Key Points
Autologous transplant provides durable remissions in DLBCL with PET+ PR.
Autologous transplant remains the standard of care for relapsed chemosensitive DLBCL.
Abstract
For relapsed chemosensitive diffuse large B-cell lymphoma (DLBCL), consolidation with autologous hematopoietic cell transplantation (auto-HCT) is a standard option. With the approval of anti-CD19 chimeric antigen receptor T cells in 2017, the Center for International Blood and Marrow Transplant Research (CIBMTR) reported an ?∼10% decrease in the number of auto-HCTs for DLBCL in the United States. Using the CIBMTR database, we identified 249 relapsed DLBCL patients undergoing auto-HCT from 2003 to 2013 with a positive positron emission tomography/computed tomography (PET/CT)+ partial response prior to transplant were identified. The study cohort was divided into 2 groups: early chemoimmunotherapy failure (ECF), defined as patients with primary refractory disease (PRefD) or relapse within 12 months of diagnosis and late chemoimmunotherapy failure, defined as patients relapsing after ≥12 months. Primary outcome was overall survival (OS). Secondary outcomes included progression-free survival (PFS) and relapse. Atotal of 182 patients had ECF, whereas 67 did not. Among ECF cohort, 79% had PRefD. The adjusted 5-year probabilities for PFS and OS (ECF vs no ECF) were not different: 41% vs 41% (P = .93) and 51% vs 63% (P = .09), respectively. On multivariate analysis, ECF patients had an increased risk for death (hazard ratio, 1.61; 95% confidence interval, 1.05-2.46; P = .03) but not for PFS or relapse. In conclusion, for relapsed chemosensitive DLBCL patients with residual PET/CT+ disease prior to auto-HCT, the adjusted 5-year PFS (41%) was comparable, irrespective of time to relapse. These data support ongoing application of auto-HCT in chemosensitive DLBCL.
Introduction
High-dose therapy and autologous hematopoietic cell transplantation (auto-HCT) are the standard of care for transplant-eligible relapsed diffuse large B-cell lymphoma (DLBCL) patients with chemosensitive disease.1-3 Demonstrating chemosensitive disease following salvage therapy, as defined by achieving complete remission (CR) or partial remission (PR) assessed by radiographic imaging,4 is the key factor in determining a patient’s eligibility for auto-HCT consolidation. In patients receiving modern rituximab and anthracycline–containing frontline therapies, early failure of therapy (within 1 year of initial diagnosis) is a well-known predictor of inferior outcome.5,6 However, several studies have shown that, despite early failure of first-line chemoimmunotherapy, diffuse large B-cell lymphoma (DLBCL) patients with chemosensitive disease after ≥1 salvage treatment can achieve durable disease control with auto-HCT consolidation.2,5,7,8 Thus, outside the setting of a clinical trial, auto-HCT remains the standard consolidation option in chemosensitive relapsed DLBCL, regardless of the timing of therapy failure.
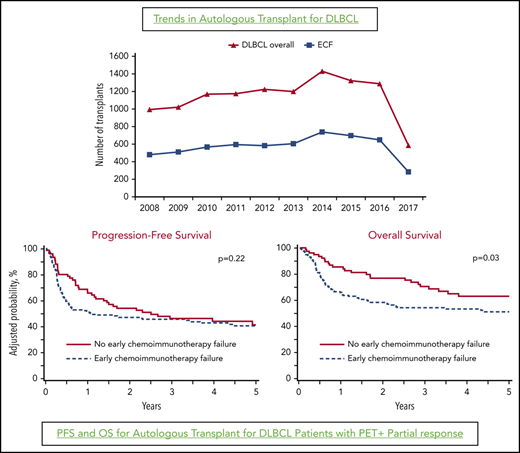
With the approval of anti-CD19 chimeric antigen receptor (CAR) T-cell therapies in 2017,9 relapsed DLBCL patients who achieve only a PR after salvage therapies with residual positron emission tomography (PET)-avid disease are increasingly being offered CAR T-cell therapy in lieu of auto-HCT. According to Center for International Blood and Marrow Transplant Research (CIBMTR) data in 2018, the number of auto-HCTs for DLBCL in the United States decreased by ∼10% from prior years(2015–2017) (Figure 1),10 potentially as a result of the application of CAR T-cell therapy for chemorefractory patients (in line with the US Food and Drug Administration label for these therapies), as well as for chemosensitive DLBCL patients not achieving a CR. Using the CIBMTR database, we report outcomes of auto-HCT in relapsed chemosensitive DLBCL patients achieving only a PET+ PR prior to auto-HCT, relative to the timing of the failure of current standard frontline chemoimmunotherapies.
Methods
Data source
The CIBMTR is a collaborative research program managed by the Medical College of Wisconsin and The National Marrow Donor Program that collects data from >380 transplant centers worldwide. Participating sites are required to report detailed data on autologous and allogeneic hematopoietic cell transplantation (HCT), with frequent updates gathered during the longitudinal follow-up of transplant patients; compliance is monitored by on-site audits. Computerized checks for discrepancies, physicians’ review of submitted data, and on-site audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. The Medical College of Wisconsin and The National Marrow Donor Program institutional review boards approved this study. Research was conducted in accordance with the Declaration of Helsinki.
Patients
Adult (age ≥18 years) DLBCL patients who achieved a PR in response to chemotherapy, and subsequently undergoing an auto-HCT between 2003 and 2018, were included in this analysis. To ensure that residual nodal disease represented viable lymphoma, patients in PR by computed tomography (CT) criteria but with a negative PET scan (n = 35) or those without an available PET scan before auto-HCT (n = 42) were excluded. All patients received rituximab-containing anthracycline-based chemoimmunotherapy in the first-line setting (rituximab, cyclophosphamide, adriamycin, vincristine, prednisone [R-CHOP] or R-dose–adjusted etoposide, prednisone, vincristine, cyclophosphamide, and adriamycin [EPOCH]). Conditioning for auto-HCT was limited to carmustine, etoposide, cytarabine, and melphalan; cyclophosphamide, carmustine, and etoposide; and busulfan and cyclophosphamide. Patients who received a bone marrow graft for transplant (n = 36), those who were treated with frontline therapy not containing rituximab (n = 201), and patients with active central nervous system involvement prior to auto-HCT (n = 9) were excluded.
Definitions and end points
Response to frontline chemoimmunotherapy and disease status prior to auto-HCT were determined using the International Working Group criteria.11,12 Early chemoimmunotherapy failure (ECF) was defined as not achieving a CR after the first line of chemoimmunotherapy or relapse/progression within 1 year of initial diagnosis, as previously reported.5,7 The positive PET status (typically performed within 1 month) before auto-HCT was as assessed by the local radiology teams in individual transplantation centers.
The primary end point was overall survival (OS). Death from any cause was considered an event, and surviving patients were censored at last follow-up. Secondary outcomes included nonrelapse mortality (NRM), relapse/progression, and progression-free survival (PFS). NRM was defined as death without evidence of prior lymphoma progression/relapse; relapse was considered a competing risk. Relapse/progression was defined as progressive lymphoma after auto-HCT or lymphoma recurrence after a CR; NRM was considered a competing risk. For PFS, a patient was considered a treatment failure at the time of progression/relapse or death from any cause. Patients alive without evidence of disease relapse or progression were censored at last follow-up.
Statistical analysis
All of the end points were compared between patients with or without ECF. Patient-, disease-, and transplant-related variables were compared between the 2 cohorts using the χ2 test for categorical variables and the Wilcoxon 2-sample test for continuous variables. The distributions of OS and PFS were estimated using the Kaplan-Meier method. The cumulative-incidence method was used to estimate NRM and relapse/progression, while accounting for competing events. Cox proportional-hazards analysis was used to identify prognostic factors for relapse, NRM, PFS, and OS using forward stepwise variable selection. No covariates violated the proportional-hazards assumption. No significant interactions between the main effect and significant covariates were found. Results are reported as hazard ratio (HR), 95% confidence interval (CI) for HR and P value. Covariates with a P value < .05 were considered statistically significant. The variables considered in multivariate analysis (MVA) are shown in supplemental Table 1 (available on the Blood Web site). All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
Results
Baseline characteristics
The study population consisted of 249 DLBCL patients in PET+ PR before auto-HCT and was divided into 2 cohorts: patients with ECF (n = 182) and patients without ECF (n = 67). The baseline characteristics between the 2 groups were comparable (Table 1), with respect to patient sex, race, Karnofsky performance score, number of prior therapy lines, bone marrow or extranodal involvement at diagnosis, residual disease bulk at HCT, and type of auto-HCT conditioning received. Median age of patients in the no-ECF cohort was significantly higher compared with patients in the ECF cohort (63 vs 57 years; P < .001). Significantly more patients with ECF had advanced-stage disease at diagnosis (74.2% vs 53.7%; P = .003). As expected, median time from diagnosis to auto-HCT was shorter in the ECF cohort (11 vs 35 months; P < .001). The median follow-up of survivors was 69 months (range, 5-123) and 53 months (range, 10-149) in the no early chemoimmunotherapy and the early chemoimmunotherapy groups, respectively.
Baseline characteristics of DLBCL patients in a PET+ PR before autologous transplantation
| . | No ECF . | ECF . | P . |
|---|---|---|---|
| No. of patients | 67 | 182 | |
| No. of centers | 39 | 64 | |
| Patient age | |||
| Μedian (range), y | 63 (39-77) | 57 (20-76) | <.001 |
| ≥65 y, n (%) | 29 (43.3) | 47 (25.8) | |
| Males | 38 (56.7) | 106 (58.2) | .83 |
| Patient race | .20 | ||
| White | 56 (83.6) | 134 (73.6) | |
| African American | 7 (10.4) | 34 (18.7) | |
| Other* | 4 (6) | 9 (4.9) | |
| Missing data | 0 | 5 (2.8) | |
| Karnofsky performance score | |||
| ≥90 | 32 (47.8) | 94 (51.6) | .83 |
| Missing data | 2 (3) | 4 (2.2) | |
| Disease at diagnosis | |||
| Stage III-IV | 36 (53.7) | 135 (74.2) | .003 |
| Missing data | 5 (7.5) | 14 (7.7) | |
| LDH | |||
| Elevated at diagnosis | 9 (13.4) | 24 (13.2) | .14 |
| Missing data | 45 (67.2) | 100 (54.9) | |
| Lines of therapy prior to HCT, median (range), n | 2 (1-5) | 2 (1-5) | .65 |
| Bone marrow involvement at diagnosis | |||
| No | 52 (77.6) | 143 (78.6) | .53 |
| Missing data | 7 (10.4) | 12 (6.6) | |
| Extranodal involvement at diagnosis | |||
| Yes | 31 (46.3) | 105 (57.7) | .24 |
| Missing data | 7 (10.4) | 12 (6.6) | |
| Time from diagnosis to HCT, median (range), mo | 35 (16-149) | 11 (3-146) | <.001 |
| Residual nodal bulk at HCT | .18 | ||
| Nonbulky (<5 cm) | 24 (35.8) | 87 (47.8) | |
| Bulky (≥5 cm) | 7 (10.5) | 22 (12.1) | |
| Not reported | 36 (53.7) | 73 (40.1) | |
| Conditioning regimen | .88 | ||
| BEAM | 55 (82.1) | 147 (80.8) | |
| Bu/Cy | 7 (10.4) | 23 (12.6) | |
| CBV | 5 (7.5) | 12 (6.6) | |
| Primary refractory after first line of therapy | 0 | 144 (79.1) | <.001 |
| Follow-up of survivors, median (range), mo | 69 (5-123) | 53 (10-149) |
| . | No ECF . | ECF . | P . |
|---|---|---|---|
| No. of patients | 67 | 182 | |
| No. of centers | 39 | 64 | |
| Patient age | |||
| Μedian (range), y | 63 (39-77) | 57 (20-76) | <.001 |
| ≥65 y, n (%) | 29 (43.3) | 47 (25.8) | |
| Males | 38 (56.7) | 106 (58.2) | .83 |
| Patient race | .20 | ||
| White | 56 (83.6) | 134 (73.6) | |
| African American | 7 (10.4) | 34 (18.7) | |
| Other* | 4 (6) | 9 (4.9) | |
| Missing data | 0 | 5 (2.8) | |
| Karnofsky performance score | |||
| ≥90 | 32 (47.8) | 94 (51.6) | .83 |
| Missing data | 2 (3) | 4 (2.2) | |
| Disease at diagnosis | |||
| Stage III-IV | 36 (53.7) | 135 (74.2) | .003 |
| Missing data | 5 (7.5) | 14 (7.7) | |
| LDH | |||
| Elevated at diagnosis | 9 (13.4) | 24 (13.2) | .14 |
| Missing data | 45 (67.2) | 100 (54.9) | |
| Lines of therapy prior to HCT, median (range), n | 2 (1-5) | 2 (1-5) | .65 |
| Bone marrow involvement at diagnosis | |||
| No | 52 (77.6) | 143 (78.6) | .53 |
| Missing data | 7 (10.4) | 12 (6.6) | |
| Extranodal involvement at diagnosis | |||
| Yes | 31 (46.3) | 105 (57.7) | .24 |
| Missing data | 7 (10.4) | 12 (6.6) | |
| Time from diagnosis to HCT, median (range), mo | 35 (16-149) | 11 (3-146) | <.001 |
| Residual nodal bulk at HCT | .18 | ||
| Nonbulky (<5 cm) | 24 (35.8) | 87 (47.8) | |
| Bulky (≥5 cm) | 7 (10.5) | 22 (12.1) | |
| Not reported | 36 (53.7) | 73 (40.1) | |
| Conditioning regimen | .88 | ||
| BEAM | 55 (82.1) | 147 (80.8) | |
| Bu/Cy | 7 (10.4) | 23 (12.6) | |
| CBV | 5 (7.5) | 12 (6.6) | |
| Primary refractory after first line of therapy | 0 | 144 (79.1) | <.001 |
| Follow-up of survivors, median (range), mo | 69 (5-123) | 53 (10-149) |
Unless otherwise noted, data are n (%).
BEAM, carmustine, etoposide, cytarabine and melphalan; Bu/Cy, busulfan/cyclophosphamide; CBV, cyclophosphamide, carmustine, and etoposide; LDH: lactate dehydrogenase.
No ECF: 3 Asian and 1 Native American. ECF: 7 Asian, 1 Pacific Islander, and 1 Native American.
NRM and relapse/progression
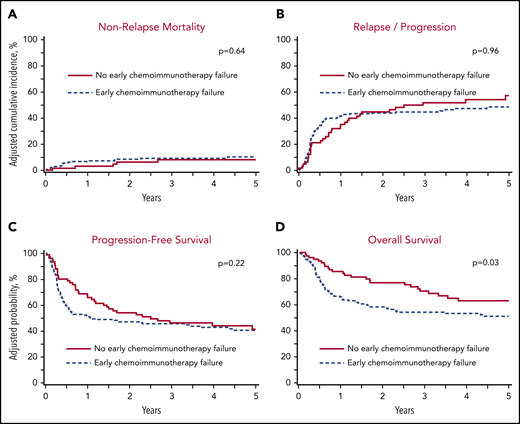
The adjusted 1-year cumulative incidence of NRM in the no-ECF and ECF cohorts was 3% (95% CI, 0.6-9.5%) vs 6.6% (95% CI, 3.6-10.8%), respectively (P = .21; Figure 2A; Table 2). On MVA, the risk of NRM was not significantly different between the no-ECF and ECF cohorts (HR, 1.01; 95% CI, 0.68-1.50; P = .96; Table 3).
Autologous transplant outcomes in DLBCL patients in a PET+ PR prior to transplant. (A) NRM. (B) Progression/relapse. (C) PFS. (D) OS.
Autologous transplant outcomes in DLBCL patients in a PET+ PR prior to transplant. (A) NRM. (B) Progression/relapse. (C) PFS. (D) OS.
Adjusted autologous transplantation outcomes
| Outcomes . | No ECF (n = 67) . | ECF (n = 182) . | P . |
|---|---|---|---|
| NRM | |||
| 1-y | 3.0 (0.6-9.5) | 6.6 (3.6-10.8) | .21 |
| 3-y | 8.0 (2.9-16.5) | 9.0 (5.4-13.9) | .80 |
| 5-y | 8.0 (2.9-16.5) | 10.1 (6.1-15.5) | .62 |
| Progression or relapse | |||
| 1-y | 34.8 (23.5-46.4) | 41.3 (34.1-48.4) | .36 |
| 3-y | 51.5 (38.3-63.1) | 44.3 (36.9-51.5) | .34 |
| 5-y | 57.0 (42.5-69.1) | 48.2 (40.3-55.7) | .27 |
| PFS | |||
| 1-y | 65.7 (54.9-76.6) | 51.1 (44.0-58.2) | .03 |
| 3-y | 46.1 (34.5-57.8) | 45.5 (38.4-52.6) | .93 |
| 5-y | 41.2 (29.0-53.5) | 40.5 (33.1-48.0) | .93 |
| OS | |||
| 1-y | 85.4 (77.4-93.3) | 65.8 (59.0-72.6) | <.001 |
| 3-y | 70.4 (59.8-81.0) | 54.0 (46.9-61.2) | .01 |
| 5-y | 62.9 (51.3-74.6) | 51.0 (43.6-58.5) | .09 |
| Outcomes . | No ECF (n = 67) . | ECF (n = 182) . | P . |
|---|---|---|---|
| NRM | |||
| 1-y | 3.0 (0.6-9.5) | 6.6 (3.6-10.8) | .21 |
| 3-y | 8.0 (2.9-16.5) | 9.0 (5.4-13.9) | .80 |
| 5-y | 8.0 (2.9-16.5) | 10.1 (6.1-15.5) | .62 |
| Progression or relapse | |||
| 1-y | 34.8 (23.5-46.4) | 41.3 (34.1-48.4) | .36 |
| 3-y | 51.5 (38.3-63.1) | 44.3 (36.9-51.5) | .34 |
| 5-y | 57.0 (42.5-69.1) | 48.2 (40.3-55.7) | .27 |
| PFS | |||
| 1-y | 65.7 (54.9-76.6) | 51.1 (44.0-58.2) | .03 |
| 3-y | 46.1 (34.5-57.8) | 45.5 (38.4-52.6) | .93 |
| 5-y | 41.2 (29.0-53.5) | 40.5 (33.1-48.0) | .93 |
| OS | |||
| 1-y | 85.4 (77.4-93.3) | 65.8 (59.0-72.6) | <.001 |
| 3-y | 70.4 (59.8-81.0) | 54.0 (46.9-61.2) | .01 |
| 5-y | 62.9 (51.3-74.6) | 51.0 (43.6-58.5) | .09 |
Data are percentage probability (95% CI).
Multivariate analysis
| Outcome . | n . | HR . | 95% CI . | Overall P . |
|---|---|---|---|---|
| Relapse/progression | ||||
| ECF | ||||
| No | 67 | 1 | .96 | |
| Yes | 182 | 1.01 | 0.68-1.50 | |
| NRM | ||||
| ECF | ||||
| No | 67 | 1 | .64 | |
| Yes | 182 | 1.24 | 0.49-3.15 | |
| PFS* | ||||
| ECF | ||||
| No | 67 | 1 | .22 | |
| Yes | 182 | 1.26 | 0.87-1.83 | |
| OS* | ||||
| ECF | ||||
| No | 67 | 1 | .03 | |
| Yes | 182 | 1.61 | 1.05-2.46 |
| Outcome . | n . | HR . | 95% CI . | Overall P . |
|---|---|---|---|---|
| Relapse/progression | ||||
| ECF | ||||
| No | 67 | 1 | .96 | |
| Yes | 182 | 1.01 | 0.68-1.50 | |
| NRM | ||||
| ECF | ||||
| No | 67 | 1 | .64 | |
| Yes | 182 | 1.24 | 0.49-3.15 | |
| PFS* | ||||
| ECF | ||||
| No | 67 | 1 | .22 | |
| Yes | 182 | 1.26 | 0.87-1.83 | |
| OS* | ||||
| ECF | ||||
| No | 67 | 1 | .03 | |
| Yes | 182 | 1.61 | 1.05-2.46 |
Adjusted for significant covariate: patient age.
The adjusted 5-year cumulative incidence of relapse/progression was 57% (95% CI, 2.5-69.1%) in the no-ECF cohort and 48.2% (95% CI, 40.3-55.7%) in the ECF cohort (P = .27; Figure 2B; Table 2). The risk of relapse/progression was not significantly different between those cohorts (HR, 1.24; 95% CI, 0.49-3.15; P = .64; Table 3).
PFS and OS
The adjusted 5-year PFS was 41.2% (95% CI, 29-53.5%) in the no-ECF cohort compared with 40.5% (95% CI, 33.1-48%) in the ECF group (P = .93; Figure 2C; Table 2). On MVA for patient, ECF was not associated with a significantly inferior PFS (HR, 1.26; 95% CI, 0.87-1.83; P = .22; Table 3).
The adjusted 5-year OS was 62.9% (95% CI, 51.3-74.6%) in the no-ECF cohort compared with 51% (95% CI, 43.6-58.5%) in the ECF group (P = .09; Figure 2D; Table 2). On MVA, after adjusting for patient, ECF was associated with a significantly higher mortality risk (HR, 1.61; 95% CI, 1.05-2.46; P = .03; Table 3).
Subgroup analysis of patients with ≥2 lines of chemotherapy
Because 35 patients with PET+ PR in our primary analysis received only 1 line of therapy before auto-HCT, we performed a subset analysis limiting the study population to patients who had received ≥2 lines of therapy before auto-HCT (Table 4). In this adjusted subset analysis, similar to the overall study findings, there was no difference in 1-year NRM or 5-year cumulative incidence rates of relapse/progression among ECF patients (n = 150) and no-ECF patients (n = 64). Although 1-year PFS favored no-ECF patients (67% vs 50%; P = .01), there was no difference in 5-year PFS. Additionally, although there was an OS benefit at 1 year, the 5-year OS was not significantly different: 62% for no-ECF patients vs 48% for ECF patients (P = .08).
Adjusted outcomes for DLBCL patients undergoing auto-HCT in PR who received ≥2 prior lines of chemotherapy
| Outcomes . | No ECF (n = 64) . | ECF (n = 150) . | P . |
|---|---|---|---|
| NRM | |||
| 1-y | 3.2 (0.06-9.9) | 7.3 (3.9-12.3) | .18 |
| 3-y | 8.4 (3-17.3) | 10.3 (6-16) | .66 |
| 5-y | 8.4 (3-17.3) | 11.6 (6.8-17.7) | .49 |
| Progression or relapse | |||
| 1-y | 33.3 (21.9-45.1) | 41.4 (33.4-49.2) | .26 |
| 3-y | 50.8 (37.3-62.7) | 45.1 (36.9-53) | .47 |
| 5-y | 56.5 (41.7-68.9) | 49.5 (40.7-57.7) | .40 |
| PFS | |||
| 1-y | 67.2 (56.2-78.2) | 49.9 (42-57.7) | .01 |
| 3-y | 46.6 (34.6-58.7) | 43.1 (35.3-50.9) | .63 |
| 5-y | 41.5 (28.7-54.2) | 37.5 (29.4-45.6) | .61 |
| OS | |||
| 1-y | 84.6 (76.2-92.9) | 63.2 (55.5-70.8) | <.001 |
| 3-y | 69.1 (58.2-80.1) | 51.9 (43.9-59.8) | .01 |
| 5-y | 61.6 (49.6-73.6) | 48.4 (40.2-56.7) | .08 |
| Outcomes . | No ECF (n = 64) . | ECF (n = 150) . | P . |
|---|---|---|---|
| NRM | |||
| 1-y | 3.2 (0.06-9.9) | 7.3 (3.9-12.3) | .18 |
| 3-y | 8.4 (3-17.3) | 10.3 (6-16) | .66 |
| 5-y | 8.4 (3-17.3) | 11.6 (6.8-17.7) | .49 |
| Progression or relapse | |||
| 1-y | 33.3 (21.9-45.1) | 41.4 (33.4-49.2) | .26 |
| 3-y | 50.8 (37.3-62.7) | 45.1 (36.9-53) | .47 |
| 5-y | 56.5 (41.7-68.9) | 49.5 (40.7-57.7) | .40 |
| PFS | |||
| 1-y | 67.2 (56.2-78.2) | 49.9 (42-57.7) | .01 |
| 3-y | 46.6 (34.6-58.7) | 43.1 (35.3-50.9) | .63 |
| 5-y | 41.5 (28.7-54.2) | 37.5 (29.4-45.6) | .61 |
| OS | |||
| 1-y | 84.6 (76.2-92.9) | 63.2 (55.5-70.8) | <.001 |
| 3-y | 69.1 (58.2-80.1) | 51.9 (43.9-59.8) | .01 |
| 5-y | 61.6 (49.6-73.6) | 48.4 (40.2-56.7) | .08 |
Data are percentage probability (95% CI). Bold values are statistically significant P values.
Subgroup analysis of patients receiving a single platinum-based salvage
A second subset analysis was performed limiting the patient population to those who received second-line therapy with a platinum-based regimen (Table 5). This included patients receiving ifosfamide-carboplatin-etoposide; gemcitabine-dexamethasone-cisplatin; dexamethasone-cytarabine-cisplatin; or gemcitabine-oxaliplatin. Among these patients (n = 120), the ECF patients (n = 40) had an adjusted 5-year PFS of 33% vs 40% for no-ECF patients (n = 80; P = .42). Similarly, although the 5-year OS favored no-ECF patients (59% vs 47%), there was no statistically significant difference (P = .22).
Adjusted outcomes for DLBCL patients undergoing auto-HCT in PR receiving platinum-based salvage regimens as second-line therapy
| Outcomes . | No ECF (n = 40) . | ECF (n = 80) . | P . |
|---|---|---|---|
| NRM | |||
| 1-y | 5.1 (0.9-15.3) | 7.5 (3-14.7) | .60 |
| 3-y | 7.7 (1.9-18.9) | 9.1 (4-17) | .79 |
| 5-y | 7.7 (1.9-18.9) | 9.1 (4-17) | .79 |
| Progression or relapse | |||
| 1-y | 32.8 (18.8-47.7) | 42.5 (31.5-53.1) | .31 |
| 3-y | 54.5 (37-69) | 48.2 (36.6-58.9) | .53 |
| 5-y | 58.3 (40-72.8) | 56.8 (43.7-67.9) | .88 |
| PFS | |||
| 1-y | 65.9 (52-79.9) | 48.4 (37-59.1) | .05 |
| 3-y | 43.8 (28.8-58.9) | 48.2 (30.4-51.7) | .77 |
| 5-y | 40.4 (25.1-55.8) | 32.7 (21.7-43.7) | .42 |
| OS | |||
| 1-y | 81.4 (69.8-93) | 60.1 (49.4-70.8) | .01 |
| 3-y | 68.5 (54.3-82.8) | 49.6 (38.4-60.7) | .04 |
| 5-y | 59.4 (43.6-75.1) | 47.2 (35.8-58.7) | .22 |
| Outcomes . | No ECF (n = 40) . | ECF (n = 80) . | P . |
|---|---|---|---|
| NRM | |||
| 1-y | 5.1 (0.9-15.3) | 7.5 (3-14.7) | .60 |
| 3-y | 7.7 (1.9-18.9) | 9.1 (4-17) | .79 |
| 5-y | 7.7 (1.9-18.9) | 9.1 (4-17) | .79 |
| Progression or relapse | |||
| 1-y | 32.8 (18.8-47.7) | 42.5 (31.5-53.1) | .31 |
| 3-y | 54.5 (37-69) | 48.2 (36.6-58.9) | .53 |
| 5-y | 58.3 (40-72.8) | 56.8 (43.7-67.9) | .88 |
| PFS | |||
| 1-y | 65.9 (52-79.9) | 48.4 (37-59.1) | .05 |
| 3-y | 43.8 (28.8-58.9) | 48.2 (30.4-51.7) | .77 |
| 5-y | 40.4 (25.1-55.8) | 32.7 (21.7-43.7) | .42 |
| OS | |||
| 1-y | 81.4 (69.8-93) | 60.1 (49.4-70.8) | .01 |
| 3-y | 68.5 (54.3-82.8) | 49.6 (38.4-60.7) | .04 |
| 5-y | 59.4 (43.6-75.1) | 47.2 (35.8-58.7) | .22 |
Includes ifosfamide, carboplatin, etoposide; gemcitabine-dexamethasone-cisplatin; gemcitabine-oxaliplatin; and dexamethasone, cytarabine, cisplatin chemotherapy regimens. Data are percentage probability (95% CI). Bold values are statistically significant P values.
Discussion
In this retrospective CIBMTR analysis, we evaluated the contemporary outcomes of auto-HCT in DLBCL patients achieving only a PET-avid PR following salvage therapies, stratified according to the timing of first-line chemoimmunotherapy failure. Our analysis shows that, in chemosensitive patients achieving only a PR following salvage attempts, auto-HCT consolidation can provide durable disease control in a sizable patient subset (5-year PFS ∼ 40%), with low NRM rates. Subset analyses limiting patients to ≥2 lines of prior chemotherapy or to patients receiving platinum-based salvage regimens resulted in similar outcomes, with no difference in PFS among ECF and no-ECF patients.
The striking decline in the number of auto-HCTs performed in the United States in 2018 (Figure 1), following the approval of anti-CD19 CAR T-cell therapies in 2017, suggests that relapsed chemosensitive DLBCL patients not achieving a CR following salvage are increasingly being offered CAR T-cell therapies in lieu of auto-HCT. No data have shown the superiority of CAR T-cell therapy over auto-HCT in DLBCL patients with established chemosensitive disease. At least 3 randomized trials in relapsed DLBCL with ECF are evaluating the current standard (chemotherapy salvage followed by auto-HCT in responding patients) vs proceeding directly with CAR T-cell therapies (without a salvage attempt) (NCT03391466, NCT03570892). The results of these important trials will inform our practice of whether applying CAR T-cell therapies instead of attempting to establish chemosensitivity with salvage therapy improves patient outcomes. However, outside of the clinical trial setting, our study cautions against abandoning auto-HCT consolidation in patients achieving only a PR following salvage therapy (with PET-avid residual disease). This is important for several reasons: (1) auto-HCT can cure ∼40% of such patients with a low risk for NRM, (2) no data are available to suggest that CAR T-cell therapy offers superior outcomes in this setting, (3) CAR T-cell therapy comes with a substantially higher cost,13 (4) although CAR T-cell therapy can clearly salvage patients relapsing after auto-HCT, the reverse sequence is generally not believed to be feasible,14 and (5) patients relapsing after CAR T-cell therapy have poor outcomes with limited options.15
There are several limitations associated with this study. First, as a registry study we are limited to the retrospective data available in the database; as a result, we cannot determine the impact of unmeasured or unknown confounders on outcomes. Next, we acknowledge that PET imaging in our study was not centrally adjudicated using the current Deauville 5-point scale, and responses reported are per-center evaluations, which can have significant heterogeneity.12 However, it is important to point out that, although the Deauville scale represents an important advance in PET interpretation, the prognostic significance of PET status (negative vs positive, as determined by radiologists in individual centers) for aggressive lymphomas, including DLBCL, has been demonstrated.16,17 DLBCL patients achieving a PR, but with residual PET activity due to viable residual disease, would typically be expected to have a Deauville score of 4. Only 1 study, to our knowledge, has reported auto-HCT outcomes for DLBCL patients relative to a pretransplant PET scan interpreted according to Deauville score.4 Interestingly, the 3-year PFS of patients in our study (44-50%; n = 249) is virtually identical to the 3-year PFS (49%) of patients with a Deauville score of 4 (n = 48) in the report by Sauter et al.4 It is unlikely that our data set contains a substantial number of patients who would be characterized as PET negative if Deauville score was applied, because the 3-year PFS of Deauville 1-3 patients following auto-HCT is expected to be ∼75%.4 Although not evidence based, we acknowledge that the bulk of residual disease (or changes in maximum standardized uptake values) is often considered when deciding to proceed (or not) with auto-HCT, even among chemosensitive patients. Although the CIBMTR registry does not capture total residual tumor volumes, the residual nodal disease in our cohort (in cases for which this information was reported by the centers) was nonbulky (defined as largest remaining lymph node < 5 cm on pre–auto-HCT PET/CT) for 88% to 90% of patients (Table 1).
The presence of a c-myc gene rearrangement alone,18 or with a bcl-2 and/or a bcl-6 gene rearrangement,19 in relapsed DLBCL or relapsed DLBCL with ≥2 clinical risk factors defined in the REFINE study20 (progression on frontline therapy, c-myc rearrangement, high National Comprehensive Cancer Network–International Prognostic Index [NCCN-IPI] at relapse) identifies patients at a particularly high risk for relapse following auto-HCT consolidation. The nature of the data captured in the registry setting precludes us from evaluating these variables in the current analysis. Investigation of alternative consolidation (eg, with CAR T-cell therapy or allogenic-HCT21,22 ) or post–auto-HCT maintenance/consolidation23 is warranted in this ultrahigh-risk, albeit small, subset of relapsed DLBCL. However, our study does include some higher-risk patients who received >1 salvage regimen and subsequently achieved long-term benefit consistent with prior reports from CORAL, which demonstrated that failure of 1 salvage regimen does not preclude auto-HCT.8
In line with the published data, our analysis identified ECF as a poor prognostic factor for OS. In the CORAL study in which standard CT scans (not PET scans) were used to evaluate the outcomes of 2 pretransplant salvage regimens, patients with early relapse (<12 months from initial diagnosis) experienced a lower response rate (46% vs 88%; P ≤. 001) and inferior 3-year PFS (20% vs 45%).5 Several subsequent registry analyses have confirmed these findings.2,7 Similarly, PFS at 1 year after auto-HCT was worse for ECF patients in our analysis, consistent with prior registry reports.7 However, although ECF patients who underwent auto-HCT in a PET+ PR had inferior OS in our analysis (relative to those without ECF), it should be noted that these patients still had a 5-year PFS of 40.5% and a 5-year OS of 51%. This represents a substantial proportion of these patients experiencing curative treatment with auto-HCT. Although the long-term follow of ZUMA-1 shows a 2-year PFS ∼40% in chemorefractory DLBCL failing at least 2 prior therapy lines,24 it is not known whether earlier application of CAR T-cell therapy (eg, second-line therapy) will provide better disease control.
In conclusion, our analysis shows that, in chemoresponsive relapsed DLBCL patients achieving only a PET-avid PR remission following salvage attempts, auto-HCT consolidation remains the standard of care (5-year PFS ∼ 40%) with low NRM rates. Results of ongoing randomized trials will define the role of CAR T-cell therapy vs auto-HCT in relapsed DLBCL with ECF.
Presented in abstract form at the virtual 2020 American Society of Clinical Oncology Annual Meeting, 9 June 2020.
Data are available at https://www.cibmtr.org/ReferenceCenter/PubList/PubDsDownload/Pages/default.aspx.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the CIBMTR. The CIBMTR is supported primarily by Public Health Service grant/cooperative agreement U24CA076518 with the National Cancer Institute (NCI)/National Institutes of Health (NIH), the National Heart, Lung, and Blood Institute (NHLBI)/NIH, and the National Institute of Allergy and Infectious Diseases (NIAID)/NIH; grant/cooperative agreement U24HL138660 with the NHLBI/NIH and the NCI/NIH; grants R21HL140314 and U01HL128568 from the NHLBI/NIH; contract HHSH250201700006C with Health Resources and Services Administration; grants N00014-18-1-2888 and N00014-17-1-2850 from the Office of Naval Research; subaward from prime contract award SC1MC31881-01-00 with Health Resources and Services Administration; subawards from prime grant awards R01HL131731 and R01HL126589 from NHLBI/NIH; and subawards from prime grant awards 5P01CA111412, R01CA152108, and 1R01CA231141 from NCI/NIH, 5R01HL129472 and 1R01HL131731 from NHLBI/NIH, and 1U01AI126612 from NIAID/NIH. The CIBMTR is also supported by commercial funds from Actinium Pharmaceuticals, Inc.; Adaptive Biotechnologies; AlloVir, Inc.; Amgen, Inc.; anonymous donation to the Medical College of Wisconsin; Anthem, Inc.; Astellas Pharma US; Atara Biotherapeutics, Inc.; BARDA; Be the Match Foundation; bluebird bio, Inc.; Boston Children’s Hospital; Bristol Myers Squibb Co.; Celgene Corp.; Children’s Hospital of Los Angeles; Chimerix, Inc.; City of Hope Medical Center; CSL Behring; CytoSen Therapeutics, Inc.; Daiichi Sankyo Co., Ltd.; Dana Farber Cancer Institute; Enterprise Science and Computing, Inc.; Fred Hutchinson Cancer Research Center; Gamida-Cell, Ltd.; Genzyme; Gilead Sciences, Inc.; GlaxoSmithKline; HistoGenetics, Inc.; Immucor; Incyte Corporation; Janssen Biotech, Inc.; Janssen Pharmaceuticals, Inc.; Janssen Research & Development, LLC; Janssen Scientific Affairs, LLC; Japan Hematopoietic Cell Transplantation Data Center; Jazz Pharmaceuticals, Inc.; Karius, Inc.; Karyopharm Therapeutics, Inc.; Kite, a Gilead Company; Kyowa Kirin; Magenta Therapeutics; Mayo Clinic and Foundation Rochester; Medac GmbH; Mediware; Memorial Sloan-Kettering Cancer Center; Merck & Company, Inc.; Mesoblast; Meso Scale Diagnostics, Inc.; Millennium, the Takeda Oncology Co.; Miltenyi Biotec, Inc.; Mundipharma EDO; National Marrow Donor Program; Novartis Oncology; Novartis Pharmaceuticals Corporation; Omeros Corporation; OncoImmune, Inc.; OptumHealth; Orca Biosystems, Inc.; PCORI; Pfizer, Inc.; Phamacyclics, LLC; PIRCHE AG; Regeneron Pharmaceuticals, Inc.; REGiMMUNE; Sanofi Genzyme; Seattle Genetics; Shire; Sobi, Inc.; Spectrum Pharmaceuticals, Inc.; St. Baldrick's Foundation; Swedish Orphan Biovitrum, Inc.; Takeda Oncology; The Medical College of Wisconsin; University of Minnesota; University of Pittsburgh; University of Texas-MD Anderson; University of Wisconsin - Madison; Viracor Eurofins; and Xenikos BV.
The views expressed in this article do not reflect the official policy or position of the NIH, the Department of the Navy, the Department of Defense, the Health Resources and Services Administration, or any other agency of the US government.
Authorship
Contribution: M.H. conceived and designed the study; C.L. and M.H. collected and assembled data; K.W.A., C.L., and M.H. analyzed data; and N.N.S. and M.H. wrote the manuscript; and all authors interpreted data and helped to revise the manuscript.
Conflict-of-interest disclosure: N.N.H. has received honoraria, travel support, and research funding from Lentigen Technology and Miltenyi Biotec; has received honoraria from Incyte and Celgene; has served on scientific advisory boards for Kite, Celgene, Lily, Verastem, and Cellectar; and has received institutional research support for clinical trials from Bristol Myers Squibb. M.H. has received research support/funding from Spectrum Pharmaceuticals and Astellas Pharma; has acted as a consultant for MedImmune LLC, Janssen Research & Development, Incyte Corporation, ADC Therapeutics, Celgene, Pharmacyclics, and Verastem; and has been a member of the speaker’s bureau for Sanofi Genzyme and AstraZeneca. T.S.F. has received research support/funding from Millennium, Novartis, Kyowa, TG Therapeutics, Portola, and Curis; has acted as a consultant for BeiGene, Genentech, Adaptive Biotechnologies, AbbVie, Verastem, Kite, MorphoSys, AstraZeneca, Pharmacyclics, and Sanofi; and has been a member of the speaker’s bureau for Genentech, Sanofi, Seattle Genetics, AstraZeneca, Celgene/Bristol Myers Squibb, and Adaptive Biotechnologies. The remaining authors declare no competing financial interests.
Correspondence: Mehdi Hamadani, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, 9200 W. Wisconsin Ave, Suite C5500, Milwaukee, WI 53226; e-mail: mhamadani@mcw.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal