It is tempting to view the immune system as a thrombosis driver,1 but the article by Obermayer et al2 in this issue of Blood provides a counterpoise through the discovery of an anticoagulant role for innate immunity. Their article linking circulating microvesicles with natural immunoglobulin M (IgM) antibodies is an intriguing story with high clinical significance.
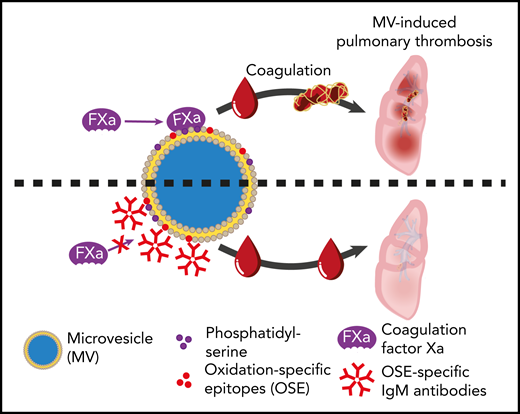
Inhibitory effect that natural IgM antibodies against oxidation-specific epitopes on microvesicles have on the ability of microvesicles to bind factor X/Xa, thereby attenuating thrombin generation and thrombosis. See Figure 4G in the article by Obermayer et al that begins on page 1406.
Inhibitory effect that natural IgM antibodies against oxidation-specific epitopes on microvesicles have on the ability of microvesicles to bind factor X/Xa, thereby attenuating thrombin generation and thrombosis. See Figure 4G in the article by Obermayer et al that begins on page 1406.
Plasma microvesicles are a heterogeneous collection of membranous cell-derived microparticles released in response to inflammatory activation, cellular stress, or apoptosis. Typically, microvesicles express phosphatidylserine on their exterior, allowing for their identification in the laboratory by flow cytometry with Annexin V. Microvesicles also tend to express the membrane glycoproteins of their cell of origin, whether they are lineage markers (eg, of platelets, endothelial cells, neutrophils, monocytes) or other functionally significant glycoproteins. Consequently, there is now a sizeable number of studies identifying specific microvesicle phenotypes as biomarkers of disease. Microvesicles are already known to contribute to thrombosis by virtue of expressing negatively charged phospholipids that facilitate prothrombinase complex assembly. Some plasma microvesicles also express tissue factor, and experiments using intravital microscopy to visualize clot formation in preclinical models have highlighted the importance of these for thrombus propagation.3 Furthermore, plasma tissue factor–expressing microvesicles are associated with thrombotic events in patients, as for example, in Behçet syndrome.4 Until now, the existence of a specific endogenous means to counter the prothrombotic effect of microvesicles has not been realized.
Natural antibodies are those germline-encoded immunoglobulins that are expressed without the need for immunization and that contribute to innate humoral immunity. They are mainly of the pentavalent IgM class and not only serve as frontline defense against infections but also have an important “vacuum cleaner” role in the waste disposal of damaged tissues. For example, IgM binds epitopes on apoptotic cells and cooperates with complement to ensure their clearance.5 A significant proportion of IgM natural antibodies react with oxidation-specific epitopes created by free radicals, and these have been extensively studied in the context of atherosclerosis and its thrombotic consequences.6,7 IgM antibodies protect against atherosclerosis in mice, and several studies have linked high total IgM and anti–oxidation-specific epitope IgM levels in humans with relative protection from ischemic strokes and acute coronary syndromes.8,9
Obermayer et al now build on their substantial portfolio of research on natural IgM antibodies, which includes work showing that some bind oxidation-specific epitopes on microvesicles.10 It already seemed likely that opsonization of microvesicles in vivo by IgM would result in complement fixation and rapid removal from the circulation via the reticulo-endothelial system. However, this new study makes it clear that IgM antibody binding to microvesicles has a more immediate safeguarding effect by inhibiting the contribution of microvesicles to the propagation of coagulation, irrespective of whether this is triggered by the intrinsic or extrinsic pathways. This ability of IgM appears not to depend on antibodies against a particular oxidation-specific epitope on microvesicles, because antibodies reacting with either malondialdehyde or phosphorylcholine had similar inhibitory effects on thrombin generation. The primary mechanism of inhibition appears to be by IgM bound to microvesicles sterically, preventing the binding of factor X/Xa to the microvesicle surface (see figure). Supporting the relevance of these in vitro observations, the group went on to show that injection of an anti-malondialdehyde IgM monoclonal antibody attenuates clot formation in a mouse model of pulmonary thrombosis. Intriguingly, the same antibodies do not affect hemostasis after tail tip injury.
This novel mechanism is likely to be particularly relevant in providing endogenous protection against thrombosis in clinical situations characterized by high levels of circulating microvesicles, such as inflammation and cancer. Of the many questions that now derive from the study, one is what the effect is of smaller IgG antibodies against oxidation-specific antibodies. These can readily be detected in serum and might possibly compete with IgM for binding microvesicles. Do they have the same ability to sterically hinder factor X/Xa, and/or might they activate proinflammatory/prothrombotic functions by ligating macrophage Fcγ receptors?
All in all, this is a fascinating piece of work. On the one hand, IgM levels, and in particular titers of IgM antibodies against epitopes on microvesicles, can now be drawn into consideration for use in risk assessments for arterial and venous thrombosis and stratification of treatment. On the other hand, there is a therapeutic potential to be tested for antibodies that neutralize the contribution of microvesicles to thrombosis, particularly if their lack of effect on hemostasis can be substantiated.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal