Key Points
Daratumumab monotherapy elicited deep and rapid hematological responses in previously treated AL patients, with a good safety profile.
Patients with a very good partial response or better can experience long-lasting responses after 6 months of daratumumab monotherapy.
Abstract
Daratumumab is a human monoclonal antibody targeting CD38, an antigen uniformly expressed by plasma cells in multiple myeloma and light-chain amyloidosis (AL). We report the results of a prospective multicenter phase 2 study of daratumumab monotherapy in AL (NCT02816476). Forty previously treated AL patients with a difference between involved and uninvolved free light chains (dFLC) >50 mg/L were included in 15 centers between September of 2016 and April of 2018. Patients received 6 28-day cycles of IV daratumumab, every week for cycles 1 and 2 and every 2 weeks for cycles 3 through 6. Median age was 69 years (range, 45-83). Twenty-six patients had ≥2 organs involved, with heart in 24 and kidney in 26. Median time from diagnosis to enrollment was 23 months (interquartile range, 4-122), with a median of 3 prior therapies (range, 1-5). At data cutoff (September of 2019), all patients discontinued therapy; 33 received the planned 6 cycles. Overall, 22 patients had hematological response, and 19 patients (47.5%) achieved very good partial response (dFLC <40 mg/L) or better. Median time to hematological response was 1 week. Patients with no response after 4 doses were unlikely to respond further. Renal and cardiac responses occurred in 8 and 7 patients, respectively. Daratumumab was well tolerated, with no unexpected adverse events. With a median follow-up of 26 months, the 2-year overall survival rate was 74% (95% confidence interval, 62-81). Daratumumab monotherapy is associated with deep and rapid hematological responses in previously treated AL patients, with a good safety profile. Further studies of daratumumab in combination regimens are warranted.
Introduction
Systemic immunoglobulin light-chain amyloidosis (AL) is a rare multisystem disease caused by the deposition of misfolded and toxic monoclonal light chains, resulting in symptomatic organ damage.1 Heart, kidney, liver, and peripheral nervous system involvement is frequent. The estimated incidence of AL is ∼12 cases per million people in France.2 AL is usually associated with a clonal plasma cell (PC) disorder with a low tumor burden (median marrow infiltration ∼7%) and recurrent cytogenetic abnormalities: t(11;14) in 53%, del 13 in 35%, and gain 1q in 24%.3 Treatment of systemic AL relies primarily on multiple myeloma (MM) regimens aimed at suppressing the underlying PC clone secreting amyloid-forming monoclonal free light chains (FLCs). Organ responses and survival outcomes are highly dependent on the depth of hematological response evaluated by serum FLC measurement.4,5 Therapy depends on disease severity and is tailored to hematological response. Melphalan, cyclophosphamide, dexamethasone, and bortezomib are the most commonly used drugs in the frontline setting. Although patients may not be refractory to bortezomib or alkylating agents at relapse, our current standard relapsed therapies are often based on immunomodulatory drugs (IMiDs): lenalidomide and pomalidomide.1 By gaining insight into the pathogenesis, the signaling pathways, and the interactions between PCs and their microenvironment, novel targeted therapies were developed in MM that made their way to the treatment of AL in a delayed fashion, given the ongoing safety concerns in this fragile population. The use of monoclonal antibodies should be of interest because of their perceived lack of toxicities.
Daratumumab is a human immunoglobulin G1ĸ monoclonal antibody that binds with high affinity to a unique epitope on CD38, a transmembrane glycoprotein. It is a targeted immunotherapy directed toward tumor cells that express high levels of CD38, such as PCs in AL. Over the last 4 years, daratumumab has emerged as a breakthrough targeted and safe therapy for patients with MM and is now approved in multiple settings (frontline and relapsed, alone or in combination). The mechanisms of action of daratumumab consist of multiple immune-mediated effects.6-11 Some retrospective cases highlighted that daratumumab in AL has a safe cardiac and renal profile, which is particularly advantageous in frail patients, and may provide a deep and rapid hematological response.12-14 Herein, we report the results of a prospective single-arm phase 2 trial of daratumumab plus low-dose corticosteroids in previously treated patients with AL.
Methods
Patients
Patients with biopsy-proven systemic AL and measurable disease, with a difference between serum involved and uninvolved FLCs (dFLC) >50 mg/L, were included in this trial provided that they were in relapse or did not reach very good partial response (VGPR; dFLC <40 mg/L) after the last therapy. Patients with concomitant symptomatic MM, bone marrow PC infiltration ≥30%, or severe cardiac disease (Mayo stage IIIb with N-terminal pro–B-type natriuretic peptide (NT-proBNP) ≥8500 ng/L) were not included. Other exclusion criteria included recurrent ventricular arrhythmias, chronic atrial fibrillation, supine systolic blood pressure <100 mm Hg, myocardial infarction within the last 6 months, left ventricular ejection fraction ≤45%, chronic obstructive pulmonary disease with a forced expiratory volume in 1 second <50% of predicted, uncontrolled or severe asthma, uncontrolled infection, positivity for HIV or active hepatitis B or C virus, liver insufficiency including total bilirubin ≥2.0 mg/dL, serum aspartate/alanine aminotransferase levels or alkaline phosphatases levels >3 times the upper limit of normal, and history of any other malignant disease in the past 3 years, with the exception of basal cell carcinoma, low-grade prostatic cancer, in situ breast carcinoma, or stage I cervical cancer. This study received approval from ethics committees and regulatory authorities. It was conducted according to the Declaration of Helsinki, the International Conference on Harmonization, and the Guidelines for Good Clinical Practice. All patients provided written informed consent prior to entering the study. The original clinical trial was registered as NCT02816476 and as EUDRACT 2016-000287-42.
Study design and treatment
This multicenter single-arm open-label phase 2 study was conducted at 14 Intergroupe Francophone du Myélome centers and at 1 center in Italy, and subjects were enrolled between September of 2016 and April of 2018. Treatment consisted of 6 (4-week) cycles of IV daratumumab (16 mg/kg) on days 1, 8, 15, and 22 for the first 2 cycles and then every other week for cycles 3 through 6. Premedication with paracetamol, antihistamines, and corticosteroids (IV methylprednisolone, 100 mg or dexamethasone, 20 mg) was administered to all patients to minimize the risk of daratumumab-associated infusion reactions. Montelukast was strongly recommended but given at the discretion of the treating physician. Unless contraindicated, all patients received antiviral therapy (eg, valacyclovir) for herpes zoster prevention and antibiotic prophylaxis (eg, amoxicillin) for bacterial infections until the completion of therapy.
Criteria for evaluation
The primary end point was VGPR or better at the completion of 6 cycles of daratumumab. Secondary end points included time to hematological response, best hematological response, cardiac and renal response, safety and tolerability, progression-free survival (PFS), and overall survival (OS). PFS was defined as time to progression or death, whatever occurred first. Initial diagnostic evaluation and response to previous therapy at enrollment were documented for all patients. Definitions of hematological response and organ response were based on the standard and updated international response criteria for amyloidosis; VGPR was defined as dFLC <40 mg/L, complete response (CR) was defined as negative serum and urine immunofixation plus normalized FLC ratio (with no bone marrow evaluation requested), and partial response (PR) was defined as a reduction >50% of the dFLC.4,5 Overall response rate (ORR) was defined as PR or better. Relapse definition was based on the start of a new line of therapy (for insufficient hematological or clinical response or organ progression), reappearance (on immunofixation) of the original monoclonal protein in the serum or urine, or an increase in the serum involved FLC (iFLC) at least a doubling from the normal range if CR or an increase in the serum involved FLC (iFLC) with at least a doubling from the normal range if CR or an increase in the serum iFLC concentration of 50%, and this must increase to a value greater than (100 mg/L) if PR.5 Safety was monitored until 30 days after the last dose of study drug, with the exception of secondary malignancies (which were monitored throughout the study). Toxicities were graded according to National Cancer Institute Common Toxicity Criteria of Adverse Events (version 4.0).
Statistical considerations
In this Fleming single-stage design, a sample size of 37 evaluable patients was required to assess whether the proportion of patients achieving at least VGPR could be increased from 0.15 (null hypothesis) to 0.30 (alternative), with a 1-sided type I error rate of 0.10 and a power of 0.8; ≥9 VGPR or better responses were required to reject the null hypothesis.15 To take into account potential loss to follow-up or nonevaluable patients, we enrolled a total of 40 subjects.
The analysis was performed on an intent-to-treat (ITT) basis and used the revised CONSORT statement for reporting randomized trials. The treated population and the safety population consisted of patients who received ≥1 dose of daratumumab.
Quantitative data were reported using median, interquartile range (IQR), and range; qualitative data were reported with frequency and percentages. Response rates were reported as point estimates with exact 95% confidence interval (CI).
Analysis used the reference date of 27 September 2019. Survival analysis used the Kaplan-Meier method to estimate PFS and OS. This is a single-arm study; thus, comparative analyses, if needed, were exploratory, based on the nonparametric Wilcoxon rank-sum test and the Fisher’s exact test. Predictive factors of hematological overall response were analyzed through logistic regression models, and for PFS and time-to-relapse after hematological ORR were assesses using Cox models. Statistical analyses were performed with SAS 9.4 (SAS Institute Inc., Cary, NC) and R 3.5.1 (https://www.R-project.org/) software.
Results
Patients
As of September of 2019, 49 patients were screened; 40 patients were included in the study and received ≥4 injections of daratumumab. Table 1 summarizes baseline patient and disease characteristics. Median age was 69 years (range, 45-83). Overall, 24 (60%) patients had cardiac involvement, 26 (65%) had renal involvement, and 26 (65%) had ≥2 organs involved. The median dFLC level at baseline was 164 mg/L (IQR, 112-334), and 16 of 40 patients had dFLC >180 mg/L. Median NT-proBNP at baseline was 916 ng/L (IQR, 285-2302). Twenty-one (52.5%) patients had creatinine clearance <60 mL/min. Median time from diagnosis was 23 months (IQR, 14-40), and median time from last line was 5 months (IQR, 1-14). Most of the patients were in advanced stages of the disease, 21 of them received >3 lines of therapy (4 lines in 6 patients and 5 lines in 1 patient), and 17 had refractory disease. Overall, 22 of 40 never reached VGPR or better before inclusion and are reported here as “refractory/poor AL responders.” The majority of patients (92.5%) had previously received bortezomib, and 32% were proteasome inhibitors refractory.
Demographics and baseline characteristics
| Characteristic . | Patients (n = 40) . |
|---|---|
| Age, median (IQR), y | 69 (63-72) |
| Male | 25 (62.5) |
| λ Light chain | 30 (75) |
| Light chain only | 18 (45) |
| ECOG performance status* | |
| 0 | 14 (35) |
| 1 | 21 (52.5) |
| 2 | 5 (12.5) |
| Time from diagnosis, median (IQR), mo | 23 (14-40) |
| Time from last treatment, median (IQR), mo | 5 (1.5-14) |
| No. of previous lines of treatment, median (IQR) | 3 (1.75-3) |
| Never reach VGPR | 22 (55) |
| Relapsed | 20 (50) |
| Refractory disease | 17 (42.5) |
| Refractory to therapy, n/N (%) | |
| Bortezomib | 12/37 (32.4) |
| IMiDs | 10/17 (58.8) |
| Melphalan | 9/19 (47.4) |
| Transplant | 0/1 |
| No. of involved organs, median (IQR) | 2 (1-3) |
| Kidney | 26 (65) |
| Heart | 24 (60) |
| Nerve | 10 (25) |
| Gastrointestinal tract | 11 (27.5) |
| Liver | 4 (10) |
| Soft tissue | 7 (17.5) |
| Mayo Clinic cardiac stage† | |
| I | 11 (27.5) |
| II | 10 (25) |
| IIIA | 19 (47.5) |
| dFLC baseline, median (IQR), mg/L | 164 (112-334) |
| Baseline NT-proBNP, median (IQR), ng/L | 917 (285-2302) |
| Baseline creatinine clearance | |
| Median (IQR) | 56 (40-82) |
| ≥60 mL/min | 19 (47.5) |
| <60 mL/min | 21 (52.5) |
| Characteristic . | Patients (n = 40) . |
|---|---|
| Age, median (IQR), y | 69 (63-72) |
| Male | 25 (62.5) |
| λ Light chain | 30 (75) |
| Light chain only | 18 (45) |
| ECOG performance status* | |
| 0 | 14 (35) |
| 1 | 21 (52.5) |
| 2 | 5 (12.5) |
| Time from diagnosis, median (IQR), mo | 23 (14-40) |
| Time from last treatment, median (IQR), mo | 5 (1.5-14) |
| No. of previous lines of treatment, median (IQR) | 3 (1.75-3) |
| Never reach VGPR | 22 (55) |
| Relapsed | 20 (50) |
| Refractory disease | 17 (42.5) |
| Refractory to therapy, n/N (%) | |
| Bortezomib | 12/37 (32.4) |
| IMiDs | 10/17 (58.8) |
| Melphalan | 9/19 (47.4) |
| Transplant | 0/1 |
| No. of involved organs, median (IQR) | 2 (1-3) |
| Kidney | 26 (65) |
| Heart | 24 (60) |
| Nerve | 10 (25) |
| Gastrointestinal tract | 11 (27.5) |
| Liver | 4 (10) |
| Soft tissue | 7 (17.5) |
| Mayo Clinic cardiac stage† | |
| I | 11 (27.5) |
| II | 10 (25) |
| IIIA | 19 (47.5) |
| dFLC baseline, median (IQR), mg/L | 164 (112-334) |
| Baseline NT-proBNP, median (IQR), ng/L | 917 (285-2302) |
| Baseline creatinine clearance | |
| Median (IQR) | 56 (40-82) |
| ≥60 mL/min | 19 (47.5) |
| <60 mL/min | 21 (52.5) |
All data are n (%), unless otherwise indicated.
ECOG, Eastern Cooperative Oncology Group.
ECOG performance status is scored on a scale from 0 to 5, with 0 indicating no symptoms and higher scores indicating increasing disability.
Based on the European Modification of the Mayo Staging system; cardiac stage was based on 2 biomarker risk factors: NT-ProBNP and high sensitivity cardiac troponin. IIIA: NT-proBNP < 8500 ng/L; note that 3 patients with normal NT-ProBNP values, but missing troponin levels, were considered to have stage I disease.
Treatment
Thirty-three (82.5%) patients completed the planned 6 cycles, with a median treatment duration of 6.0 months (IQR, 5.5-6.2): 7 patients discontinued study treatment because of organ progression and/or absence of hematological response (n = 6), and 1 patient discontinued treatment because of lung cancer. No one stopped study treatment or died as a result of drug toxicity.
Response evaluation
Hematological response
At the completion of therapy (6 cycles of daratumumab) or last evaluation, 19 of 40 patients achieved VGPR or better, with an estimate at 47.5% (95% CI, 31.5-63.9): 3 patients (7.5%) achieved CR and 16 patients (40%) achieved VGPR, thus rejecting the null hypothesis (P < .001). Of note, 2 patients were assessed as VGPR because they missed urine immunofixation, and 2 patients were assessed as VGPR because they did not normalize their FLC ratio with a normal iFLC. Three (7.5%) additional patients were in PR for an ORR of 55% (95% CI, 38.5-70.7). Thirteen patients (32%) achieved a normal iFLC at the end of treatment, and 9 (22%) patients achieved dFLC <10 mg/L.16 Considering the best response, at any time point, the ITT estimate of ORR was 70% (95% CI, 53.5-83.4), and 23 patients achieved VGPR or CR (n = 6). The median time to hematological response was 1 week, with 19 patients achieving at least PR after 1 injection, and 13 reaching VGPR. The median reduction in dFLC after 1 dose of daratumumab was 49% for the whole group. After 4 weeks, 18 patients (45%) were in VGPR or better, and 15 did not reach PR. Only 2 patients further achieved a transient PR after 3 cycles; however, at the end of therapy, none of these 15 patients were in PR or better. Responses over time are depicted in Figure 1. Three patients with PR improved the depth of response to VGPR after cycle 2 and cycle 3. Only 3 patients achieved CR at the completion of 6 cycles.
Swimmer plot for hematological response (ITT). EOT, end of therapy; M, month; NR, no response; PD, progressive disease; †, dead; ★, dFLC <10 mg/L; ↕, end of daratumumab.
Swimmer plot for hematological response (ITT). EOT, end of therapy; M, month; NR, no response; PD, progressive disease; †, dead; ★, dFLC <10 mg/L; ↕, end of daratumumab.
Considering predictive factors of hematological response (Table 2), only the dFLC level and percentage reduction after 1 injection were predictive (whatever the time of response), with a median dFLC after 1 dose of 38 mg/L (IQR, 26-69) and a 63% reduction in the 22 responders vs 137 mg/L (IQR, 82-238) and a 24% reduction in the 15 nonresponders (P < .001 for both). Refractoriness to previous therapies did not have an impact on response; ORR was 67% in patients with PI-refractory disease and 59% in IMiD-refractory disease.
Predictive factors for hematological ORR
| . | Nonresponders (n = 18) . | Responders (n = 22) . | P . |
|---|---|---|---|
| Age, median (IQR), y | 69 (68-72) | 69 (62-75) | .92 |
| Male sex | 10 (55.6) | 15 (68.2) | .52 |
| No previous lines of treatment, median (IQR) | 2 (1.25-3) | 3 (2-3) | .50 |
| Refractory/poor AL responders | 13 (72.2) | 9 (40.9) | .062 |
| Light chain λ | 15 (83.3) | 15 (68.2) | .46 |
| Light chain only | 7 (38.9) | 11 (50.0) | .54 |
| ECOG performance status | |||
| 0 | 8 (44.4) | 6 (27.3) | .33 |
| 1 | 7 (38.9) | 14 (63.6) | |
| 2 | 3 (16.7) | 2 (9.1) | |
| Bone marrow PCs, median % (IQR) | 3.0 (1.3-6.5) | 3.0 (1.0-5.0) | .61 |
| dFLC at baseline, median (IQR) | 169 (112-308) | 145 (115-336) | .88 |
| dFLC <180 mg/L | 10 (55.6) | 13 (59.0) | 1.00 |
| dFLC after 1 injection, median (IQR), mg/L | 137 (82-238) | 38 (26-69) | <.001 |
| dFLC decrease after 1 injection, % | 24 | 63 | <.001 |
| . | Nonresponders (n = 18) . | Responders (n = 22) . | P . |
|---|---|---|---|
| Age, median (IQR), y | 69 (68-72) | 69 (62-75) | .92 |
| Male sex | 10 (55.6) | 15 (68.2) | .52 |
| No previous lines of treatment, median (IQR) | 2 (1.25-3) | 3 (2-3) | .50 |
| Refractory/poor AL responders | 13 (72.2) | 9 (40.9) | .062 |
| Light chain λ | 15 (83.3) | 15 (68.2) | .46 |
| Light chain only | 7 (38.9) | 11 (50.0) | .54 |
| ECOG performance status | |||
| 0 | 8 (44.4) | 6 (27.3) | .33 |
| 1 | 7 (38.9) | 14 (63.6) | |
| 2 | 3 (16.7) | 2 (9.1) | |
| Bone marrow PCs, median % (IQR) | 3.0 (1.3-6.5) | 3.0 (1.0-5.0) | .61 |
| dFLC at baseline, median (IQR) | 169 (112-308) | 145 (115-336) | .88 |
| dFLC <180 mg/L | 10 (55.6) | 13 (59.0) | 1.00 |
| dFLC after 1 injection, median (IQR), mg/L | 137 (82-238) | 38 (26-69) | <.001 |
| dFLC decrease after 1 injection, % | 24 | 63 | <.001 |
All data are n (%), unless otherwise indicated.
ECOG, Eastern Cooperative Oncology Group.
Organ response
During follow-up, 6 of 24 (25%) patients with baseline cardiac involvement had a cardiac response (defined by a 30% decrease in NT-proBNP if baseline >650 ng/L). One other patient had a decrease in septum thickness >2 mm. Considering the 26 patients with renal involvement, 8 (31%) had a renal response (defined as a 30% decrease in proteinuria without a 25% decrease in creatinine clearance) at the completion of therapy (n = 7) or at the 3-month follow-up assessment (n = 1).
Safety
Overall, 12 serious adverse events (AEs) were reported in 8 patients, including 3 deaths, 1 patient with septicemia, and 1 patient with bradycardia; none were considered treatment related. Thirteen patients had grade 3/4 AEs; 4 grade 3 AEs were considered treatment related: cutaneous rash after first infusion that did not recur in 2 patients and leucopenia and orthostatic hypotension in 1 patient each. The most common AEs were grade 1/2 infusion reactions after the first dose in 17 (42.5%) patients. No grade 4 or 5 therapy-related AEs were recorded. Reported AEs of interest or those that occurred in >5% of patients are listed in Table 3.
Reported treatment-emergent AEs according to MedDRA, all grades, >5% safety population, or of clinical significance
| System organ class (MedDRA) . | TEAEs, n . | Patients . | |
|---|---|---|---|
| n . | % . | ||
| Infections and infestations | 28 | 22 | 55.0 |
| Bronchitis | 9 | 9 | 22.5 |
| Pneumonia | 4 | 4 | 10.0 |
| Urinary tract infection | 3 | 3 | 7.5 |
| Rhinitis | 3 | 3 | 7.5 |
| Skin infection | 3 | 2 | 5.0 |
| General disorders and administration site conditions | 39 | 21 | 52.5 |
| Asthenia | 10 | 10 | 25.0 |
| Edema | 10 | 8 | 20.0 |
| Fever | 6 | 6 | 15.0 |
| Inflammation | 3 | 3 | 7.5 |
| Chills | 3 | 3 | 7.5 |
| Chest pain | 4 | 3 | 7.5 |
| Gastrointestinal disorders | 31 | 17 | 42.5 |
| Diarrhea | 11 | 9 | 22.5 |
| Constipation | 6 | 6 | 15.0 |
| Nausea | 5 | 5 | 12.5 |
| Vomiting | 4 | 4 | 10.0 |
| Abdominal pain | 3 | 3 | 7.5 |
| Respiratory, thoracic, and mediastinal disorders | 17 | 12 | 30.0 |
| Cough | 8 | 7 | 17.5 |
| Dyspnea | 6 | 4 | 10.0 |
| Rhinorrhea | 2 | 2 | 5.0 |
| Pleural effusion | 1 | 1 | 2.5 |
| Bronchospasm (IRR) | 1 | 1 | 2.5 |
| Nervous system disorders | 14 | 11 | 27.5 |
| Neuropathy peripheral | 5 | 5 | 12.5 |
| Headache | 4 | 4 | 10.0 |
| Tremor | 2 | 2 | 5.0 |
| Ruptured cerebral aneurysm | 1 | 1 | 2.5 |
| Vascular disorders | 13 | 11 | 27.5 |
| Hypertension | 5 | 4 | 10.0 |
| Hypotension | 2 | 2 | 5.0 |
| Orthostatic hypotension | 3 | 2 | 5.0 |
| Injury, poisoning, and procedural complications | 8 | 7 | 17.5 |
| Infusion-related reaction | 5 | 5 | 12.5 |
| Fall | 2 | 2 | 5.0 |
| Musculoskeletal and connective tissue disorders | 10 | 6 | 15.0 |
| Pain | 6 | 4 | 10.0 |
| Cardiac disorders | 10 | 6 | 15.0 |
| Arrhythmia | 6 | 3 | 7.5 |
| Heart failure | 2 | 1 | 2.5 |
| Angina pectoris | 1 | 1 | 2.5 |
| Cardiac arrest | 1 | 1 | 2.5 |
| Investigations | 10 | 4 | 10.0 |
| Blood creatinine increased | 3 | 1 | 2.5 |
| Metabolism and nutrition disorders | 7 | 6 | 15.0 |
| Hyperkalemia | 3 | 3 | 7.5 |
| Psychiatric disorders | 6 | 5 | 12.5 |
| Skin and subcutaneous tissue disorders | 5 | 5 | 12.5 |
| Blood and lymphatic system disorders | 5 | 4 | 10.0 |
| Anemia | 2 | 2 | 5.0 |
| Eye disorders | 4 | 4 | 10.0 |
| Blurred vision | 2 | 2 | 5.0 |
| Congenital, familial, and genetic disorders | 2 | 2 | 5.0 |
| Macroglossia | 2 | 2 | 5.0 |
| Neoplasms: benign, malignant, and unspecified (including cysts and polyps) | 2 | 2 | 5.0 |
| Lung adenocarcinoma | 1 | 1 | 2.5 |
| Colon cancer | 1 | 1 | 2.5 |
| Ear and labyrinth disorders | 2 | 2 | 5.0 |
| Renal and urinary disorders | 1 | 1 | 2.5 |
| Renal failure | 1 | 1 | 2.5 |
| Surgical and medical procedures | 1 | 1 | 2.5 |
| Cardiac pacemaker insertion | 1 | 1 | 2.5 |
| System organ class (MedDRA) . | TEAEs, n . | Patients . | |
|---|---|---|---|
| n . | % . | ||
| Infections and infestations | 28 | 22 | 55.0 |
| Bronchitis | 9 | 9 | 22.5 |
| Pneumonia | 4 | 4 | 10.0 |
| Urinary tract infection | 3 | 3 | 7.5 |
| Rhinitis | 3 | 3 | 7.5 |
| Skin infection | 3 | 2 | 5.0 |
| General disorders and administration site conditions | 39 | 21 | 52.5 |
| Asthenia | 10 | 10 | 25.0 |
| Edema | 10 | 8 | 20.0 |
| Fever | 6 | 6 | 15.0 |
| Inflammation | 3 | 3 | 7.5 |
| Chills | 3 | 3 | 7.5 |
| Chest pain | 4 | 3 | 7.5 |
| Gastrointestinal disorders | 31 | 17 | 42.5 |
| Diarrhea | 11 | 9 | 22.5 |
| Constipation | 6 | 6 | 15.0 |
| Nausea | 5 | 5 | 12.5 |
| Vomiting | 4 | 4 | 10.0 |
| Abdominal pain | 3 | 3 | 7.5 |
| Respiratory, thoracic, and mediastinal disorders | 17 | 12 | 30.0 |
| Cough | 8 | 7 | 17.5 |
| Dyspnea | 6 | 4 | 10.0 |
| Rhinorrhea | 2 | 2 | 5.0 |
| Pleural effusion | 1 | 1 | 2.5 |
| Bronchospasm (IRR) | 1 | 1 | 2.5 |
| Nervous system disorders | 14 | 11 | 27.5 |
| Neuropathy peripheral | 5 | 5 | 12.5 |
| Headache | 4 | 4 | 10.0 |
| Tremor | 2 | 2 | 5.0 |
| Ruptured cerebral aneurysm | 1 | 1 | 2.5 |
| Vascular disorders | 13 | 11 | 27.5 |
| Hypertension | 5 | 4 | 10.0 |
| Hypotension | 2 | 2 | 5.0 |
| Orthostatic hypotension | 3 | 2 | 5.0 |
| Injury, poisoning, and procedural complications | 8 | 7 | 17.5 |
| Infusion-related reaction | 5 | 5 | 12.5 |
| Fall | 2 | 2 | 5.0 |
| Musculoskeletal and connective tissue disorders | 10 | 6 | 15.0 |
| Pain | 6 | 4 | 10.0 |
| Cardiac disorders | 10 | 6 | 15.0 |
| Arrhythmia | 6 | 3 | 7.5 |
| Heart failure | 2 | 1 | 2.5 |
| Angina pectoris | 1 | 1 | 2.5 |
| Cardiac arrest | 1 | 1 | 2.5 |
| Investigations | 10 | 4 | 10.0 |
| Blood creatinine increased | 3 | 1 | 2.5 |
| Metabolism and nutrition disorders | 7 | 6 | 15.0 |
| Hyperkalemia | 3 | 3 | 7.5 |
| Psychiatric disorders | 6 | 5 | 12.5 |
| Skin and subcutaneous tissue disorders | 5 | 5 | 12.5 |
| Blood and lymphatic system disorders | 5 | 4 | 10.0 |
| Anemia | 2 | 2 | 5.0 |
| Eye disorders | 4 | 4 | 10.0 |
| Blurred vision | 2 | 2 | 5.0 |
| Congenital, familial, and genetic disorders | 2 | 2 | 5.0 |
| Macroglossia | 2 | 2 | 5.0 |
| Neoplasms: benign, malignant, and unspecified (including cysts and polyps) | 2 | 2 | 5.0 |
| Lung adenocarcinoma | 1 | 1 | 2.5 |
| Colon cancer | 1 | 1 | 2.5 |
| Ear and labyrinth disorders | 2 | 2 | 5.0 |
| Renal and urinary disorders | 1 | 1 | 2.5 |
| Renal failure | 1 | 1 | 2.5 |
| Surgical and medical procedures | 1 | 1 | 2.5 |
| Cardiac pacemaker insertion | 1 | 1 | 2.5 |
IRR, infusion related reaction; MedDRA, Medical Dictionary for Regulatory Activities; TEAE, treatment-emergent AE.
Survival outcomes and durability of response
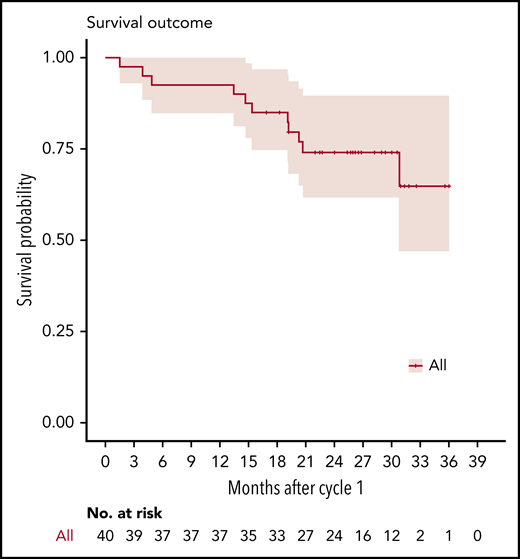
At the reference date, no patient was lost to follow-up. The median actual follow-up was 26.4 months (IQR, 19.3-30.1). Overall, 11 patients died, and 24 patients started a new therapy because of unsatisfactory response (n = 17) or hematological progression and/or organ progression (n = 7; 3 renal). None of the responding patients relapsed or progressed before completion of the planned 6 months of treatment. We observed 3 deaths while on therapy because of disease progression (n = 2) or lung cancer (n = 1), as well as 8 deaths during follow-up because of disease progression (n = 7) or colon cancer (n = 1). The median OS was not reached, whereas median PFS estimate was 24.8 months (lower bound of 95% CI, 15.7). The 2-year OS estimate was 74.2% (95% CI, 61.6-89.4) (Figure 2), and 2-year PFS rate was 51.2% (95% CI, 37.6-69.8).
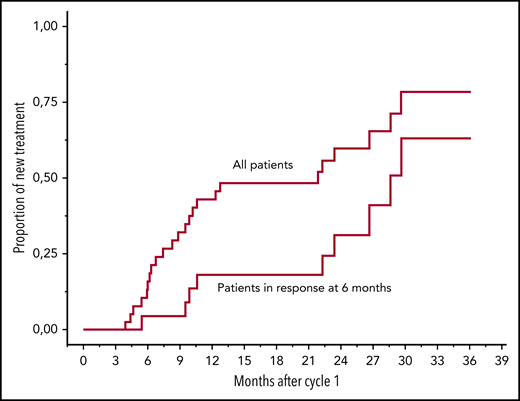
With a median follow-up after the end of treatment of the 22 responding patients of 16.0 months (IQR, 6.4-20.7), 9 patients required a new line of therapy: 5 patients for hematological progression, and 4 patients who did not meet the criteria for hematological relapse but based on the decision of the treating physician. Patients with VGPR or better were less likely to receive a subsequent line of treatment (P = .039). Evolution of iFLC measurements over time in these 22 patients is presented in Table 4. In a landmark analysis at 6 months, median time to next treatment (TTNT) in responding patients was 24 months. Figure 3 shows TTNT for the entire cohort (excluding 3 patients who died early) and for the 22 patients with a response at 6 months.
iFLC levels in the 22 responding patients at baseline and during posttreatment follow-up
| Patient . | Relapsed . | Involved type . | Involved LC at baseline . | Involved LC at EOT . | 3 mo post-EOT . | 6 mo post-EOT . | 9 mo post-EOT . | 12 mo post-EOT . | 15 mo post-EOT . | 18 mo post-EOT . | 21 mo post-EOT . | 24 mo post-EOT . | 28 mo post-EOT . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | λ | 190 | 27.4 | 26.9 | 30 | 32.7 | 38.2 | 89.83* | Start subsequent therapy | |||
| 2 | 0 | λ | 380 | 20 | 32.8 | 40.4 | 41.9 | 51.3 | 60 | 52.8 | 81.5 | ||
| 3 | 1 | κ | 135 | 57 | Start subsequent therapy | ||||||||
| 4 | 1 | λ | 87 | 44.7 | 102* | Start subsequent therapy | |||||||
| 5 | 0 | λ | 85 | 6.6 | 6.48 | 10.3 | |||||||
| 6 | 0 | κ | 153 | 26 | 28.1 | 28.4 | 37.4 | 57 | 58 | 78 | |||
| 7 | 0 | λ | 151 | 6 | 6.2 | 6.02 | 6.33 | 10 | 9 | ||||
| 8 | 1 | λ | 141 | 20.8 | 24.2 | 28.9 | 34.7 | 67* | Start subsequent therapy | ||||
| 9 | 0 | λ | 165 | 41.5 | 37.5 | 38.8 | 48 | 90 | |||||
| 10 | 1 | κ | 528 | 10 | 7.3 | 13.3 | 11.5 | 11.5 | 12.5 | 654* | Start subsequent therapy | ||
| 11 | 0 | λ | 475 | 4 | 9 | 6 | 7.1 | 12.9 | 18.1 | ||||
| 12 | 1 | λ | 1280 | 226 | 293 | 158 | 211 | 237* | Start subsequent therapy | ||||
| 13 | 0 | λ | 311 | 10.8 | 14.6 | 97.5 | Death not related to AL | ||||||
| 14 | 0 | κ | 141 | 46 | 47 | 49 | 48 | 66.5 | 46 | 45 | 56.5 | 50.6 | 63 |
| 15 | 1 | κ | 79 | 29.3 | 46.6 | 43.1 | 51.3 | 97* | 108* | Start subsequent therapy | |||
| 16 | 1 | λ | 363 | 130 | 253* | Start subsequent therapy | |||||||
| 17 | 0 | κ | 96 | 2.9 | 6.67 | 9.42 | 20.7 | 18.2 | 17.2 | 17.6 | |||
| 18 | 0 | λ | 301 | 16.1 | 34.4 | 54.2 | 62.4 | 76.2 | 73.7 | 72 | |||
| 19 | 1 | λ | 136 | 20.5 | 123* | Start subsequent therapy | |||||||
| 20 | 0 | λ | 71 | 22.7 | 30.5 | 31 | 40.2 | 33.3 | |||||
| 21 | 0 | λ | 263 | 13.1 | 19.2 | 31.3 | 27.1 | 27.3 | |||||
| 22 | 0 | κ | 392 | 9.3 | 17.5 | 34 | 24.5 | 24.2 | |||||
| Patient . | Relapsed . | Involved type . | Involved LC at baseline . | Involved LC at EOT . | 3 mo post-EOT . | 6 mo post-EOT . | 9 mo post-EOT . | 12 mo post-EOT . | 15 mo post-EOT . | 18 mo post-EOT . | 21 mo post-EOT . | 24 mo post-EOT . | 28 mo post-EOT . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | λ | 190 | 27.4 | 26.9 | 30 | 32.7 | 38.2 | 89.83* | Start subsequent therapy | |||
| 2 | 0 | λ | 380 | 20 | 32.8 | 40.4 | 41.9 | 51.3 | 60 | 52.8 | 81.5 | ||
| 3 | 1 | κ | 135 | 57 | Start subsequent therapy | ||||||||
| 4 | 1 | λ | 87 | 44.7 | 102* | Start subsequent therapy | |||||||
| 5 | 0 | λ | 85 | 6.6 | 6.48 | 10.3 | |||||||
| 6 | 0 | κ | 153 | 26 | 28.1 | 28.4 | 37.4 | 57 | 58 | 78 | |||
| 7 | 0 | λ | 151 | 6 | 6.2 | 6.02 | 6.33 | 10 | 9 | ||||
| 8 | 1 | λ | 141 | 20.8 | 24.2 | 28.9 | 34.7 | 67* | Start subsequent therapy | ||||
| 9 | 0 | λ | 165 | 41.5 | 37.5 | 38.8 | 48 | 90 | |||||
| 10 | 1 | κ | 528 | 10 | 7.3 | 13.3 | 11.5 | 11.5 | 12.5 | 654* | Start subsequent therapy | ||
| 11 | 0 | λ | 475 | 4 | 9 | 6 | 7.1 | 12.9 | 18.1 | ||||
| 12 | 1 | λ | 1280 | 226 | 293 | 158 | 211 | 237* | Start subsequent therapy | ||||
| 13 | 0 | λ | 311 | 10.8 | 14.6 | 97.5 | Death not related to AL | ||||||
| 14 | 0 | κ | 141 | 46 | 47 | 49 | 48 | 66.5 | 46 | 45 | 56.5 | 50.6 | 63 |
| 15 | 1 | κ | 79 | 29.3 | 46.6 | 43.1 | 51.3 | 97* | 108* | Start subsequent therapy | |||
| 16 | 1 | λ | 363 | 130 | 253* | Start subsequent therapy | |||||||
| 17 | 0 | κ | 96 | 2.9 | 6.67 | 9.42 | 20.7 | 18.2 | 17.2 | 17.6 | |||
| 18 | 0 | λ | 301 | 16.1 | 34.4 | 54.2 | 62.4 | 76.2 | 73.7 | 72 | |||
| 19 | 1 | λ | 136 | 20.5 | 123* | Start subsequent therapy | |||||||
| 20 | 0 | λ | 71 | 22.7 | 30.5 | 31 | 40.2 | 33.3 | |||||
| 21 | 0 | λ | 263 | 13.1 | 19.2 | 31.3 | 27.1 | 27.3 | |||||
| 22 | 0 | κ | 392 | 9.3 | 17.5 | 34 | 24.5 | 24.2 | |||||
EOT, end of treatment.
Hematologic progression.
Time to next treatment for all patients, excluding 3 patients who died before 5 months of follow-up, and patients with response at 6 months (n = 22).
Time to next treatment for all patients, excluding 3 patients who died before 5 months of follow-up, and patients with response at 6 months (n = 22).
Other than the type of response, there was no predictive factor for relapse (supplemental Table 1, available on the Blood Web site). Of note, 3 of the 13 patients (23%) whose iFLC level normalized relapsed; only 1 was among the 9 patients (11%) with dFLC < 10 mg/L.
Discussion
In light-chain AL, deep hematological response is a key determinant of survival and organ responses, as shown by improved outcomes in the last several years with more efficient regimens.17 Single-agent daratumumab in this prospective phase 2 study provided encouraging results in 40 previously treated patients with AL. Responses were deep and rapid. A VGPR or better was achieved by 47% of patients at the 6-month time point; this figure increased to 57.5% if considering best response at any time during the treatment period.
The ORRs observed in our study are lower than those in previous retrospective studies of daratumumab in AL. This may be due to the high number of previous lines of treatment before inclusion in our study, as well as the fact that more than half of the patients had never achieved a VGPR.18-20 However, the ORR in AL is better than the 30% reported for daratumumab monotherapy in MM,21 suggesting that PCs in AL could have a particular sensitivity to daratumumab or that the immune microenvironment is more efficient. We did not find any patient baseline characteristic that was predictive for hematological response. The most striking result in this study was the rapidity of hematological response. Half of the patients achieved a clonal response after only 1 injection of daratumumab, typically with a dramatic drop in dFLC (median, 63% for responders). Interestingly, the response achieved after 1 injection was predictive of final response, with a median dFLC of 38 mg/L after 1 injection in patients with clonal response at 6 months compared with 137 mg/L in nonresponders (Table 2). Median time to VGPR or better was 8.5 days. Of note, no partial response after 4 doses predicted for no response at 6 months. Because patients who did not reach a response after 4 injections have a very low chance of reaching a subsequent response, we should consider adding another drug for those patients or switching to a different regimen. Rapidity of response is particularly important for the survival of patients with very severe amyloidotic organ compromise, particularly in patients in stage IIIB of the modified Mayo Clinic staging.22 Currently, a phase 2 prospective study has begun with subcutaneous daratumumab monotherapy (NCT04131309) in newly diagnosed stage IIIB patients.
Daratumumab, as anticipated, appears to have a good safety profile, and we mostly observed nonsevere AEs; the most common was infusion-related reaction with the first dose. No patient stopped treatment because of side effects, and no unexpected toxicity was observed. Patients with cardiac AL were able to tolerate IV infusion of daratumumab.
The median follow-up after the end of treatment of the 22 patients who achieved hematological response was 16 months; 9 needed a subsequent line of therapy, with a median TTNT of 24 months (Table 4). One could argue that patients should also benefit from daratumumab maintenance, as proposed in the prospective phase 2 study by the Boston group.23 When we designed this protocol, we chose to stop daratumumab after 6 months of treatment, considering that responses in AL are usually much longer than in MM. The depth of the response seems to have an influence on the response’s duration, with a trend toward less relapse in patients who attain VGPR or better (P = .039), a normal iFLC level (P = .094), or a very low dFLC. Therefore, the question of duration of treatment has to be solved by further studies. No patient in our study had an upgraded response after 3 cycles. When looking at the efficacy of treatment in AL, a key point is organ responses; it is usually dependent on achieving deep hematological responses, provided that the treatment does not alter macrophage functions (and clearance of amyloid deposits). In our study, with regard to the high number of cardiac and renal responses within a relatively short follow-up, we can conclude that daratumumab did not interfere with amyloid deposit clearance, although macrophages express CD38. Finally, survival outcomes are encouraging in this relapsed population, and three quarters of patients were alive at 2 years, supporting a role for this drug in relapsing or refractory patients. This study has some limitations. First, this is a single-arm trial; it is well known that estimates from such cohorts should not be overinterpreted and that comparative trials are needed, even in such small populations. Criteria for organ responses were not all centrally assessed, and some measure bias could have been introduced; however, we used standard and updated international response criteria for AL that have been recommended worldwide. Last, predictive analyses of response in this small cohort lacked statistical power, which is why we only performed univariate analysis.
In summary, daratumumab monotherapy in this prospective phase 2 study provided encouraging efficacy and safety results in previously treated patients with AL. Clonal responses were deep and rapid, even after the first infusion; 47.5% achieved VGPR or better, and one third of patients normalized their involved light chain serum level. Further studies of daratumumab-based associations in AL patients are warranted. A multicenter international randomized phase 3 trial (ANDROMEDA[AMY3001] Study) is ongoing; it is testing bortezomib, cyclophosphamide, and dexamethasone, with or without subcutaneous daratumumab (NCT03201965), in de novo AL patients.
Data sharing requests should be sent to Arnaud Jaccard (arnaud.jaccard@chu-limoges.fr).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank individual research teams at all participating study sites, especially all members of the French Network for AL/Intergroupe Francophone du Myélome and the referral center for AL in Pavia (G.M. and G.P.). They are also indebted to Simon Gibbs and Jean Yves Mary for critical reading of the manuscript and to the research team in Limoges (Abdelslam Bentaleb, Florence Bosselut, Pascale Duret-Blanc, Sabrina Crépin, and Claire Villeneuve). Daratumumab was provided by Janssen.
This work was supported by Programme Hospitalier de Recherche Clinique and Janssen.
Authorship
Contribution: A.J. and M.R. designed the study; D.L. and S.C. analyzed the data and performed the statistical analyses; and M.R., S.C. and A.J. wrote the manuscript; and all authors participated in analyzing and interpreting the data, checked the final version of the manuscript, and are fully responsible for the content and editorial decisions pertaining to this manuscript.
Conflict-of-interest disclosure: M.R., G.M., B.A., AM.S., A.P., L.K., B.R., A.H., L.F., E.B., C.T., G.P., and F.B. have participated in lectures for Janssen. M.R. has served on advisory boards for Janssen, Celgene, Takeda and Amgen, has received research funding from Janssen and Takeda, and has had travel, accommodations, or other expenses paid for or reimbursed by Janssen and Celgene, Takeda, and Amgen. A.J. has served in a consulting or advisory role for Janssen and has received honoraria from, received research funding from, and had travel, accommodations, or other expenses paid for or reimbursed by Janssen and Celgene. The remaining authors declare no competing financial interests.
Correspondence: Arnaud Jaccard, Centre de Référence pour l'Amylose AL et Autres Maladies de Dépôts d'Immunoglobulines Monoclonales, Service d’Hématologie Clinique et de Thérapie Cellulaire, CHU Limoges, Limoges 8700, France; e-mail: arnaud.jaccard@chu-limoges.fr.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal