Key Points
Daratumumab can achieve good results in advanced AL amyloidosis, but is less effective in nephrotic patients with high free light chains.
Translocation t(11;14) is beneficial, and myeloma-like hyperdiploidy is adverse for outcome with daratumumab therapy in AL.
Abstract
Daratumumab has shown promising first results in systemic amyloid light-chain (AL) amyloidosis. We analyzed a consecutive series of 168 patients with advanced AL receiving either daratumumab/dexamethasone (DD, n = 106) or daratumumab/bortezomib/dexamethasone (DVD, n = 62). DD achieved a remission rate (RR) of 64% and a very good hematologic remission (VGHR) rate of 48% after 3 months. Median hematologic event-free survival (hemEFS) was 11.8 months and median overall survival (OS) was 25.6 months. DVD achieved a 66% RR and a 55% VGHR rate. Median hemEFS was 19.1 months and median OS had not been reached. Cardiac organ responses were noted in 22% with DD and 26% with DVD after 6 months. Infectious complications were common (Common Terminology Criteria [CTC] grade 3/4: DD 16%, DVD 18%) and likely related to a high rate of lymphocytopenia (CTC grade 3/4: DD 20%, DVD 17%). On univariable analysis, hyperdiploidy and gain 1q21 conferred an adverse factor for OS and hemEFS with DD, whereas translocation t(11;14) was associated with a better hemEFS. N-terminal prohormone of brain natriuretic peptide >8500 ng/L could not be overcome for survival with each regimen. Multivariable Cox regression analysis revealed plasma cell dyscrasia (difference between serum free light chains [dFLC]) >180 mg/L as an overall strong negative prognostic factor. Additionally, nephrotic-range albuminuria with an albumin-to-creatinine-ratio (ACR) >220 mg/mmol was a significantly adverse factor for hemEFS (hazard ratio, 2.1 and 3.1) with DD and DVD. Daratumumab salvage therapy produced good results and remission rates challenging any therapy in advanced AL. Outcome is adversely influenced by the activity of the underlying plasma cell dyscrasia (dFLC) and nephrotic-range albuminuria (ACR).
Introduction
The monoclonal IgG1 κ antibody daratumumab (Darzalex) was first reported to be effective and safe in refractory multiple myeloma (MM).1,2 Thereafter it was approved as third-line therapy after exposure to a proteasome inhibitor (PI) and an immunomodulatory drug (IMID) in Germany in 2016. Effectiveness and safety in systemic amyloid light-chain (AL) amyloidosis was first reported in August 2016 and confirmed in a series of 25 patients in 2017.3,4 Daratumumab combination therapy with bortezomib and dexamethasone (DVD) was approved as a second-line therapy for MM in 2017 after reporting superior overall response rates compared with bortezomib and dexamethasone.5 A phase 3 trial is currently assessing the effectiveness and safety of the addition of subcutaneous daratumumab to a standard first-line therapy with bortezomib, cyclophosphamide, and dexamethasone in AL.6
Cardiac biomarkers and the difference between serum free light chains (dFLC) are established prognostic markers for overall survival (OS) at first diagnosis in AL and have recently been confirmed as prognostic ahead of second-line therapy.7-11 Furthermore, impaired renal function also negatively affects OS, but proteinuria does not.12 Translocation t(11;14) has been reported as adverse for outcome with bortezomib and IMID-based regimens in AL first-line therapy.13,14 So far, there are no published data available to predict response to daratumumab in AL.
The performance of monoclonal antibodies in patients with nephrotic syndrome still remains uncertain.15 A case report about the successful treatment with the monoclonal antibody elotuzumab in combination with lenalidomide/dexamethasone has recently been published in a nephrotic patient with AL and MM.16
Methods
Objective
This study aims to analyze the efficacy of daratumumab in patients with symptomatic systemic AL and to find factors predicting outcome with daratumumab/dexamethasone (DD) and confirm these with DVD. Prospective urine analysis for the detection of daratumumab was initiated after detecting monoclonal IgG κ in the urine of 4 AL λ patients suffering from nephrotic-range albuminuria (see supplemental Data, available on the Blood Web site).
Study design
Between June 2016 and July 2019, we initiated 168 consecutive patients with symptomatic AL and internal organ involvement on either DD (n = 106) or DVD (n = 62, starting July 2017) as salvage therapy after persistence or recurrence of the underlying clonal plasma cell disorder. The choice of treatment was primarily based on the approval status for DD and DVD for MM in Germany. All patients fulfilling approval criteria for DD were routinely started on this regimen. DVD was chosen whenever a patient met eligibility criteria for DVD, but not DD. Without any data available to support use of DVD in systemic AL, patients with an underlying monoclonal gammopathy not classifying as MM were mainly started on DD as health care providers would solely grant cost coverage for this regimen. Additionally, patients with severe polyneuropathy deemed ineligible for bortezomib only received DD. All patients were either treated at our center or by local hematologists, under our guidance. Clinical data and biomarkers were obtained ahead of daratumumab initiation. Interphase fluorescence in situ hybridization (iFISH) results stem from primary analysis routinely performed at first diagnosis. For iFISH cytogenetic analysis as previously described: see supplement.17 Follow-up data were obtained from consultations with our center or reported from local hematologists. For OS analysis, we additionally contacted our patients or their general practitioners by phone call or via e-mail exchange.
Clinical organ involvement was assessed according to international consensus criteria.18 For univariable and multivariable analysis the established cutoffs for survival in AL dFLC >180 mg/L, estimated glomerular filtration rate (eGFR) <50 mL/min/1.73 m2 and N-terminal prohormone of brain natriuretic peptide (NT-ProBNP) >8500 ng/L were used.7,10-12 To address the effect of nephrotic-range albuminuria, the Kidney Disease Outcomes Quality Initiative guideline suggested cutoff of 220 mg/mmol for albumin-to-creatinine ratio (ACR) for nephrotic syndrome was chosen.19
Missing data at daratumumab initiation were imputed according to expert knowledge for the cutoffs dFLC 180 mg/L in 8 patients, NT-ProBNP 8500 ng/L in 25 patients, and ACR 220 mg/mmol in 12 of the 168 patients (supplemental Data).
We prospectively analyzed 24-hour urine samples from 20 patients receiving daratumumab between January and March 2019. One patient provided 2 urine samples. Patients collected urine immediately after the end of daratumumab infusion for 24 hours and shipped the samples to our center via courier service. Probes were analyzed for ACR, albuminuria, proteinuria, and immunofixation. Urine electrophoresis was performed when more than 100 mg of total protein was detected to identify and measure a daratumumab peak. Screening was computer-assisted (supplemental Data).
Patients gave informed written consent for data and biomaterial analysis in accordance with the Declaration of Helsinki. Approval was granted by the Ethics Committee of the University of Heidelberg.
Treatment
Daratumumab 16 mg/kg total body weight was intravenously applied as a 500- to 1000-mL infusion after standard premedication with antihistamines and dexamethasone 20 mg 8 times weekly, 8 times every other week, and from then every 4 weeks. DVD patients additionally received 35-day cycles of bortezomib 1.0 mg/m2 or 1.3 mg/m2 subcutaneously applied on days 1, 8, 15, and 22.
Response evaluation
For hematologic event-free survival (hemEFS), a hematologic progress with the sole exception of loss of complete remission (CR), the switch to a different regimen because of insufficient treatment response and death were classified as an event. A patient was considered refractory to a drug when the last response was stable disease or progressive disease under therapy. The achievement of a partial remission (PR) or better was considered a response. A very good hematologic remission (VGHR) was either classified as a very good partial remission or better, for all patients with a dFLC >50 mg/L, or as a low-dFLC PR or better for all patients with a dFLC between 20 and 50 mg/L ahead of DD (n = 10) or DVD (n = 7).8,9,20 Hematologic remission (HR) assessment was performed with an intention-to-treat (ITT) approach after 3 months. Nine patients with a dFLC <20 mg/L and 4 patients evaluated with the Siemens free light-chain assay were not evaluated for HR. Nine further patients were alive and not assessed at 3 months and therefore could not be analyzed. Patients with cardiac organ response becoming eligible for consolidation therapy with high-dose melphalan were censored from analysis on the day of high-dose melphalan (n = 1 for DD; n = 3 for DVD). A summary of OS and hemEFS events, additional remission assessments after 6 months, and after 3 and 6 months excluding early deaths within the first 3 months can be found within the supplement material.
Organ response assessment was performed after 3, 6, and 12 months according to updated response criteria (ITT) in patients with cardiac or renal involvement fulfilling organ response eligibility criteria at start of daratumumab therapy.20,21 All patients receiving a next-line therapy for insufficient treatment response were classified as nonresponders for organ response assessment. Patients with missing NT-ProBNP levels or missing 24-hour proteinuria before initiation of daratumumab were deemed ineligible for organ response evaluation.
Statistical analysis
Statistical analysis was performed using R version 3.6.1 on a ×86_64-Apple-Darwin15.6.0 platform. Continuous data are described by their median and range. The Kruskal-Wallis test was used to test differences in continuous variables and the Fisher exact test was used to test differences in categorical variables between 2 groups.
OS and hemEFS curves were constructed according to Kaplan-Meier (KM) estimates. The median estimated time of observation was calculated based on the median time to censoring (reverse KM).22 Univariable and multivariable Cox and logistic models were fitted to evaluate the influence of possible prognostic factors on OS and hemEFS and remission, respectively. The proportional hazards assumption was tested as proposed by Grambsch and Therneau.23 To illustrate the results of the Cox models, hazard ratios and corresponding 95% confidence intervals (95% CI) were calculated. For the logistic models, odds ratios and corresponding 95% CIs were reported.
All statistical tests were 2-sided. Results with values of P ≤ .05 were considered statistically significant.
Results
Patient characteristics
Table 1 shows baseline characteristics of the DD and DVD cohorts at diagnosis and ahead of daratumumab initiation. Significant differences between groups were apparent in time from first and last therapy, age, number of previous lines of therapy, melphalan and IMID exposure, and IMID refractoriness. Monoclonal gammopathies were significantly underrepresented within the DVD group and consecutively the DVD group had a significantly higher bone marrow plasma cell (BMPC) percentage. There were also similarities present as both cohorts had similar dFLC levels at diagnosis and ahead of daratumumab, rates of refractoriness to PI, cytogenetic aberrations, and advanced cardiac and renal involvement.
Patient characteristics
| Variable . | DD (106 patients) . | DVD (62 patients) . | P . |
|---|---|---|---|
| At first diagnosis | |||
| λ subtype | 90 (85%) | 47 (76%) | .15 |
| Ig heavy chain present | 44 (42%) | 33 (53%) | .37 |
| Male sex | 73 (69%) | 33 (53%) | .05 |
| dFLC, mg/L | 236 (22-9600)* | 247 (0-2237)* | .74 |
| Symptomatic MM | 10 (9%) | 5 (8%) | 1.00 |
| Smoldering MM | 76 (72%) | 53 (86%) | .06 |
| Monoclonal gammopathy | 20 (19%) | 4 (7%) | .04 |
| BMPC percentage | 13 (2-84) | 19.5 (8-81) | .0014 |
| Translocation t(11;14) | 53/85 (62%) | 23/43 (54%) | .35 |
| Gain of 1q21 | 25/83 (30%) | 10/40 (25%) | .67 |
| Hyperdiploidy | 12/82 (15%) | 6/40 (15%) | 1.00 |
| MM high-risk aberrations | 8/85 (9%) | 4/41 (10%) | 1.00 |
| Deletion 13q14 | 28/84 (33%) | 12/41 (29%) | .69 |
| Before daratumumab | |||
| Age, y | 65 (36-81) | 60 (38-79) | .03 |
| Time from first therapy, mo | 29 (0-143) | 5 (0-104) | <.0001 |
| Time from last therapy, mo | 2 (0-54) | 1 (0-72) | .0009 |
| No. of previous therapies | 2 (1-7) | 1 (1-7) | .0005 |
| Refractory to last therapy | 59 (56%) | 34 (55%) | 1.00 |
| PI exposed | 97 (92%) | 59 (95%) | .54 |
| PI refractory | 52 (54%) | 33 (56%) | .63 |
| IMID exposed | 77 (73%) | 3 (5%) | <.0001 |
| IMID refractory | 42 (40%) | 1 (2%) | <.0001 |
| Melphalan exposed | 69 (65%) | 13 (21%) | <.0001 |
| Previous autologous transplant | 24 (23%) | 5 (8%) | .019 |
| Number of organs involved | 2 (1-5) | 3 (1-5) | .16 |
| >2 organs involved | 38 (36%) | 32 (52%) | .05 |
| Cardiac involvement | 88 (83%) | 54 (87%) | .52 |
| Renal involvement | 67 (63%) | 40 (65%) | 1.00 |
| Hepatic involvement | 15 (14%) | 15 (24%) | .14 |
| Soft-tissue involvement | 42 (40%) | 28 (45%) | .52 |
| Gastrointestinal involvement | 21 (20%) | 12 (19%) | 1.00 |
| Neuropathic involvement | 16 (15%) | 3 (5%) | .05 |
| dFLC in mg/L | 136 (0-3108)† | 117 (0-1997)† | .47 |
| dFLC >180 mg/L | 38 (36%) | 23 (37%) | .87 |
| NT-ProBNP in ng/L | 4155 (105-294 788)‡ | 5475 (78-127 410)‡ | .90 |
| NT-ProBNP >8500 ng/L | 36 (34%) | 25 (40%) | .41 |
| ACR, mg/mmol | 71 (0-13 415)§ | 194 (0-1434)§ | .44 |
| ACR >220mg/mmol | 48 (45%) | 32 (52%) | .52 |
| eGFR, mL/min/1.73 m2 (all patients not on dialysis) | 51.5 (12-126)|| | 53.5 (11-106)|| | .62 |
| eGFR <50 mL/min/1.73 m2 | 57 (54%)¶ | 33 (53%)¶ | 1.00 |
| Patients on dialysis | 15 (14%) | 6 (10%) | .47 |
| Daily proteinuria, mg/d | 1606 (0-20 160)# | 2701(57-18 852)# | .45 |
| Variable . | DD (106 patients) . | DVD (62 patients) . | P . |
|---|---|---|---|
| At first diagnosis | |||
| λ subtype | 90 (85%) | 47 (76%) | .15 |
| Ig heavy chain present | 44 (42%) | 33 (53%) | .37 |
| Male sex | 73 (69%) | 33 (53%) | .05 |
| dFLC, mg/L | 236 (22-9600)* | 247 (0-2237)* | .74 |
| Symptomatic MM | 10 (9%) | 5 (8%) | 1.00 |
| Smoldering MM | 76 (72%) | 53 (86%) | .06 |
| Monoclonal gammopathy | 20 (19%) | 4 (7%) | .04 |
| BMPC percentage | 13 (2-84) | 19.5 (8-81) | .0014 |
| Translocation t(11;14) | 53/85 (62%) | 23/43 (54%) | .35 |
| Gain of 1q21 | 25/83 (30%) | 10/40 (25%) | .67 |
| Hyperdiploidy | 12/82 (15%) | 6/40 (15%) | 1.00 |
| MM high-risk aberrations | 8/85 (9%) | 4/41 (10%) | 1.00 |
| Deletion 13q14 | 28/84 (33%) | 12/41 (29%) | .69 |
| Before daratumumab | |||
| Age, y | 65 (36-81) | 60 (38-79) | .03 |
| Time from first therapy, mo | 29 (0-143) | 5 (0-104) | <.0001 |
| Time from last therapy, mo | 2 (0-54) | 1 (0-72) | .0009 |
| No. of previous therapies | 2 (1-7) | 1 (1-7) | .0005 |
| Refractory to last therapy | 59 (56%) | 34 (55%) | 1.00 |
| PI exposed | 97 (92%) | 59 (95%) | .54 |
| PI refractory | 52 (54%) | 33 (56%) | .63 |
| IMID exposed | 77 (73%) | 3 (5%) | <.0001 |
| IMID refractory | 42 (40%) | 1 (2%) | <.0001 |
| Melphalan exposed | 69 (65%) | 13 (21%) | <.0001 |
| Previous autologous transplant | 24 (23%) | 5 (8%) | .019 |
| Number of organs involved | 2 (1-5) | 3 (1-5) | .16 |
| >2 organs involved | 38 (36%) | 32 (52%) | .05 |
| Cardiac involvement | 88 (83%) | 54 (87%) | .52 |
| Renal involvement | 67 (63%) | 40 (65%) | 1.00 |
| Hepatic involvement | 15 (14%) | 15 (24%) | .14 |
| Soft-tissue involvement | 42 (40%) | 28 (45%) | .52 |
| Gastrointestinal involvement | 21 (20%) | 12 (19%) | 1.00 |
| Neuropathic involvement | 16 (15%) | 3 (5%) | .05 |
| dFLC in mg/L | 136 (0-3108)† | 117 (0-1997)† | .47 |
| dFLC >180 mg/L | 38 (36%) | 23 (37%) | .87 |
| NT-ProBNP in ng/L | 4155 (105-294 788)‡ | 5475 (78-127 410)‡ | .90 |
| NT-ProBNP >8500 ng/L | 36 (34%) | 25 (40%) | .41 |
| ACR, mg/mmol | 71 (0-13 415)§ | 194 (0-1434)§ | .44 |
| ACR >220mg/mmol | 48 (45%) | 32 (52%) | .52 |
| eGFR, mL/min/1.73 m2 (all patients not on dialysis) | 51.5 (12-126)|| | 53.5 (11-106)|| | .62 |
| eGFR <50 mL/min/1.73 m2 | 57 (54%)¶ | 33 (53%)¶ | 1.00 |
| Patients on dialysis | 15 (14%) | 6 (10%) | .47 |
| Daily proteinuria, mg/d | 1606 (0-20 160)# | 2701(57-18 852)# | .45 |
Numbers with percentages in parentheses. Percentages were calculated for all 106 patients receiving DD and all 62 patients receiving DVD unless otherwise stated in numbers section. Medians with ranges in brackets for all patients unless otherwise stated. P values to test for differences between DD and DVD were calculated with the Kruskal-Wallis test for continuous variables, and the Fisher exact test for categorical variables. Statistically significant results (P < .05) are bold.
6 DD and 2 DVD not available.
4 DD and 4 DVD patients with only involved serum free-light chain available or not evaluated with binding site test.
18 DD and 7 DVD not available.
9 DD and 3 DVD not available.
For patients not on dialysis.
Patients on dialysis classified as <50 mL/min/1.73 m2.
17 DD and 10 DVD not available.
Treatment application
On the data cutoff date, 30 November 2019, patients in the DD group were given a median of 14 daratumumab infusions (range, 2-35) and 79% (84/106) of patients received at least 6 infusions of daratumumab without an interruption of more than 2 weeks within the first 3 months. Patients in the DVD group were given a median of 14 daratumumab infusions (range, 1-29) and 87% (54/62) of patients completed at least 6 infusions within the first 3 months.
Complications with DD and DVD
Upper and lower respiratory tract infections were the most frequent infectious complications. Infections and atrial fibrillation were common in responders and non-responders (Table 2). Congestive heart failure typically manifested within the first 8 dosages of weekly infusions, whereas severe infectious complications occurred at any treatment interval. Infusion-related reactions solely arose with the first dosage. A high rate of lymphocytopenia was detected with both DD and DVD.
Complications according to CTCAE, version 5.024
| Type . | Any grade . | Grade 3/4 . | Grade 5 . | |||
|---|---|---|---|---|---|---|
| . | DD . | DVD . | DD . | DVD . | DD . | DVD . |
| Infection | 37% (39) | 31% (19) | 16% (17) | 18% (11) | 6% (6) | 3% (2) |
| IRR | 7% (7) | 5% (3) | 2% (2) | — | — | — |
| CHF | NA | NA | 8% (9) | 10% (6) | NA | NA |
| Hyperglycemia | NA | NA | 3% (3) | — | — | — |
| Atrial fibrillation | 5% (5) | 6% (4) | — | — | — | — |
| Nausea | 4% (4) | 2% (1) | — | — | — | — |
| Diarrhea | 10% (11) | 2% (1) | 1% (1) | — | — | — |
| Polyneuropathy | — | 3% (2) | — | — | — | — |
| Other* | NA | NA | 4% (4) | 2% (1) | — | — |
| Lymphocytopenia | 53% (47/89) | 57% (30/53) | 20% (18/89) | 17% (9/53) | NA | NA |
| Neutropenia | 2% (2/90) | 0% (0/52) | — | — | — | — |
| Thrombocytopenia | 19% (18/94) | 22% (12/55) | 0% (0/94) | 2% (1/55) | — | — |
| Anemia | 50% (39/78) | 52% (29/56) | 3% (2/78) | 4% (2/56) | — | — |
| Type . | Any grade . | Grade 3/4 . | Grade 5 . | |||
|---|---|---|---|---|---|---|
| . | DD . | DVD . | DD . | DVD . | DD . | DVD . |
| Infection | 37% (39) | 31% (19) | 16% (17) | 18% (11) | 6% (6) | 3% (2) |
| IRR | 7% (7) | 5% (3) | 2% (2) | — | — | — |
| CHF | NA | NA | 8% (9) | 10% (6) | NA | NA |
| Hyperglycemia | NA | NA | 3% (3) | — | — | — |
| Atrial fibrillation | 5% (5) | 6% (4) | — | — | — | — |
| Nausea | 4% (4) | 2% (1) | — | — | — | — |
| Diarrhea | 10% (11) | 2% (1) | 1% (1) | — | — | — |
| Polyneuropathy | — | 3% (2) | — | — | — | — |
| Other* | NA | NA | 4% (4) | 2% (1) | — | — |
| Lymphocytopenia | 53% (47/89) | 57% (30/53) | 20% (18/89) | 17% (9/53) | NA | NA |
| Neutropenia | 2% (2/90) | 0% (0/52) | — | — | — | — |
| Thrombocytopenia | 19% (18/94) | 22% (12/55) | 0% (0/94) | 2% (1/55) | — | — |
| Anemia | 50% (39/78) | 52% (29/56) | 3% (2/78) | 4% (2/56) | — | — |
Percentages with numbers in parentheses. Percentages were calculated for all 106 patients receiving DD and all 62 patients receiving DVD unless otherwise stated. *Includes 3 patients receiving DD with transient ischemic attack/stroke, 1 patient receiving DD with a coronary infarction, and 1 patient receiving DVD with Leriche syndrome.
CHF, congestive heart failure; IRR, infusion-related reaction; NA, not assessed
DD results
OS, hemEFS, and HR
Please refer to Figure 1. After a median follow-up of 21.2 months, 25 patients were still receiving daratumumab and 17 patients were off therapy without a hematologic event. Forty-four patients had died.
KM plots for hemEFS and OS for DD and DVD. For the DD group, median OS was 25.6 months and hemEFS 11.8 months. After 12 months, 68% of patients were still alive and 47% were without a hemEFS event (median follow-up for DD was 22.2 months). For the DVD group, median OS had not yet been reached and hemEFS was 19.1 months. After 12 months, 73% of patients were still alive and 52% were without a hemEFS event (median follow-up time for DVD was 16.7 months).
KM plots for hemEFS and OS for DD and DVD. For the DD group, median OS was 25.6 months and hemEFS 11.8 months. After 12 months, 68% of patients were still alive and 47% were without a hemEFS event (median follow-up for DD was 22.2 months). For the DVD group, median OS had not yet been reached and hemEFS was 19.1 months. After 12 months, 73% of patients were still alive and 52% were without a hemEFS event (median follow-up time for DVD was 16.7 months).
The following hematologic events occurred between treatment initiation and last follow-up: death (n = 26), next-line therapy (n = 21), and hematologic progression (n = 17) with 10 patients progressing after achieving at least a PR (on median after 20.5 months [range, 7.1-24.2 months]). All patients with hematologic progression either had renal involvement (n = 12), mainly with an ACR >220 mg/mmol (n = 9) and/or a dFLC >180 mg/L (n = 11).
Median OS was 25.6 months and hemEFS was 11.8 months. After 12 months, 68% of patients were alive and 47% were without a hemEFS event. The overall response rate (ORR; ITT) after 3 months was 64% (59/92), the VGHR rate was 48% (44/92), and the CR rate was 8% (7/92).
Organ response assessment
Seventy-three patients with cardiac involvement had baseline NT-ProBNP levels higher than 650 ng/L and 44 patients with renal involvement not on dialysis had a baseline 24-hour proteinuria over 0.5 g/d (Table 3). Apart from 2 patients in hematologically stable disease and initial dFLC <50 mg/L, all organ responders were either in HR or patients with an initial dFLC <20 mg/L.
Organ response assessment (ITT)
| . | Responders* . | Nonresponders† . | Deaths‡ . | Missing/alive§ . |
|---|---|---|---|---|
| DD | ||||
| Cardiac 3 mo | 10% (7) | 51% (37) | 10% (7) | 30% (22) |
| Cardiac 6 mo | 22% (15) | 35% (24) | 22% (15) | 21% (14) |
| Cardiac 12 mo | 18% (11) | 31% (19) | 33% (20) | 18% (11) |
| Renal 3 mo | 20% (9) | 45% (20) | 2% (1) | 32% (14) |
| Renal 6 mo | 24% (10) | 38% (16) | 10% (4) | 29% (12) |
| Renal 12 mo | 14% (5) | 51% (18) | 26% (9) | 9% (3) |
| DVD | ||||
| Cardiac 3 mo | 28% (13) | 48% (22) | 13% (6) | 11% (5) |
| Cardiac 6 mo | 26% (11) | 44% (19) | 19% (8) | 12% (5) |
| Cardiac 12 mo | 25% (9) | 39% (14) | 28% (10) | 8% (3) |
| Renal 3 mo | 10% (3) | 56% (18) | 3% (1) | 31% (10) |
| Renal 6 mo | 24% (7) | 41% (12) | 7% (2) | 28% (8) |
| Renal 12 mo | 27% (7) | 42% (11) | 27% (7) | 4% (1) |
| . | Responders* . | Nonresponders† . | Deaths‡ . | Missing/alive§ . |
|---|---|---|---|---|
| DD | ||||
| Cardiac 3 mo | 10% (7) | 51% (37) | 10% (7) | 30% (22) |
| Cardiac 6 mo | 22% (15) | 35% (24) | 22% (15) | 21% (14) |
| Cardiac 12 mo | 18% (11) | 31% (19) | 33% (20) | 18% (11) |
| Renal 3 mo | 20% (9) | 45% (20) | 2% (1) | 32% (14) |
| Renal 6 mo | 24% (10) | 38% (16) | 10% (4) | 29% (12) |
| Renal 12 mo | 14% (5) | 51% (18) | 26% (9) | 9% (3) |
| DVD | ||||
| Cardiac 3 mo | 28% (13) | 48% (22) | 13% (6) | 11% (5) |
| Cardiac 6 mo | 26% (11) | 44% (19) | 19% (8) | 12% (5) |
| Cardiac 12 mo | 25% (9) | 39% (14) | 28% (10) | 8% (3) |
| Renal 3 mo | 10% (3) | 56% (18) | 3% (1) | 31% (10) |
| Renal 6 mo | 24% (7) | 41% (12) | 7% (2) | 28% (8) |
| Renal 12 mo | 27% (7) | 42% (11) | 27% (7) | 4% (1) |
Percentages with numbers in parentheses. Percentages at 3 months were calculated for 73 cardiac and 44 renal response assessable patients receiving DD and for 46 cardiac and 32 renal response assessable patients receiving DVD. Percentages at 6 months were calculated for 68 cardiac and 42 renal response assessable patients receiving DD and for 43 cardiac and 29 renal response assessable patients receiving DVD. Percentages at 12 months were calculated for 61 cardiac and 35 renal response assessable patients receiving DD and for 36 cardiac and 26 renal response assessable patients receiving DVD.
NT-ProBNP decrease of at least 30% and ≥300 ng/L measured at 3, 6, and 12 months compared with start of daratumumab for cardiac responders or decrease of proteinuria by ≥30% or <0.5 g/d without renal progression for renal responders.
NT-ProBNP assessed at 3, 6, and 12 months not fulfilling cardiac response criteria or proteinuria and eGFR assessed at landmark not fulfilling renal response criteria.
Patients fulfilling organ response eligibility criteria at start of daratumumab dead at 3, 6, and 12 months.
Patients fulfilling organ response eligibility criteria at start of daratumumab alive at 3, 6, and 12 months not assessed for organ response.
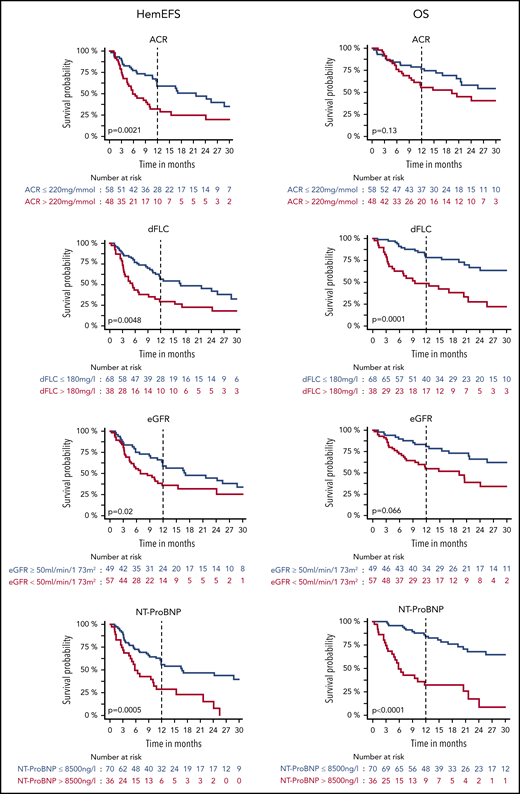
Univariable analysis of prognostic factors
Univariable analysis revealed that patients had reduced OS, hemEFS, and VGHR rates when dFLC was >180 mg/L or eGFR was <50 mL/min/1.73 m2 (Figures 2 and 3; Tables 4 and 5). ACR >220 mg/mmol was prognostically relevant for hemEFS and VGHR rates, NT-ProBNP >8500 ng/L for OS and hemEFS, and age for OS. Patients refractory to last PI had higher dFLC values ahead of DD (median, 165 mg/L vs 127 mg/L; P = .04) and a significantly lower VGHR rate.
HemEFS and OS for specific subgroups with DD. KM plots for hemEFS (left) and OS (right) stratified by cutoffs for ACR, dFLC, eGFR, and NT-ProBNP. P values between groups listed in KM plots.
HemEFS and OS for specific subgroups with DD. KM plots for hemEFS (left) and OS (right) stratified by cutoffs for ACR, dFLC, eGFR, and NT-ProBNP. P values between groups listed in KM plots.
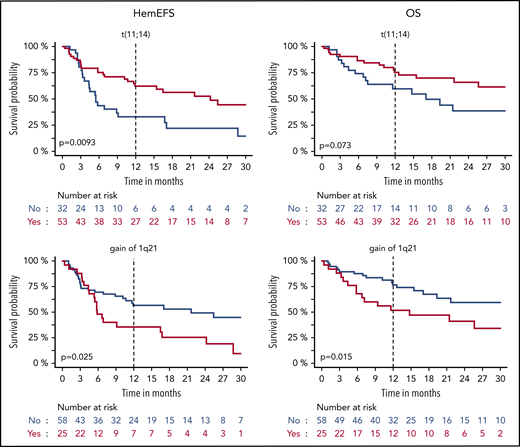
HemEFS and OS for specific subgroups with DD. KM plots for hemEFS (left) and OS (right) stratified by presence of translocation t(11;14) and gain of 1q21. P values between groups listed in KM plots.
HemEFS and OS for specific subgroups with DD. KM plots for hemEFS (left) and OS (right) stratified by presence of translocation t(11;14) and gain of 1q21. P values between groups listed in KM plots.
Overall remission and VGHR rates (ITT)
| . | ACR >220 mg/mmol . | ACR ≤220 mg/mmol . | Any ACR . | |||
|---|---|---|---|---|---|---|
| . | ORR . | VGHR rate . | ORR . | VGHR rate . | ORR . | VGHR rate . |
| DD any dFLC | 54% (21/39) | 36% (14/39) | 72% (38/53) | 60% (32/53) | 64% (59/92) | 48% (44/92) |
| DD dFLC >180 mg/L | 38% (5/13) | 8% (1/13) | 68% (15/22) | 41% (9/22) | 57% (20/35) | 29% (10/35) |
| DD dFLC ≤180 mg/L | 62% (16/26) | 50% (13/26) | 74% (23/31) | 68% (21/31) | 68% (39/57) | 60% (34/57) |
| DVD any dFLC | 54% (13/24) | 46% (11/24) | 76% (22/29) | 62% (18/29) | 66% (35/53) | 55% (29/53) |
| DVD dFLC >180 mg/L | 30% (3/10) | 10% (1/10) | 70% (7/10) | 40% (4/10) | 50% (10/20) | 25% (5/20) |
| DVD dFLC ≤180 mg/L | 71% (10/14) | 71% (10/14) | 79% (15/19) | 74% (14/19) | 76% (25/33) | 73% (24/33) |
| . | ACR >220 mg/mmol . | ACR ≤220 mg/mmol . | Any ACR . | |||
|---|---|---|---|---|---|---|
| . | ORR . | VGHR rate . | ORR . | VGHR rate . | ORR . | VGHR rate . |
| DD any dFLC | 54% (21/39) | 36% (14/39) | 72% (38/53) | 60% (32/53) | 64% (59/92) | 48% (44/92) |
| DD dFLC >180 mg/L | 38% (5/13) | 8% (1/13) | 68% (15/22) | 41% (9/22) | 57% (20/35) | 29% (10/35) |
| DD dFLC ≤180 mg/L | 62% (16/26) | 50% (13/26) | 74% (23/31) | 68% (21/31) | 68% (39/57) | 60% (34/57) |
| DVD any dFLC | 54% (13/24) | 46% (11/24) | 76% (22/29) | 62% (18/29) | 66% (35/53) | 55% (29/53) |
| DVD dFLC >180 mg/L | 30% (3/10) | 10% (1/10) | 70% (7/10) | 40% (4/10) | 50% (10/20) | 25% (5/20) |
| DVD dFLC ≤180 mg/L | 71% (10/14) | 71% (10/14) | 79% (15/19) | 74% (14/19) | 76% (25/33) | 73% (24/33) |
Percentages with numbers in parentheses. ORR and VGHR rates all calculated with ITT.
Results for univariable and multivariable Cox regression analysis for DD
| DD . | OS . | HemEFS . | VGHR rate . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Univariable analysis | |||||||||
| dFLC >180 mg/L | 3.39 | 1.86-6.18 | .0001 | 2.05 | 1.24-3.36 | .0048 | 0.27 | 0.11-0.65 | .0046 |
| ACR >220 mg/mmol | 1.58 | 0.87-2.87 | .13 | 2.21 | 1.33-3.66 | .0021 | 0.39 | 0.17-0.91 | .032 |
| NT-ProBNP >8500 ng/L | 5.57 | 3.01-10.3 | <.0001 | 2.52 | 1.50-4.21 | .0005 | 0.64 | 0.26-1.52 | .31 |
| eGFR <50 mL/min/1.73 m2 | 2.38 | 1.27-4.45 | .0066 | 1.83 | 1.10-3.05 | .020 | 0.38 | 0.16-0.87 | .024 |
| Age, y | 1.03 | 1.00-1.07 | .034 | 1.00 | 0.98-1.03 | .73 | 1.03 | 0.99-1.08 | .16 |
| Male | 1.35 | 0.69-2.62 | .38 | 0.70 | 0.42-1.17 | .18 | 1.07 | 0.45-2.59 | .88 |
| No. of organs | 1.08 | 0.83-1.40 | .59 | 1.02 | 0.81-1.28 | .86 | 1.05 | 0.71 to 1.58 | .79 |
| No. of previous therapies | 1.02 | 0.82-1.25 | .89 | 1.13 | 0.94-1.36 | .19 | 0.94 | 0.69-1.26 | .66 |
| Refractory to last therapy | 0.70 | 0.39-1.27 | .24 | 1.25 | 0.75-2.07 | .39 | 0.60 | 0.26-1.37 | .23 |
| Refractory to last PI | 1.32 | 0.71-2.45 | .39 | 1.53 | 0.91-2.60 | .11 | 0.15 | 0.05-0.38 | .0001 |
| Translocation t(11;14) | 0.54 | 0.27-1.06 | .074 | 0.47 | 0.26-0.83 | .0093 | 2.16 | 0.84-5.80 | .12 |
| Gain of 1q21 | 2.34 | 1.18-4.65 | .015 | 1.95 | 1.09-3.51 | .025 | 0.88 | 0.32-2.41 | .80 |
| Hyperdiploidy | 2.95 | 1.31-6.63 | .0089 | 2.43 | 1.20-4.94 | .014 | 0.09 | 0.00-0.52 | .026 |
| MM high-risk aberrations | 0.95 | 0.33-2.71 | .92 | 1.82 | 0.81-4.09 | .14 | 0.97 | 0.17-5.56 | .97 |
| Deletion 13q14 | 0.90 | 0.44-1.87 | .78 | 1.43 | 0.79-2.58 | .24 | 2.10 | 0.79-5.83 | .14 |
| Multivariable analysis | |||||||||
| dFLC >180 mg/L | 4.21 | 2.26-7.83 | <.0001 | 2.51 | 1.49-4.24 | .0006 | 0.17 | 0.06-0.46 | .0009 |
| ACR >220 mg/mmol | 1.36 | 0.65-2.84 | .42 | 2.07 | 1.12-3.83 | .020 | 0.47 | 0.16-1.34 | .16 |
| NT-ProBNP >8500 ng/L | 5.78 | 2.94-11.35 | <.0001 | 2.21 | 1.26-3.88 | .0056 | 0.78 | 0.27-2.23 | .65 |
| eGFR <50 mL/min/1.73 m2 | 1.50 | 0.67-3.35 | .32 | 1.08 | 0.57-2.05 | .82 | 0.46 | 0.16-1.33 | .15 |
| Age, y | 1.02 | 0.99-1.05 | .14 | 1.00 | 0.97-1.02 | .87 | 1.05 | 1.00-1.11 | .05 |
| DD . | OS . | HemEFS . | VGHR rate . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Univariable analysis | |||||||||
| dFLC >180 mg/L | 3.39 | 1.86-6.18 | .0001 | 2.05 | 1.24-3.36 | .0048 | 0.27 | 0.11-0.65 | .0046 |
| ACR >220 mg/mmol | 1.58 | 0.87-2.87 | .13 | 2.21 | 1.33-3.66 | .0021 | 0.39 | 0.17-0.91 | .032 |
| NT-ProBNP >8500 ng/L | 5.57 | 3.01-10.3 | <.0001 | 2.52 | 1.50-4.21 | .0005 | 0.64 | 0.26-1.52 | .31 |
| eGFR <50 mL/min/1.73 m2 | 2.38 | 1.27-4.45 | .0066 | 1.83 | 1.10-3.05 | .020 | 0.38 | 0.16-0.87 | .024 |
| Age, y | 1.03 | 1.00-1.07 | .034 | 1.00 | 0.98-1.03 | .73 | 1.03 | 0.99-1.08 | .16 |
| Male | 1.35 | 0.69-2.62 | .38 | 0.70 | 0.42-1.17 | .18 | 1.07 | 0.45-2.59 | .88 |
| No. of organs | 1.08 | 0.83-1.40 | .59 | 1.02 | 0.81-1.28 | .86 | 1.05 | 0.71 to 1.58 | .79 |
| No. of previous therapies | 1.02 | 0.82-1.25 | .89 | 1.13 | 0.94-1.36 | .19 | 0.94 | 0.69-1.26 | .66 |
| Refractory to last therapy | 0.70 | 0.39-1.27 | .24 | 1.25 | 0.75-2.07 | .39 | 0.60 | 0.26-1.37 | .23 |
| Refractory to last PI | 1.32 | 0.71-2.45 | .39 | 1.53 | 0.91-2.60 | .11 | 0.15 | 0.05-0.38 | .0001 |
| Translocation t(11;14) | 0.54 | 0.27-1.06 | .074 | 0.47 | 0.26-0.83 | .0093 | 2.16 | 0.84-5.80 | .12 |
| Gain of 1q21 | 2.34 | 1.18-4.65 | .015 | 1.95 | 1.09-3.51 | .025 | 0.88 | 0.32-2.41 | .80 |
| Hyperdiploidy | 2.95 | 1.31-6.63 | .0089 | 2.43 | 1.20-4.94 | .014 | 0.09 | 0.00-0.52 | .026 |
| MM high-risk aberrations | 0.95 | 0.33-2.71 | .92 | 1.82 | 0.81-4.09 | .14 | 0.97 | 0.17-5.56 | .97 |
| Deletion 13q14 | 0.90 | 0.44-1.87 | .78 | 1.43 | 0.79-2.58 | .24 | 2.10 | 0.79-5.83 | .14 |
| Multivariable analysis | |||||||||
| dFLC >180 mg/L | 4.21 | 2.26-7.83 | <.0001 | 2.51 | 1.49-4.24 | .0006 | 0.17 | 0.06-0.46 | .0009 |
| ACR >220 mg/mmol | 1.36 | 0.65-2.84 | .42 | 2.07 | 1.12-3.83 | .020 | 0.47 | 0.16-1.34 | .16 |
| NT-ProBNP >8500 ng/L | 5.78 | 2.94-11.35 | <.0001 | 2.21 | 1.26-3.88 | .0056 | 0.78 | 0.27-2.23 | .65 |
| eGFR <50 mL/min/1.73 m2 | 1.50 | 0.67-3.35 | .32 | 1.08 | 0.57-2.05 | .82 | 0.46 | 0.16-1.33 | .15 |
| Age, y | 1.02 | 0.99-1.05 | .14 | 1.00 | 0.97-1.02 | .87 | 1.05 | 1.00-1.11 | .05 |
HR, hazard ratio. Statistically significant results (P < .05) are bold.
HemEFS was lower in renal patients compared with nonrenal patients (P = .0504). Patients with renal AL and an ACR <220 mg/mmol had an ITT-ORR of 72% (13/18) and a VGHR rate of 67% (12/18).
Translocation t(11;14) was associated with a significantly better hemEFS and trended toward a better OS and VGHR rate. Hyperdiploidy was associated with a significantly higher dFLC ahead of DD (median dFLC, 272 mg/L; P = .013) and worse hemEFS, OS, and a lower VGHR rate. Gain of 1q21 was associated with a worse hemEFS and OS. One patient with deletion17p13 also had t(11;14), gain of 1q21, and hyperdiploidy. No other patient was hyperdiploidic with a concurrent t(11;14). Hyperdiploidy was present among the other 24 patients with gain of 1q21 in 7 cases and t(11;14) in another 7 cases.
Multivariable analysis of prognostic factors
In multivariable analysis of hemEFS, OS, and VGHR rate, we tested the univariably significant and established cutoffs dFLC >180 mg/L, NT-proBNP >8500 ng/L, and eGFR <50 mL/min/1.73 m2 in combination with age as a standard parameter and, additionally, ACR >220 mg/mmol due to univariable results for hemEFS (Table 5).
Applying full Cox regression models, we found that dFLC >180 mg/L, NT-ProBNP >8500 ng/L, and an ACR >220 mg/mmol were prognostic for hemEFS. Furthermore, dFLC >180 mg/L and NT-ProBNP >8500 ng/L were prognostic for OS and in addition dFLC >180 mg/L was prognostic for VGHR rate.
DVD results
OS, hemEFS, and HR
After a median follow-up of 16.7 months for the DVD group, 14 patients were still receiving DVD, 7 patients solely received daratumumab, and 13 patients were off therapy without a hematologic event (Figure 1). Sixteen patients had died.
The following hematologic events occurred between treatment initiation and last follow-up: death (n = 11), next-line therapy (n = 13), and hematologic progression (n = 5) with 3 patients progressing after achieving at least a PR (after 1.6, 19.0, and 22.1 months).
Median OS had not yet been reached and hemEFS was 19.1 months. After 12 months, 73% of patients were still alive and 52% were without a hemEFS event. Three months after treatment initiation, the ORR (ITT) was 66% (35/53), the VGHR rate was 55% (29/53), and the CR rate was 11% (6/53).
Organ response assessment
Forty-four patients with cardiac involvement had baseline NT-ProBNP levels higher than 650 ng/L and 32 patients with renal involvement not on dialysis had a baseline 24-hour proteinuria over 0.5 g/d (Table 4). One renal and 2 cardiac organ responders after 3 months and 2 renal organ responders after 6 months achieved hematologically stable disease. All other organ responders were either in HR or patients with an initial dFLC <20 mg/L.
Univariable analysis of prognostic factors
Univariable analysis revealed that patients had reduced OS, hemEFS, and VGHR rate when dFLC was >180 mg/L (Figures 4 and 5; Tables 3 and 6). NT-ProBNP >8500 ng/L was prognostically relevant for OS and hemEFS. ACR >220 mg/mmol trended toward a worse hemEFS. Male sex was beneficial, and the number of organs clinically involved was adverse for VGHR rate and hemEFS. HemEFS was significantly lower in renal AL patients (P = .035). Patients with renal AL and an ACR <220 mg/mmol achieved an ITT-ORR/VGHR rate of only 38% (3/8) as 2 patients in VGHR had died before month 3.
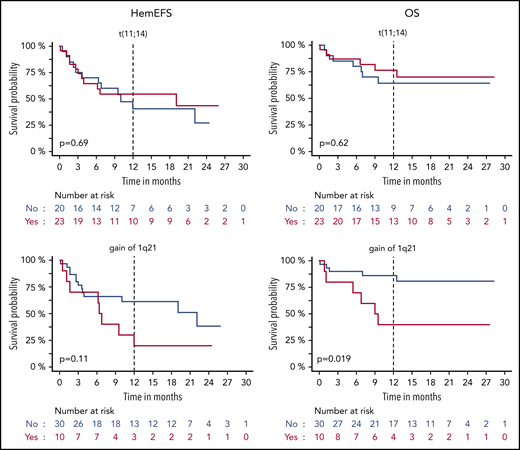
HemEFS and OS for specific subgroups with DVD. KM plots for hemEFS (left) and OS (right) stratified by cutoffs for ACR, dFLC, eGFR, and NT-ProBNP. P values between groups listed in KM plots.
HemEFS and OS for specific subgroups with DVD. KM plots for hemEFS (left) and OS (right) stratified by cutoffs for ACR, dFLC, eGFR, and NT-ProBNP. P values between groups listed in KM plots.
HemEFS and OS for specific subgroups with DVD. KM plots for hemEFS (left) and OS (right) stratified by presence of translocation t(11;14) and gain of 1q21. P values between groups listed in KM plots.
HemEFS and OS for specific subgroups with DVD. KM plots for hemEFS (left) and OS (right) stratified by presence of translocation t(11;14) and gain of 1q21. P values between groups listed in KM plots.
Results for univariable and multivariable Cox regression analysis for DVD
| DVD . | OS . | HemEFS . | VGHR rate . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Univariable analysis | |||||||||
| dFLC >180 mg/L | 4.43 | 1.54-12.78 | .0058 | 3.49 | 1.63-7.47 | .0013 | 0.12 | 0.03-0.42 | .0013 |
| ACR >220 mg/mmol | 2.15 | 0.75-6.19 | .16 | 1.93 | 0.91-4.09 | .088 | 0.52 | 0.17-1.54 | .24 |
| NT-ProBNP >8500 ng/L | 8.38 | 2.38-29.51 | .0009 | 2.36 | 1.13-4.93 | .022 | 0.45 | 0.14-1.33 | .15 |
| eGFR < 50 mL/min/1.73 m2 | 1.50 | 0.54-4.12 | .43 | 1.11 | 0.53-2.31 | .79 | 1.10 | 0.37-3.30 | .86 |
| Age, y | 1.00 | 0.95-1.05 | .93 | 0.99 | 0.95-1.03 | .57 | 1.01 | 0.95-1.07 | .80 |
| Male | 0.39 | 0.14-1.12 | .081 | 0.41 | 0.19-0.88 | .023 | 5.25 | 1.68-17.96 | .0057 |
| No. of organs | 1.40 | 0.91-2.13 | .12 | 1.57 | 1.13-2.18 | .0069 | 0.54 | 0.30-0.92 | .033 |
| No. of therapies | 0.93 | 0.56-1.57 | .79 | 0.75 | 0.45-1.24 | .26 | 1.81 | 0.89-5.89 | .21 |
| Refractory to last therapy | 2.25 | 0.78-6.49 | .13 | 2.22 | 1.03-4.80 | .042 | 0.35 | 0.11-1.06 | .070 |
| Refractory to last PI | 1.94 | 0.66-5.69 | .23 | 2.26 | 1.02-4.96 | .043 | 0,43 | 0.13-1.33 | .15 |
| Translocation t(11;14) | 0.76 | 0.26-2.26 | .62 | 0.85 | 0.37-1.92 | .69 | 1.23 | 0.34-4.49 | .75 |
| Gain of 1q21 | 4.14 | 1.26-13.59 | .019 | 2.07 | 0.86-5.01 | .11 | 0.30 | 0.04-1.66 | .19 |
| Hyperdiploidy | 0.55 | 0.07-4.27 | .56 | 1.22 | 0.41-3.65 | .72 | 0.58 | 0.07-4.01 | .58 |
| MM high-risk aberrations | — | — | — | 3.80 | 1.23-11.69 | .020 | 0.26 | 0.01-2.27 | .26 |
| Deletion 13q14 | 1.58 | 0.46-5.40 | .47 | 1.19 | 0.46-3.11 | .72 | 0.86 | 0.19-3.78 | .84 |
| Multivariable analysis | |||||||||
| dFLC >180 mg/L | — | — | — | 3.40 | 1.55-7.48 | .0023 | 0.13 | 0.03-0.49 | .0034 |
| ACR >220 mg/mmol | — | — | — | 3.06 | 1.18-7.93 | .021 | 0.32 | 0.06-1.37 | .14 |
| NT-ProBNP >8500 ng/L | — | — | — | 2.39 | 1.10-5.16 | .027 | 0.40 | 0.10-1.58 | .20 |
| eGFR <50 mL/min/1.73 m2 | — | — | — | 0.57 | 0.22-1.49 | .25 | 2.03 | 0.48-9.61 | .35 |
| Age, y | — | — | — | 1.00 | 0.96-1.05 | .93 | 0.98 | 0.91-1.05 | .65 |
| DVD . | OS . | HemEFS . | VGHR rate . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Univariable analysis | |||||||||
| dFLC >180 mg/L | 4.43 | 1.54-12.78 | .0058 | 3.49 | 1.63-7.47 | .0013 | 0.12 | 0.03-0.42 | .0013 |
| ACR >220 mg/mmol | 2.15 | 0.75-6.19 | .16 | 1.93 | 0.91-4.09 | .088 | 0.52 | 0.17-1.54 | .24 |
| NT-ProBNP >8500 ng/L | 8.38 | 2.38-29.51 | .0009 | 2.36 | 1.13-4.93 | .022 | 0.45 | 0.14-1.33 | .15 |
| eGFR < 50 mL/min/1.73 m2 | 1.50 | 0.54-4.12 | .43 | 1.11 | 0.53-2.31 | .79 | 1.10 | 0.37-3.30 | .86 |
| Age, y | 1.00 | 0.95-1.05 | .93 | 0.99 | 0.95-1.03 | .57 | 1.01 | 0.95-1.07 | .80 |
| Male | 0.39 | 0.14-1.12 | .081 | 0.41 | 0.19-0.88 | .023 | 5.25 | 1.68-17.96 | .0057 |
| No. of organs | 1.40 | 0.91-2.13 | .12 | 1.57 | 1.13-2.18 | .0069 | 0.54 | 0.30-0.92 | .033 |
| No. of therapies | 0.93 | 0.56-1.57 | .79 | 0.75 | 0.45-1.24 | .26 | 1.81 | 0.89-5.89 | .21 |
| Refractory to last therapy | 2.25 | 0.78-6.49 | .13 | 2.22 | 1.03-4.80 | .042 | 0.35 | 0.11-1.06 | .070 |
| Refractory to last PI | 1.94 | 0.66-5.69 | .23 | 2.26 | 1.02-4.96 | .043 | 0,43 | 0.13-1.33 | .15 |
| Translocation t(11;14) | 0.76 | 0.26-2.26 | .62 | 0.85 | 0.37-1.92 | .69 | 1.23 | 0.34-4.49 | .75 |
| Gain of 1q21 | 4.14 | 1.26-13.59 | .019 | 2.07 | 0.86-5.01 | .11 | 0.30 | 0.04-1.66 | .19 |
| Hyperdiploidy | 0.55 | 0.07-4.27 | .56 | 1.22 | 0.41-3.65 | .72 | 0.58 | 0.07-4.01 | .58 |
| MM high-risk aberrations | — | — | — | 3.80 | 1.23-11.69 | .020 | 0.26 | 0.01-2.27 | .26 |
| Deletion 13q14 | 1.58 | 0.46-5.40 | .47 | 1.19 | 0.46-3.11 | .72 | 0.86 | 0.19-3.78 | .84 |
| Multivariable analysis | |||||||||
| dFLC >180 mg/L | — | — | — | 3.40 | 1.55-7.48 | .0023 | 0.13 | 0.03-0.49 | .0034 |
| ACR >220 mg/mmol | — | — | — | 3.06 | 1.18-7.93 | .021 | 0.32 | 0.06-1.37 | .14 |
| NT-ProBNP >8500 ng/L | — | — | — | 2.39 | 1.10-5.16 | .027 | 0.40 | 0.10-1.58 | .20 |
| eGFR <50 mL/min/1.73 m2 | — | — | — | 0.57 | 0.22-1.49 | .25 | 2.03 | 0.48-9.61 | .35 |
| Age, y | — | — | — | 1.00 | 0.96-1.05 | .93 | 0.98 | 0.91-1.05 | .65 |
Univariable OS analysis for MM high-risk aberrations and multivariable OS analysis were not performed because of an insufficient number of events. Statistically significant results (P < .05) are bold.
Gain of 1q21 was associated with a significantly worse OS and MM high-risk aberrations with a shorter hemEFS. No significant effects were detected for translocation t(11;14) and hyperdiploidy. There were no patients with t(11;14) and concurrent hyperdiploidy. Among the 10 patients with gain of 1q21, there was an overlap with hyperdiploidy in 4 and with t(11;14) in 2 patients. Refractoriness to last therapy was adversely correlated with hemEFS. The most recent treatment regimen was PI based for 82% (28/34) of the patients refractory to last therapy. Nevertheless, 17 patients had previously responded with a VGHR to last PI treatment.
Multivariable analysis of prognostic factors
The previously tested combination of factors for DD was used for multivariable analysis of hemEFS and VGHR rate (Table 6). OS analysis was not performed because of an insufficient number of events. Applying full Cox regression models, we found that dFLC >180 mg/L, NT-ProBNP >8500 ng/L, and an ACR >220mg/mmol were prognostic for hemEFS. Furthermore, dFLC >180 mg/L was prognostic for VGHR rate.
Discussion
In our study, we present 2 large cohorts of patients with advanced AL amyloidosis treated with daratumumab. We achieved an ITT-overall response 3 months after treatment initiation in 2 of 3 patients and a VGHR in every second patient with each combination in a population with >50% bortezomib refractoriness. For DD, median OS was 25.6 months and hemEFS was 11.8 months. DVD achieved slightly better results with median OS not reached and median hemEFS 19.1 months. Nevertheless, with DD primarily being available as a third-line regimen and DVD being used as a second-line regimen, within our population, which meant substantial discrepancies in age, number of previous therapies and time from first therapy, outcome and efficacy should not directly be compared. The previously established negative prognostic factors dFLC >180 mg/L and NT-ProBNP >8500 ng/L could not be overcome with daratumumab salvage therapy. Additionally, we were able to first describe nephrotic-range albuminuria with an ACR >220 mg/mmol as adverse for outcome and response to a monoclonal antibody in antineoplastic therapy and could confirm this finding with uni- and multivariable analyses in a second cohort of patients. Baseline iFISH cytogenetics still remained relevant ahead of salvage therapy as translocation t(11;14) patients had longer and hyperdiploidic patients had shorter hemEFS with DD.
A dFLC >180 mg/L was adversely correlated with OS, hemEFS, and VGHR in patients treated with DD and DVD. This is in accordance with the pivotal MM trials for daratumumab in which patients with higher BMPC percentages had lower remission rates.25 Patients with a dFLC >180 mg/L should also have higher BMPC percentages since a significant correlation between BMPC percentage and dFLC has been reported in AL.26
NT-ProBNP >8500 ng/L was first described as a highly adverse prognostic factor in first-line therapy and has recently been confirmed together with dFLC >180 mg/L ahead of second-line therapy.10,11 DD could not overcome this trend with a 1-year survival rate of only 32% for patients with NT-ProBNP >8500 ng/L. DVD used mostly as second-line therapy achieved slightly better results with a 1-year survival rate of 48%.
In the present study, we also show for the first time that a monoclonal antibody in antineoplastic therapy has inferior results for hemEFS and VGHR rate in patients with nephrotic-range albuminuria (Figures 2 and 4). In our prospective urine analysis, we detected a loss of daratumumab using urine electrophoresis in 2 patients with nephrotic-range albuminuria. Furthermore, we suspected daratumumab in the urine of 5 additional AL λ patients with nephrotic-range albuminuria resulting from newly positive urine immunofixation for IgG κ. A massive short-term loss, as previously described for rituximab in a nephrotic child, was not detectable.27 However, with urine electrophoresis, we are possibly underestimating the loss of daratumumab because immunofixation traced a monoclonal IgG κ without a visible daratumumab peak. A study in patients with nephrotic syndrome detected substantial loss of rituximab through excretion and in patients with lower proteinuria using an immunological method for antibody quantification.28 A steady loss of daratumumab in nephrotic patients might result in lower maximal through concentrations that have been reported less efficient in MM.29 This could, especially in patients with larger plasma cell clones, cause insufficient target saturation and therefore lower ORR. Fittingly, for patients with an ACR >220 mg/mmol and a dFLC >180 mg/L, we only saw an ORR of 38% with DD, 30% with DVD, and, with each regimen, just 1 VGHR. Furthermore, all patients progressing with DD after at least 3 months either had AL with renal involvement and/or dFLC >180 mg/L, further supporting the hypothesis of an insufficient target saturation with monthly daratumumab infusions. Simultaneous consecutive daratumumab levels in blood and urine from patients with and without nephrotic-range albuminuria could possibly verify our hypothesis. These data will hopefully be provided by the ANDROMEDA AL (A Study to Evaluate the Efficacy and Safety of Daratumumab in Combination With Cyclophosphamide, Bortezomib and Dexamethasone [CyBorD] Compared to CyBorD Alone in Newly Diagnosed Systemic AL Amyloidosis) trial (ClinicalTrials.gov Identifier: NCT03201965) with subcutaneous daratumumab. Interestingly, for IgM-related AL, which is commonly treated with rituximab, a low serum albumin probably representing substantial albuminuria, was described as an adverse factor for OS.30 A follow-up of this study regarding hemEFS and response to rituximab stratified by ACR >220 mg/mmol might hence confirm our findings in another antibody-based treatment of systemic AL.
With baseline iFISH cytogenetics, we observed a better hemEFS for patients with translocation t(11;14) and worse hemEFS for patients with hyperdiploidy treated with DD. In AL, t(11;14) has been reported significantly more common in monoclonal gammopathy and less frequent in overt MM.31 Therefore, improved hemEFS for DD in t(11;14) might be caused by a less MM-like plasma cell dyscrasia. Alternatively, higher CD38 expression present in t(11;14)-positive AL has been described with better in vitro anti-CD38-mediated cytotoxicity and positively associated with PR rates in MM.32-34 Hyperdiploidy was associated with an adverse OS and a lower VGHR rate and has been reported more prevalent in AL with symptomatic MM and appears to have less CD38 expression in symptomatic MM.31,32 With DVD, outcome was independent from prevalence of t(11;14) possibly related to non-t(11;14) patients still benefiting from bortezomib.13,14 Myeloma high-risk aberrations that have been reported adverse with daratumumab in MM also showed an impaired hemEFS for DVD.7 Nevertheless, with only 4 patients in the DVD group exhibiting high-risk aberrations these findings are immature. Gain of 1q21 was adverse for OS with DD and DVD and negative for hemEFS with DD. Interestingly, similar results were previously reported by our group for melphalan/dexamethasone.35
Our ITT 3-month remission rates in advanced AL are, as expected, lower compared with reported best remission rates.4,36 This is probably attributed to our patient population that has higher dFLC and NT-ProBNP values ahead of daratumumab and therefore confers a population with a higher risk of treatment failure. Compared with other cohorts with advanced AL receiving therapy, we achieved equally good or better HR rates.37-43
The low number of cardiac organ responders at 3 months should, in our opinion, be credited to the following factors: weekly dexamethasone and additional intravenous fluids were administered in patients with chronic heart failure who might actually benefit from fluid restriction.44 Cardiac organ damage might have been too severe and especially preserved within our DD cohort and organ response rates have previously been reported low with advanced cardiac AL.10 Currently used organ response assessments for AL have been established in first-line therapy and therefore might not be the right tool as cardiac damage might have solidified over time.23 The higher cardiac organ response rates after 3 months with the earlier applied primarily second-line regimen DVD could explain the difference. Importantly, the ITT 12-month organ response rates are comparable to results for bortezomib front-line therapy with less severe cardiac AL.37
Treatment application was mostly well tolerated with only 6% infusion-related reactions resulting in 2 unplanned hospital admissions and are substantially lower compared with the 42% to 45% infusion-related reactions to daratumumab in symptomatic MM.2,5 The high number of unplanned hospital admissions of 35% for DD and 29% for DVD, with 16% being infection-related with DD and 18% with DVD, is well in line with the reported 33% serious adverse events and 5 cases of pneumonia in the daratumumab dose-escalation trial in symptomatic MM.1 A recently published French study including 15 AL patients treated with DD and similar characteristics to our patient cohorts also reported 30% grade 3 adverse events, 7 cases of pneumonia, and 1 case of infection-related death.45 Similar patient cohorts treated with IMID-, melphalan-, or bortezomib-based regimens have mainly been reported with comparable infectious complications.38-43 A study with substantially less toxicity might have underreported.37 Our infection-related mortality of 6% with DD and 3% with DVD compared with 2% in the Daratumumab Monotherapy in Patients With Treatment-Refractory Multiple Myeloma trial for MM and <1% in the Addition of Daratumumab to Combination of Bortezomib and Dexamethasone in Participants With Relapsed or Refractory Multiple Myeloma trial for MM must be attributed to advanced cardiac AL and its associated high mortality.2,5 We are considering the high rate of lymphocytopenia causative for recurrent upper respiratory tract infections and consecutively secondary lower respiratory tract infections resulting in hospital admissions. Our lymphocytopenia rates are substantially higher than reported rates in MM.5,29
The low rate of polyneuropathy within the DVD group can be attributed to us solely applying weekly bortezomib and excluding patients with severe polyneuropathy.
Overall, toxicity was relevant and has caused us not to use the approved first-line combination for transplant-ineligible MM patients with daratumumab, bortezomib, melphalan, and prednisone in advanced AL.46
In summary, daratumumab salvage therapy produced good results and remission rates challenging any therapy in advanced AL. Nevertheless, high early mortality in severe cardiac AL still remained prevalent. We did not detect a clinically meaningful difference between DD and DVD and therefore plead to start patients with advanced AL without severe polyneuropathy or PI refractoriness on DVD.
We confirmed the 2 established factors dFLC >180 mg/L and NT-ProBNP > 8500 ng/L as adverse factors in daratumumab for AL and identified an ACR >220 mg/mmol as prognostic for response to therapy and hemEFS. Whether tighter infusion schedules or higher daratumumab dosages can achieve better results in nephrotic-range albuminuria should be addressed in a trial for nephrotic patients with monoclonal gammopathy of renal significance or systemic AL.47
Limited content of this manuscript was presented during the XVIth International Symposium on Amyloidosis in Kumamoto, Japan (oral presentation on March 27, 2018) and during the 59th Annual Meeting of the American Society of Hematology in Atlanta, GA (poster presentation on December 9, 2017).
For original data, please contact stefan.schoenland@med.uni-heidelberg.de.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank their patients and their families, local hematologists, general practitioners, and hospital staff for their participation in this study.
Authorship
Contribution: C.R.K., U.H., and S.O.S. undertook conception and design; C.R.K., T.D., K.V., A.C., T.H., H.G., A.S., D.H., A.J., S.W., C.M.-T., U.H., and S.O.S. provided study materials or patients; C.R.K., T.D., K.V., A.C., T.H., U.H., and S.O.S. collected and assembled data; C.R.K., T.T., A.B., T.D., K.V., A.C., T.H., H.G., A.S., D.H., A.J., S.W., J.B., C.M.-T., U.H., and S.O.S. undertook data analysis and interpretation; and all authors wrote the manuscript or revised it critically for important intellectual content.
Conflict-of-interest disclosure: H.G. discloses research support from Amgen, BMS, Celgene, Chugai, Dietmar-Hopp-Stiftung, Janssen, John Hopkins University, Molecular Partners, MSD, Mundipharma, Novartis, Sanofi, Takeda; serves on the advisory boards for Adaptive Biotechnology, Amgen, BMS, Celgene, Janssen, Sanofi, Takeda; and has received honoraria from ArtTempi, BMS, Celgene, Chugai, Janssen, Novarti, and Sanofi. C.M.T. has received research funding from Pfizer, Janssen, Daichi and Bioline and serves on advisory boards for Janssen, Bioline and Daichi. T.H. has received travel grants from Janssen, serves on the advisory boards for Janssen, and has received honoraria from Janssen. U.H. has received travel grants from Janssen, Prothena, and Pfizer; served on the advisory boards for Pfizer and Prothena; and has received honoraria from Janssen, Pfizer, Alnylam, and Akcea. S.O.S. has received travel grants from Janssen, MSD, Prothena and Takeda; served on the advisory boards for Janssen and Prothena; has received honoraria from Janssen, Prothena, and Takeda; and received research funding from Sanofi and Janssen. C.R.K. has received honoraria from MSD and Gilead. The remaining authors declare no competing financial interests.
Correspondence: Stefan O. Schönland, Medical Department V, Amyloidosis Center, Heidelberg University Hospital, Im Neuenheimer Feld 410, 69120 Heidelberg, Germany; e-mail: stefan.schoenland@med.uni-heidelberg.de; and Ute Hegenbart, Medical Department V, Amyloidosis Center, Heidelberg University Hospital, Im Neuenheimer Feld 410, 69120 Heidelberg, Germany; e-mail: ute.hegenbart@med.uni-heidelberg.de.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal