Key Points
IV arginine therapy increases mitochondrial activity and decreases oxidative stress in children with SCD and vaso-occlusive pain.
A dose-dependent impact of arginine therapy on mitochondrial function is a novel mechanism of action not previously described in SCD.
Abstract
Altered mitochondrial function occurs in sickle cell disease (SCD), due in part to low nitric oxide (NO) bioavailability. Arginine, the substrate for NO production, becomes acutely deficient in SCD patients with vaso-occlusive pain episodes (VOE). To determine if arginine improves mitochondrial function, 12 children with SCD-VOE (13.6 ± 3 years; 67% male; 75% hemoglobin-SS) were randomized to 1 of 3 arginine doses: (1) 100 mg/kg IV 3 times/day (TID); (2) loading dose (200 mg/kg) then 100 mg/kg TID; or (3) loading dose (200 mg/kg) followed by continuous infusion (300 mg/kg per day) until discharge. Platelet-rich plasma mitochondrial activity, protein expression, and protein-carbonyls were measured from emergency department (ED) presentation vs discharge. All VOE subjects at ED presentation had significantly decreased complex-V activity compared to a steady-state cohort. Notably, complex-V activity was increased at discharge in subjects from all 3 arginine-dosing schemes; greatest increase occurred with a loading dose (P < .001). Although complex-IV and citrate synthase activities were similar in VOE platelets vs steady state, enzyme activities were significantly increased in VOE subjects after arginine-loading dose treatment. Arginine also decreased protein-carbonyl levels across all treatment doses (P < .01), suggesting a decrease in oxidative stress. Arginine therapy increases mitochondrial activity and reduces oxidative stress in children with SCD/VOE. This trial was registered at www.clinicaltrials.gov as #NCT02536170.
Introduction
Pain is the hallmark of sickle cell disease (SCD), with vaso-occlusive pain episodes (VOE) being the leading cause of emergency department (ED) visits and hospitalizations.1,2 There are currently no standard-of-care therapies that specifically target underlying mechanisms of acute VOE in the ED or during hospitalization, with symptomatic interventions limited to analgesia and hydration.3-5 Nitric oxide (NO), enzymatically produced from its obligate substrate l-arginine, is a potent vasodilator and exerts multiple effects on vascular and circulating blood cells, including the inhibition of platelet aggregation, downregulation of adhesion molecules, and modulation of ischemia-reperfusion injury, which are all pathways adversely affected during VOE.6
SCD is an “arginine deficiency syndrome”6-10 associated with early mortality11,12 in addition to pain.13,14 Increased arginase activity from both inflammatory triggers and more significantly from erythrocyte-arginase release during hemolysis,11 leads to low arginine bioavailability. Although mechanisms of arginine dysregulation are complex and multifactorial,6,7,9 they can be overcome through arginine supplementation, a phenomenon known as the “arginine paradox.”15 In transgenic mouse models of SCD, arginine supplementation inhibits the red cell Gardos channels, reduces red cell density,16 improves perfusion, and reduces lung injury, microvascular vaso-occlusion, and mortality.17-20 We and others have found that SCD patients admitted with VOE have acutely depleted l-arginine and NO levels13,14 that correlate to pain severity.13,14 We have also demonstrated that l-arginine therapy increases NO metabolites in children with SCD hospitalized with VOE in a dose-dependent manner.21 Additionally, arginine therapy (100 mg/kg per dose) given 3 times a day (TID) for 5 days reduced opioid use by >54% and was associated with lower pain scores at discharge compared with placebo in a randomized, double-blinded, placebo-controlled trial (RCT) in children with SCD/VOE requiring hospitalization.22 Mechanisms remain unclear, but may be due in part to stimulation of NO production.
Shiva and colleagues have reported altered mitochondrial function in platelets of SCD patients compared with healthy controls.23 Platelets from SCD patients show decreased mitochondrial complex-V activity, which potentiates mitochondrial membrane potential, leading to increased oxidant production from the electron transport chain. This dysfunction is propagated by hemolysis and causes platelet activation as demonstrated by in vitro studies. Consistent with this, platelet mitochondrial dysfunction correlates with platelet activation and hemolysis in vivo.23
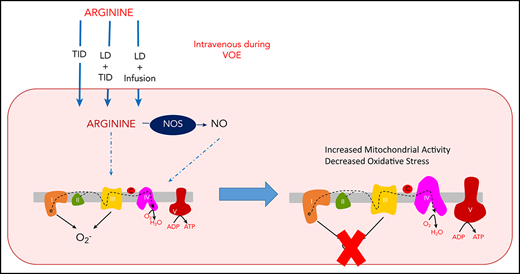
Both arginine and NO have a mechanistic impact on mitochondrial function. Promising reports of IV and oral arginine use for complications of mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes have been described.24,25 Physiological levels of NO can inhibit mitochondrial respiration at complex IV; however, this is fully reversible once NO bioavailability diminishes.26,27 NO-derived species can also reversibly modify complex-I by S-nitrosation, which has been shown to attenuate mitochondrial oxidant production.28,29 Additionally, NO can also increase mitochondrial function through the stimulation of biogenesis.30 Enhancement of biogenesis and attenuation of oxidant production may be beneficial in platelets of SCD patients. We therefore hypothesized that arginine therapy may improve mitochondrial function during SCD/VOE.
Study design
This is a single-center, prospective, randomized, open-label intervention study of children with SCD on hydroxyurea, hospitalized for VOE and requiring parenteral opioids, designed to explore the impact of arginine therapy on mitochondrial function. Children with a diagnosis of SCD (homozygous hemoglobin S [HbSS] or Sβ0-thalassemia) age 3 to 21 years with VOE requiring parenteral opioids and hospital admission were recruited from the ED and inpatient wards as a convenience sample during times when the principal investigator/coinvestigators and a study coordinator were on-site and available to consent, a legal guardian was present, and the research pharmacist was available to perform the randomization. Patients were consented within 24 hours of receiving their first dose of parenteral opioids in the ED.
Subjects were randomized to treatment using 1 of 3 dosing schemes of IV l-arginine therapy: (1) 100 mg/kg TID (n = 4); (2) loading dose (200 mg/kg) then 100 mg/kg TID (n = 4); or (3) loading dose (200 mg/kg) followed by continuous infusion (300 mg/kg per day, n = 4). Arginine therapy was administered until hospital discharge or for a maximum of 7 days for subjects with prolonged hospital stays, whichever came first. Block randomization was performed by the research pharmacist. Exclusion criteria included hepatic/renal insufficiency, acidosis, hemoglobin <5 g/dL or immediate need for emergent erythrocyte transfusion, previous randomization into this study, pregnancy, mental status changes/concern for stroke, or known arginine allergy. A Children’s Healthcare of Atlanta clinical practice guideline for VOE based on the 2014 National Heart, Lung, and Blood Institute guidelines5 was followed in the ED and during hospitalization.
Platelet-rich plasma was isolated and stored from blood samples obtained at VOE-baseline (day of ED presentation for pain) and on the day of hospital discharge for each subject. Mitochondrial activity (complex IV: site of oxygen consumption; complex V: site of ATP production; citrate synthase: matrix protein) and protein carbonyls and malondialdehyde (biomarkers of protein and lipid oxidation, respectively) were measured spectrophotometrically as previously described23 ; mitochondrial DNA was measured as previously described31 and compared across the 3 arginine dosing schemes. The study protocol was approved by the Emory University institutional review board and University of Pittsburgh Institutional Review Board for steady-state subjects; written informed consent was obtained for all patients enrolled, and assent was obtained from all children age ≥7 years. The study was under an active Investigational New Drug #66943 issued by the US Food and Drug Administration, held by the sponsor: Claudia R. Morris. Significance was tested by 1- or 2-way analysis of variance as appropriate and significance was noted when P < .05.
Results and discussion
Twelve children with SCD, mean age of 13.6 ± 3 years, 67% male, 75% HbSS, were randomized into this study. There were no unexpected adverse events or serious adverse events related to study drug.
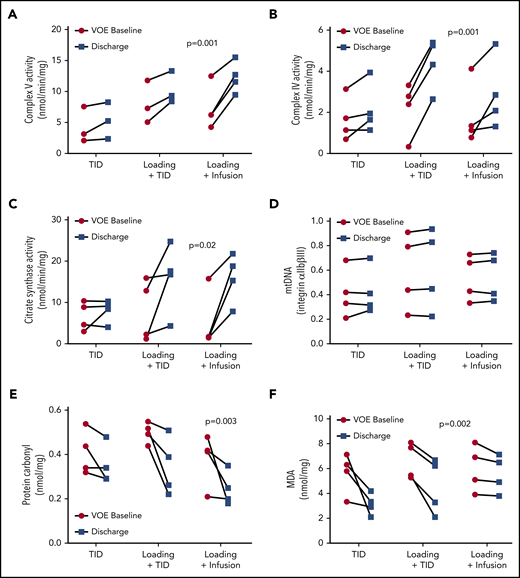
Compared with a cohort of SCD patients in steady state23 (23.11 ± 3 years, 56% male, 100% HbSS; 80% on hydroxyurea), all VOE subjects’ platelets had significantly decreased complex V activity (Figure 1A), but no change in complex IV (Figure 1B) and citrate synthase activity (data not shown). Notably, complex V activity was increased at discharge in subjects with VOE treated with arginine in all 3 dosing schemes (Figure 2A; P < .001). The activities of complex IV and citrate synthase were also significantly increased in VOE subjects after arginine treatment when using a loading dose (Figure 2A-C; P < .01). A significantly greater increase in complex IV and complex V activities, but not citrate synthase, was noted with the use of a loading dose (supplemental Figure 1, available on the Blood Web site). These changes are likely not due to increased mitochondrial number because quantification of mitochondrial DNA before and after arginine treatment was not different (Figure 2D). Arginine therapy also significantly decreased levels of protein carbonyls (Figure 2E; P < .01) and malondialdehyde (Figure 2F; P < .01) in platelet-rich plasma across all treatment arms, suggesting a decrease in oxidative stress.
Differences in mitochondrial activity in patients with sickle cell disease at steady state compared to patients experiencing acute pain. (A) Complex V and (B) complex IV activity in patients with sickle cell disease at steady-state (SS, no pain) compared with patients experiencing a moderate-to-severe VOE on the day of an ED visit. Compared with a cohort of SCD patients in SS, all VOE subjects had significantly decreased complex V activity; however, complex IV activities were similar in SS platelets vs VOE.
Differences in mitochondrial activity in patients with sickle cell disease at steady state compared to patients experiencing acute pain. (A) Complex V and (B) complex IV activity in patients with sickle cell disease at steady-state (SS, no pain) compared with patients experiencing a moderate-to-severe VOE on the day of an ED visit. Compared with a cohort of SCD patients in SS, all VOE subjects had significantly decreased complex V activity; however, complex IV activities were similar in SS platelets vs VOE.
Impact of parenteral arginine therapy on mitochondrial activity and biomarkers of oxidative stress in children with sickle cell disease during acute vaso-occlusive pain episodes at admission compared to discharge. (A) Enzymatic activities of complex V, (B) complex IV, and (C) citrate synthase in platelet-rich plasma on the day of ED presentation for pain (VOE-baseline) compared with the day of hospital discharge (discharge) across 3 IV arginine dosing schemes: 100 mg/kg per dose TID vs a loading dose (200 mg/kg) followed by 10 0mg/kg per dose TID (loading + TID) vs a loading dose followed by a continuous infusion (300 mg/kg per day) (loading + CI). (D) Mitochondrial DNA, (E) protein carbonyl levels, and (F) malondialdehyde (MDA) levels across 3 arginine dosing schemes at VOE-Baseline compared with Discharge. Notably, complex-V activity, low at VOE-baseline compared with steady-state, was increased at discharge in subjects with VOE treated with arginine across all 3 dosing schemes, with greatest increase noted when using a loading dose (P < .001). Although complex IV and citrate synthase activities were not changed in VOE platelets vs steady state, the activities of these enzymes were significantly increased in VOE subjects after arginine treatment when using a loading dose (P < .01). These changes are not due to increased mitochondrial number because quantification of mitochondrial DNA before and after IV arginine was not different. Arginine therapy also significantly decrease levels of protein carbonyls and malondialdehyde in platelet-rich plasma across all treatment doses (P < .01), suggesting a decrease in oxidative stress. All panels show n = 4 subjects for each treatment arm. (A) There is substantial overlap between 2 subjects in both the TID and load + TID arms.
Impact of parenteral arginine therapy on mitochondrial activity and biomarkers of oxidative stress in children with sickle cell disease during acute vaso-occlusive pain episodes at admission compared to discharge. (A) Enzymatic activities of complex V, (B) complex IV, and (C) citrate synthase in platelet-rich plasma on the day of ED presentation for pain (VOE-baseline) compared with the day of hospital discharge (discharge) across 3 IV arginine dosing schemes: 100 mg/kg per dose TID vs a loading dose (200 mg/kg) followed by 10 0mg/kg per dose TID (loading + TID) vs a loading dose followed by a continuous infusion (300 mg/kg per day) (loading + CI). (D) Mitochondrial DNA, (E) protein carbonyl levels, and (F) malondialdehyde (MDA) levels across 3 arginine dosing schemes at VOE-Baseline compared with Discharge. Notably, complex-V activity, low at VOE-baseline compared with steady-state, was increased at discharge in subjects with VOE treated with arginine across all 3 dosing schemes, with greatest increase noted when using a loading dose (P < .001). Although complex IV and citrate synthase activities were not changed in VOE platelets vs steady state, the activities of these enzymes were significantly increased in VOE subjects after arginine treatment when using a loading dose (P < .01). These changes are not due to increased mitochondrial number because quantification of mitochondrial DNA before and after IV arginine was not different. Arginine therapy also significantly decrease levels of protein carbonyls and malondialdehyde in platelet-rich plasma across all treatment doses (P < .01), suggesting a decrease in oxidative stress. All panels show n = 4 subjects for each treatment arm. (A) There is substantial overlap between 2 subjects in both the TID and load + TID arms.
Limitations
Although this study is limited by a small sample size per study arm, improvements in all indices of mitochondrial function are dramatic and statistically significant. Lack of a control arm is another limitation; it is possible that abnormal mitochondrial function and oxidative stress during VOE may improve as the pain event resolves. However, the substantial differences noted in the 2 arginine arms that received a loading dose compared with those treated with the standard dose suggests a dose-dependent response of arginine on mitochondrial function beyond simply VOE resolution. Future studies will also monitor platelet activation to determine whether arginine-dependent modulation of platelet number or thrombotic activation contributes to the mitochondrial alterations observed here.
Conclusions
These data demonstrate for the first time that arginine therapy increases mitochondrial activity and decreases oxidative stress in children with SCD/VOE. Our prior study demonstrated that in SCD patients, inhibited complex V activity leads to increased oxidant production.23 Thus, enhanced complex V activity may decrease mitochondrial oxidant production, attenuating platelet activation and generalized oxidative damage. Oxidative stress, which propagates inflammation and neuronal excitability, has been closely associated with pain in SCD.32,33 Hence, this novel mechanism of action may contribute to the opioid-sparing effect and decreased pain reported after IV arginine therapy during VOE in our phase 2 RCT that used the standard dose22 ; a loading dose may enhance benefits. In addition to decreased oxidants, increases in both complex-IV and complex-V activity could be indicative of enhanced capacity for oxidative phosphorylation, yielding more ATP for cellular regenerative processes. However, more in-depth study is required to test these speculations. Notably, the results presented here may have implications for mitochondrial function in cell types beyond platelets, including erythrocytes, which aberrantly retain mitochondria in SCD patients.34 Erythrocytic mitochondria contribute to hemolysis in SCD through their significant rate of oxidant production, whereas mitophagic drugs decrease erythrocytic mitochondria and attenuate hemolysis and organ damage in SCD murine models.34 Although the effect of arginine on mitophagy is currently unclear, it is plausible that arginine is used to generate NO within the erythrocytes, which contain endothelial nitric oxide synthase,35 to decrease mitochondrial oxidants. Future study will focus more specifically on the NO-dependent and independent mechanisms by which arginine regulates mitochondrial function/number.
Platelet mitochondrial function is determined by a simple blood test and may serve as an objective biological outcome measure for future clinical trials. A Pediatric Emergency Care Applied Research Network-endorsed, phase 3, placebo-controlled multicenter RCT of arginine therapy is being planned and will use a loading dose of IV arginine based on these results.
Requests for data sharing should be e-mailed to the corresponding author.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported, in part, by grants from the National Institutes of Health (NIH) National Heart, Lung, and Blood Institute (R34HL122557) (C.R.M.), NIH National Center for Complementary and Integrative Health (K24AT009893) (C.R.M.), NIH National Heart, Lung, and Blood Institute (R01HL133003-01A1) (S.S.), Vitalant, and the Hemophilia Center of Western Pennsylvania (S.S.).
Authorship
Contribution: C.R.M. designed research question, wrote the study protocol, obtained funding, obtained informed consent/assent, analyzed and interpreted the data with S.S., and wrote the manuscript; L.A.S.B. assisted with study protocol development, supervised sample processing, assisted with interpretation of the data, and critically reviewed the manuscript; M.R. processed and analyzed biological samples, assisted with interpretation of the data, and critically reviewed the manuscript; C.D.D. assisted with study protocol design, patient enrollment and consent, and interpretation of the data, and critically reviewed the manuscript; P.A.L. assisted with study protocol design, patient enrollment and consent, and interpretation of the data, and critically reviewed the manuscript; A.W. assisted with patient enrollment, obtained samples for processing, assisted with maintenance of the study database, assisted with regulatory oversight, and critically reviewed the manuscript; P.K. assisted with screening and enrollment of patients, obtained and processed biological samples, assisted with regulatory oversight including US Food and Drug Administration Investigational New Drug maintenance and reporting for this study, and critically reviewed the manuscript; F.H. processed and analyzed biological samples, assisted with interpretation of the data, and critically reviewed the manuscript; S.M. assisted with data entry, data analysis, data interpretation, and critically reviewed the manuscript; R.D.M. assisted with data entry, data analysis, and data interpretation and critically reviewed the manuscript; J.F. assisted with statistical design, data analysis, data interpretation, and writing the manuscript; and S.S. designed research question with C.R.M., supervised sample processing, analyzed and interpreted the data, and assisted with writing the manuscript.
Conflict-of-interest disclosure: C.R.M. is the inventor or coinventor of several University of California San Francisco-Benioff Children’s Hospital Oakland patents/patent-pending applications that include nutritional supplements and biomarkers of cardiovascular disease related to arginine bioavailability, is an inventor of an Emory University School of Medicine patent application for a nutritional supplement, is a consultant for Pfizer, and has received research support from MAST Therapeutics, the US Food and Drug Administration, and the National Institutes of Health. C.D.D. has received research support from Pfizer. The remaining authors declare no competing financial interests.
Correspondence: Claudia R. Morris, Division of Pediatric Emergency Medicine, Department of Pediatrics and Emergency Medicine, Emory University School of Medicine, 1760 Haygood Dr NE, W458, Atlanta, GA 30322; e-mail: claudia.r.morris@emory.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal