Percentages of IgM+ MVs of WT (n = 15) and Siglec-G−/− (n = 18) mice within annexin V–positive events measured by flow cytometry. Bars represent mean ± SEM. (F) Spontaneous TG of platelet-poor plasma from WT and Siglec-G−/− mice. Average TG curves and peak heights of pooled platelet-poor plasma of WT vs Siglec-G−/− mice. Columns and error bars or solid lines and light-colored areas represent the mean ± SEM of each group. (D-F) **P < .01, ***P < .005, Mann-Whitney U test. RLU, relative light units.
To investigate whether endogenous IgM antibodies bound to MVs affect their coagulatory potential, we assessed the presence of IgM on circulating annexin V–positive MVs of healthy volunteers (n = 22) using flow cytometry (supplemental Figure 1F). MVs were isolated and divided into IgMlow and IgMhigh based on the mean percentage of IgM-positive MVs (Figure 1C). MVs were used to induce TG in the equivalent volume of pooled MV-free plasma. Although the total amount of MVs did not significantly differ between the 2 groups (supplemental Figure 1G), IgMhigh MVs induced TG to a significantly lower degree compared with IgMlow MVs (Figure 1D; supplemental Figure 1H). Similar results were obtained when MVs were dichotomized into IgMhigh and IgMlow MVs within the subset of CD41a+ platelet MVs that are considered to represent the majority of circulating MVs (supplemental Figure 1I).
To further characterize the capacity of endogenous IgM antibodies to modulate coagulation, we characterized circulating MVs from mice lacking sialic acid-binding immunoglobulin-like lectin G (SiglecG−/−), which possess elevated levels of total and OSE-specific IgM.31 SiglecG−/− mice exhibited a significantly higher percentage of IgM+ MVs compared with wild-type (WT) mice (Figure 1E), whereas the total number of MVs was not different between the 2 groups of mice (supplemental Figure 1J). Platelet-poor plasma of SiglecG−/− mice still containing endogenous MVs exhibited significantly reduced TG compared with plasma of WT mice (Figure 1F; supplemental Figure 1K).
OSE-specific natural IgM LR04 inhibits MV-sensitive plasma coagulation and factor X binding to MVs
We have previously shown that the majority of endogenous IgM antibodies bound to circulating MVs have specificity for OSEs; OSE-specific natural IgM antibodies, in particular with specificity for MDA epitopes, bind circulating MVs.35
To address whether OSE-specific natural IgM antibodies modulate coagulation similarly to endogenous IgM, we investigated the effects of LR04, a previously characterized monoclonal MDA-specific IgM antibody50,56 on coagulation. LR04 has been shown to bind circulating and in vitro generated MVs,35 which we confirmed for the platelet-derived MVs (PMV) used (supplemental Figure 2A). To test the effect of LR04 on TG, PMVs were preincubated with increasing concentrations of either LR04 or an isotype control antibody and added to MV-free plasma. LR04 significantly inhibited initiation (lag time) and propagation (peak height) of PMV-triggered TG (Figure 2A; supplemental Figure 2B).
Natural IgM antibodies inhibit MV-sensitive plasma coagulation and factor Xa (FXa) binding to MVs. (A) Effect of the MDA-specific natural IgM antibody (ab) LR04 on TG. TG curves of MV-depleted plasma, triggered by platelet-derived MVs (2 µg/mL), which were preincubated with 2 concentrations of LR04 or an isotype control ab (0, 25, or 50 µg/mL). (B-C) Inhibitory effect of the LR04-specific peptide mimotope P2. Flow cytometry histograms depict binding of LR04 to platelet-derived MVs in the presence or absence of P2 (100 µg/mL) (B); and TG curves of MV-depleted plasma triggered by platelet-derived MVs (2 µg/mL) incubated with LR04 (25 µg/mL) in the presence of increasing concentrations of P2 (0, 12, 25, 50, or 100 µg/mL) (C). (D) TG curves of MV-depleted plasma triggered by platelet-derived MVs (2 µg/mL), which were preincubated with 25 µg/mL of the MDA-specific natural IgM LR04 or NA17, the phosphocholine-specific natural IgM E06, or an isotype control ab. (E) TG curves of MV-depleted plasma triggered by THP-1 cell-derived MVs (2 µg/mL). MVs were preincubated with LR04 or an isotype control ab (25 or 50 µg/mL) or a TF-blocking ab (HTF-1, 10 µg/mL). (F) Activated partial thromboplastin time (aPTT) and prothrombin time (PT) of platelet-poor plasma that was preincubated with increasing amounts of LR04 or an isotype control ab (0, 12, 25, or 50 µg/mL). (G) Effect of LR04 on THP-1/MV–triggered (2 µg/mL) TG of MV-depleted plasma deficient for single coagulation factors (FVII, FVIII, FIX, FXI, and FXII). Shown is the relative reduction of peak height after preincubation of MVs with LR04 (25 µg/mL) compared with the peak height of each respective plasma. (H) Flow cytometry–based size profiles of platelet-derived MVs after incubation (30 minutes, 37°C) with LR04 (25 µg/mL), isotype control ab (25 µg/mL), or concanavalin A (ConA) (5 µg/mL). (I) Inhibition of the binding of plasma-derived factor X/Xa to MVs by increasing concentrations of LR04. Symbols represent the mean ± SEM of 4 independent experiments. *P < .05, **P < .01, two-way analysis of variance, Bonferroni’s multiple comparisons test. In panels A through G, plots depict representative experiments of least 4 experiments. APC-H, allophycocyanin; FSC-H, forward scatter.
Natural IgM antibodies inhibit MV-sensitive plasma coagulation and factor Xa (FXa) binding to MVs. (A) Effect of the MDA-specific natural IgM antibody (ab) LR04 on TG. TG curves of MV-depleted plasma, triggered by platelet-derived MVs (2 µg/mL), which were preincubated with 2 concentrations of LR04 or an isotype control ab (0, 25, or 50 µg/mL). (B-C) Inhibitory effect of the LR04-specific peptide mimotope P2. Flow cytometry histograms depict binding of LR04 to platelet-derived MVs in the presence or absence of P2 (100 µg/mL) (B); and TG curves of MV-depleted plasma triggered by platelet-derived MVs (2 µg/mL) incubated with LR04 (25 µg/mL) in the presence of increasing concentrations of P2 (0, 12, 25, 50, or 100 µg/mL) (C). (D) TG curves of MV-depleted plasma triggered by platelet-derived MVs (2 µg/mL), which were preincubated with 25 µg/mL of the MDA-specific natural IgM LR04 or NA17, the phosphocholine-specific natural IgM E06, or an isotype control ab. (E) TG curves of MV-depleted plasma triggered by THP-1 cell-derived MVs (2 µg/mL). MVs were preincubated with LR04 or an isotype control ab (25 or 50 µg/mL) or a TF-blocking ab (HTF-1, 10 µg/mL). (F) Activated partial thromboplastin time (aPTT) and prothrombin time (PT) of platelet-poor plasma that was preincubated with increasing amounts of LR04 or an isotype control ab (0, 12, 25, or 50 µg/mL). (G) Effect of LR04 on THP-1/MV–triggered (2 µg/mL) TG of MV-depleted plasma deficient for single coagulation factors (FVII, FVIII, FIX, FXI, and FXII). Shown is the relative reduction of peak height after preincubation of MVs with LR04 (25 µg/mL) compared with the peak height of each respective plasma. (H) Flow cytometry–based size profiles of platelet-derived MVs after incubation (30 minutes, 37°C) with LR04 (25 µg/mL), isotype control ab (25 µg/mL), or concanavalin A (ConA) (5 µg/mL). (I) Inhibition of the binding of plasma-derived factor X/Xa to MVs by increasing concentrations of LR04. Symbols represent the mean ± SEM of 4 independent experiments. *P < .05, **P < .01, two-way analysis of variance, Bonferroni’s multiple comparisons test. In panels A through G, plots depict representative experiments of least 4 experiments. APC-H, allophycocyanin; FSC-H, forward scatter.
Preincubation with a previously described peptide mimotope (P2) of MDA that is specifically bound by LR0450 led to a significant inhibition of LR04 binding to PMVs (Figure 2B). Consistent with that action, preincubation with increasing concentrations of P2 led to a dose-dependent inhibition of the anticoagulatory effects of LR04 in PMV-triggered TG (Figure 2C; supplemental Figure 2C). Similarly to LR04, different monoclonal IgM antibodies with specificity for MDA (NA17) or the phosphocholine headgroup of oxidized phospholipids (E06) also inhibited TG (Figure 2D; supplemental Figure 2D). This finding is consistent with the previously reported presence of both types of OSEs on MVs.
To test whether LR04 inhibits TG in the presence of exogenous MV-associated TF, we used TF+ MVs generated from activated THP-1 cells, which are also bound by LR04 (supplemental Figure 2E), as a trigger. LR04 resulted in a dose-dependent reduction of THP1-MV triggered peak TG without affecting initiation (lag time), whereas a TF-blocking antibody (HTF-1) significantly delayed lag time (Figure 2E; supplemental Figure 2F), indicating that MDA-specific IgM antibodies limit the propagation of MV-dependent coagulation, irrespective of the presence of TF.
We next investigated whether LR04 also has an effect on clotting tests that is not dependent on the presence of MVs.55 We used aPTT and prothrombin time (PT) tests, which rely on the addition of exogenous phospholipids. Preincubation of pooled platelet-poor plasma with LR04 had no effect on aPTT or PT (Figure 2F). This outcome suggests that LR04 exerts its anticoagulatory effects by interacting with MVs, rather than by directly inhibiting the activity of certain coagulation factors. To further test this hypothesis, we evaluated the effect of LR04 on THP-1/MV–induced coagulation in plasmas with selective deficiency of individual coagulation factors of the extrinsic or intrinsic pathway (FV, FVII, FVIII, FIX, FXI, and FXII). A reduction of TG (peak height) by LR04 was observed in all deficient plasmas tested (Figure 2G), suggesting that LR04 does not selectively affect either the intrinsic or extrinsic arm of the coagulation cascade.
To investigate whether the anticoagulatory effect is caused by promoting aggregation of MVs, and thereby reducing the procoagulant surface, we assessed the size profile of MVs after incubation with LR04 or concanavalin A, which has been previously described as causing MV aggregation.57 Although concanavalin A caused a significant shift in the size profile of MVs, no measurable shift occurred after incubation with LR04 (Figure 2H; supplemental Figure 2G). We next tested whether LR04 affects the interaction between MVs and components of the common coagulation pathway. Because the binding of factor X/Xa to phospholipid surfaces (eg, MVs) is a crucial step in the formation of the prothrombinase complex, we tested whether LR04 could directly inhibit factor Xa binding to the phospholipids of MVs. To address this theory, we established an enzyme-linked immunosorbent assay for the detection of factor Xa binding to immobilized MVs. Purified factor Xa bound to immobilized MVs in a concentration-dependent manner. In contrast to LR04,35 no binding of factor Xa to MDA-modified bovine serum albumin was observed (supplemental Figure 2H). Furthermore, we reported the binding of LR04 to immobilized MVs (supplemental Figure 2I), which could be inhibited with the MDA peptide mimotope P2 (supplemental Figure 2J). To assess whether LR04 can compete with plasma-derived factor X/Xa binding to MVs, we established a competition enzyme-linked immunosorbent assay in which plasma was added to immobilized MVs (supplemental Figure 2K). Preincubation of immobilized MVs with LR04 but not an isotype control antibody significantly reduced binding of plasma-derived factor X/Xa to MVs in a concentration-dependent manner (Figure 2I).
LR04 delays initiation and propagation in whole blood clotting without affecting platelet aggregation
Activation of blood cells during coagulation results in the production of MVs, which in turn contributes to the propagation of blood clotting. Indeed, we observed dramatically higher numbers of MVs in serum compared with plasma of the same donor (supplemental Figure 3A). Using rotational thromboelastometry, we therefore tested the effect of LR04 on whole blood coagulation during which MVs are continuously released. Addition of LR04 but not an isotype control antibody significantly increased clotting time, clot formation time, and time of maximum clot firmness while significantly decreasing maximum clot firmness (Figure 3A-D). The strongest effect was observed in clot formation time, a measure of clotting propagation, which is consistent with the effects seen in TG. This effect could depend on the circulating MVs present, as well as affecting MVs that are generated during the clotting process.
LR04 delays whole blood clotting without affecting platelet aggregation. (A-D) Effect of LR04 on whole blood clotting using rotational thromboelastometry. Freshly drawn blood of healthy individuals (n = 11) was incubated for 20 minutes with 25 µg/mL of either LR04 or an isotype (iso) control antibody before initiating rotational thromboelastometry in the nonactivated method mode. (A) Clotting time, (B) clot formation time, (C) time of maximum clot firmness, and (D) maximum clot firmness. Bars represent the mean of each group. **P < .01, ***P < .005, Wilcoxon matched-pairs signed-rank test. (E-G) Effect of LR04 on platelet aggregation. Washed platelets isolated from healthy volunteers (n = 11-15) were preincubated for 20 minutes with LR04 or an IgM control antibody (25 µg/mL) before activation with thrombin (E), collagen (F), or adenosine 5′-diphosphate (ADP) (G). Shown are representative aggregation curves. au, arbitrary units.
LR04 delays whole blood clotting without affecting platelet aggregation. (A-D) Effect of LR04 on whole blood clotting using rotational thromboelastometry. Freshly drawn blood of healthy individuals (n = 11) was incubated for 20 minutes with 25 µg/mL of either LR04 or an isotype (iso) control antibody before initiating rotational thromboelastometry in the nonactivated method mode. (A) Clotting time, (B) clot formation time, (C) time of maximum clot firmness, and (D) maximum clot firmness. Bars represent the mean of each group. **P < .01, ***P < .005, Wilcoxon matched-pairs signed-rank test. (E-G) Effect of LR04 on platelet aggregation. Washed platelets isolated from healthy volunteers (n = 11-15) were preincubated for 20 minutes with LR04 or an IgM control antibody (25 µg/mL) before activation with thrombin (E), collagen (F), or adenosine 5′-diphosphate (ADP) (G). Shown are representative aggregation curves. au, arbitrary units.
Thrombocyte aggregation assays were performed to test the effect of LR04 on platelet function. Neither LR04 nor an isotype control antibody affected the aggregation of platelets isolated from healthy volunteers (Figure 3E-G; supplemental Figure 3B-C).
LR04 protects mice from MV-induced pulmonary thrombosis but does not affect hemostasis
We next tested whether LR04 could modulate hemostasis and MV-driven thrombosis in vivo. To assess the potential influence of LR04 on hemostasis, a murine tail bleeding assay was performed.58 After anesthesia, mice received injections with either LR04 or an isotype control antibody, and bleeding time was assessed after tail tip amputation. We observed no differences in bleeding time (Figure 4A) between the groups injected with either LR04 or the isotype. However, hemostasis is mediated largely by platelet aggregation and extravascular TF, whereas the contribution of circulating MVs is debated.44
LR04 does not affect tail bleeding time but protects mice in an MV-induced pulmonary thrombosis model. (A) Tail bleeding times of mice injected with LR04 (n = 8) or an isotype control antibody (n = 6), 25 µg/mL of estimated total blood volume. Columns represent mean ± SEM. (B) Representative microscopic fluorescent image of lung sections of mice injected with a mixture containing epinephrine (60 ng/g bw) and fluorescently labeled HPAF-MVs (0.1 µg/g bw). Nuclei were stained with 4′,6-diamidino-2-phenylindole (blue) and platelets with an anti-CD41 antibody (red), injected labeled MVs (green) (20× magnification). Arrows indicate thrombi (fibrin = bright pink). Kaplan-Meier survival curves of mice intravenously injected with a mixture containing epinephrine (60 ng/g bw) and HPAF-MVs (0.1 µg/g bw) (n = 22 per group) (C) or epinephrine (60 ng/g bw) and collagen (3 µg/g bw) (n = 5 per group) (D). The mixture was preincubated with LR04 or an isotype control antibody, 25 µg/mL of estimated total blood volume. Mice were observed for a maximum duration of 30 minutes. ***P < .005, log-rank (Mantel-Cox) test. (E) Representative microscopic images of picro-Mallory–stained lung sections of mice, which were injected with a mixture of epinephrine (60 ng/g bw) and HPAF-MVs (0.1 µg/g bw).The mixture was preincubated either with 25 µg/mL estimated blood volume (2.25 µg/g bw) of LR04 or isotype control antibody. 5× magnification and 20× magnification (top left), arrows indicate fibrin deposits. (F) Numbers of thrombi per visual field, counted at 10× magnification (n = 15 per group). Columns represent mean ± SEM. *P < .05, Mann-Whitney U test. (G) Summary cartoon (details are provided in the text). n.s., not significant.
LR04 does not affect tail bleeding time but protects mice in an MV-induced pulmonary thrombosis model. (A) Tail bleeding times of mice injected with LR04 (n = 8) or an isotype control antibody (n = 6), 25 µg/mL of estimated total blood volume. Columns represent mean ± SEM. (B) Representative microscopic fluorescent image of lung sections of mice injected with a mixture containing epinephrine (60 ng/g bw) and fluorescently labeled HPAF-MVs (0.1 µg/g bw). Nuclei were stained with 4′,6-diamidino-2-phenylindole (blue) and platelets with an anti-CD41 antibody (red), injected labeled MVs (green) (20× magnification). Arrows indicate thrombi (fibrin = bright pink). Kaplan-Meier survival curves of mice intravenously injected with a mixture containing epinephrine (60 ng/g bw) and HPAF-MVs (0.1 µg/g bw) (n = 22 per group) (C) or epinephrine (60 ng/g bw) and collagen (3 µg/g bw) (n = 5 per group) (D). The mixture was preincubated with LR04 or an isotype control antibody, 25 µg/mL of estimated total blood volume. Mice were observed for a maximum duration of 30 minutes. ***P < .005, log-rank (Mantel-Cox) test. (E) Representative microscopic images of picro-Mallory–stained lung sections of mice, which were injected with a mixture of epinephrine (60 ng/g bw) and HPAF-MVs (0.1 µg/g bw).The mixture was preincubated either with 25 µg/mL estimated blood volume (2.25 µg/g bw) of LR04 or isotype control antibody. 5× magnification and 20× magnification (top left), arrows indicate fibrin deposits. (F) Numbers of thrombi per visual field, counted at 10× magnification (n = 15 per group). Columns represent mean ± SEM. *P < .05, Mann-Whitney U test. (G) Summary cartoon (details are provided in the text). n.s., not significant.
To assess the effect on MV-driven coagulatory processes in vivo, we adapted an established model of MV-induced murine pulmonary thrombosis,52 using epinephrine together with MVs released by the pancreatic cancer cell line HPAF-II as a trigger, which possess a strong procoagulatory activity.59 The rapid occurrence of thrombotic events in this model allows assessment of the direct anticoagulatory properties of IgM antibodies, independent of their known anti-inflammatory effects. The isolated HPAF-MVs displayed high TF positivity (supplemental Figure 4A) as well as LR04 binding (supplemental Figure 4B), and induced TG in MV-free plasma already after the addition of low concentrations. Preincubation with LR04 led to a strong inhibition of TG peak height (supplemental Figure 4C).
We next tested the prothrombotic effect of HPAF-MVs in vivo. Coinjection of HPAF-MVs and epinephrine, but not epinephrine alone, led to death within 10 minutes (supplemental Figure 4D). Microscopic evaluation of the lungs of these animals revealed the presence of thrombi, as indicated by deposition of fibrin (supplemental Figure 4E) and platelets (supplemental Figure 4F-G). Importantly, we observed the presence of injected fluorescently labeled HPAF-MVs within thrombi (Figure 4B). Coinjection of HPAF-MVs with LR04 but not an isotype control significantly rescued mice from death due to pulmonary thrombosis (Figure 4C). However, LR04 had no effect when collagen was used as a prothrombotic trigger (Figure 4D), further illustrating the MV-dependent mode of action of these antibodies. Importantly, the number of thrombi was significantly lower in lung sections of animals that were coinjected with MVs and LR04 compared with MVs and isotype control (Figure 4E-F).
Discussion
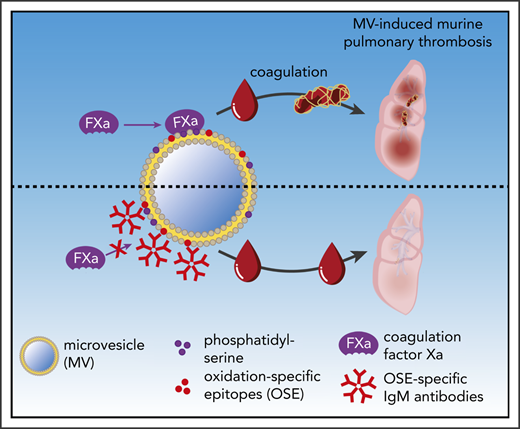
Circulating MVs are considered to be critical mediators of coagulation, and their increased levels and procoagulatory potential have been reported in cardiovascular disease and other pathologic states associated with increased risk for thrombotic events.38,45,48 The current study identified natural IgM antibodies, and in particular IgM antibodies that bind to OSEs, as important modulators of the procoagulatory properties of MVs (Figure 4G). We found that depletion of IgM antibodies from plasma increases its coagulatory capacity and that the presence of IgM on circulating MVs is associated with a lower coagulatory potential. These findings identify IgM antibodies as a protective factor with a direct anticoagulatory function.
Interestingly, splenectomy has been shown to lead to an elevated long-term risk of venous thrombosis60 and an increased rate of myocardial infarctions.61 However, the mechanisms behind these observations have been elusive. Notably, splenectomy results in decreased IgM levels,62 which might explain the prothrombotic state of these patients. In line with this, we and others have recently shown that low levels of OSE IgM antibodies are associated with an increased risk of venous thrombosis.27,63 Thus, our findings offer an explanation for the high thrombotic risk associated with low IgM levels. Moreover, elevated numbers of circulating MVs in splenectomized individuals have been suggested to contribute to a prothrombotic state.64
High levels of IgM antibodies, and IgM antibodies with a specificity for OSEs in particular, have also been shown to be inversely associated with atherosclerotic cardiovascular events.25,26,28 The protective effect of OSE-specific IgM antibodies in atherosclerotic cardiovascular disease has largely been attributed to their capacity to inhibit atherogenesis by neutralizing the proinflammatory effects of oxidized phospholipids, blocking the scavenger receptor–mediated uptake of oxidized LDL, and promoting the clearance of apoptotic cells.16,29,65 Of note, we have shown that MDA-specific IgM antibodies inhibit the ability of MVs to induce interleukin-8 production by monocytes.35 The anticoagulatory effect of OSE-specific IgM antibodies that we describe here identifies a novel function that is not directly linked to the antibodies’ ability to neutralize the proinflammatory effects of lipid peroxidation products. Oxidized phospholipids, such as phosphatidylethanolamine and phosphatidylcholine, have been shown to increase the phosphatidylserine accessibility of membranes to coagulation factors by changing the size and curvature of liposomes.66 Similar effects may occur on the surface of MVs, which are a major source of procoagulant phospholipids in vivo.38 Therefore, the recognition of lipid oxidation products present on the surface of MVs may modulate their procoagulant potential. Indeed, we found that MV-dependent TG is delayed by the addition of different OSE-specific IgM antibodies binding either MDA (LR04 and NA17) or the phosphocholine headgroup of oxidized phospholipids (E06) but not a control IgM antibody. Notably, we previously showed that one-third of the natural IgM in plasma has specificity for OSE and that most MV-associated IgM binds to MDA- or copper-oxidized LDL, the cognate antigens of NA17, LR04, or E06, respectively.30,35 Because IgM antibodies with specificity for different OSEs mediate a similar anticoagulatory function, their combined effects may be even more potent.
Importantly, LR04 delayed TG (in particular, its propagation) triggered by both TF+ and TF– MV, showing that the effect occurs after initiation by both the extrinsic and intrinsic pathways. This scenario indicates that LR04 interferes with MVs interacting with the common pathway of the coagulation cascade. A key event in the common pathway is factor X binding to negatively charged phospholipids as found on MVs, leading to the assembly of the prothrombinase complex.67 This explanation is supported by our finding that LR04 competes binding of factor X/Xa to immobilized MVs, whereas it does not cause MV aggregation. The fact that factor Xa itself does not directly bind to MDA epitopes, which are recognized by LR04, indicates that binding of LR04 to MVs indirectly interferes with the phosphatidylserine accessibility for factor Xa on MV surfaces and therefore decreases their procoagulatory capacity.
LR04 also delayed clot formation, most prominently clot formation time (ie, propagation), in the dynamic situation of constant MV production that occurs during whole blood clotting, which depends on both plasmatic and cellular components of coagulation. Of note, LR04 did not affect aggregation of isolated platelets, providing further support for the notion that the effect of LR04 relies on the interaction between MVs and plasmatic coagulation components. Importantly, the antithrombotic effect of LR04 was also observed in vivo, when MVs were used to induce pulmonary thrombosis in mice but not when collagen was used. These data further illustrate the specificity of the anticoagulatory effect of LR04 for MV-driven coagulation in contrast to collagen-induced clot formation that primarily depends on platelets.
Notably, OSE-specific IgM antibodies had no effect on clotting assays that depend on the addition of exogenous phospholipids and are not sensitive to MVs (aPTT). This finding is in contrast to antiphospholipid syndrome antibodies that recognize the phospholipid cardiolipin and/or β2-glycoprotein 1, and are associated with an increased risk of thrombosis but prolong phospholipid-dependent clotting times in vitro.68 Furthermore, epidemiologically, OSE-specific IgM antibodies are associated with lower risk of arterial25,26,28 and venous27,63 thrombotic events. Thus, these natural IgM antibodies represent a different group of antibodies that confer MV-dependent anticoagulatory effects both in vitro and in vivo.
Our findings have several clinical implications. First, assessing OSE-specific IgM levels could improve patient stratification within at-risk populations to decide on the necessity or intensity of anticoagulatory therapies. This might be particularly important in thrombotic pathologies in which MVs are considered to contribute to the thrombotic burden, such as cancer-associated thrombosis49 and acute coronary syndrome.69 Second, the therapeutic potential of OSE-specific IgM antibodies could be explored by both developing therapeutic monoclonal antibodies and identifying factors that stimulate production of OSE-specific natural IgM antibodies.
Requests for original data may be submitted to the corresponding author (Christoph J. Binder; e-mail: christoph.binder@meduniwien.ac.at).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by the SFB-54 “InThro” of the Austrian Science Fund, the Christian Doppler Laboratory for Innovative Therapy Approaches in Sepsis, and Cell Communication in Health and Disease of the Austrian Science Fund.
Authorship
Contribution: G.O., T.A., and W.S. conducted experiments and performed data analysis; G.O., T.A., and C.J.B. designed experiments and wrote the article; G.O. and T.A. handled all in vivo experiments; L.G. and T.A. established and performed histologic treatment and analysis of mouse lungs; S.T. performed histologic analysis of mouse lungs; F.P. designed, conducted, and supervised the flow cytometry experiments; M.S. performed and supervised the rotational thromboelastometry experiments; W.S. conducted thrombocyte aggregation experiments and analysis; F.P., C.A., I.P., B.J., A.A., and N.M. contributed to writing and critical evaluation of the article; and C.J.B. and T.A. supervised and coordinated the effort.
Conflict-of-interest disclosure: C.J.B. is a board member of Technoclone. The remaining authors declare no competing financial interests.
Correspondence: Christoph J. Binder, Medical University of Vienna, Department of Laboratory Medicine, Lazarettgasse 14, AKH BT25/2, 1090 Vienna, Austria; e-mail: christoph.binder@meduniwien.ac.at.
REFERENCES
Author notes
G.O. and T.A. contributed equally to this study.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal