Cell-based therapies are emerging as potent agents against cancer and other diseases, but are uniquely uncontrolled "living drugs". For example, chimeric antigen receptor (CAR) T cells can effectively target hematologic malignancies yet pose a risk for toxic hyperactivation. Future cell-based therapies, including CAR T cells, could be improved by incorporating specific and reversible control systems. However, clinically suitable ON- and OFF-switches engineered from non-immunogenic human polypeptide sequences and regulated by non-immunosuppressive FDA-approved drugs are needed. Here we report the engineering of a robust lenalidomide-responsive degron tag, which we then used to construct a degradable CAR affording reversible OFF-switch functional control at clinically relevant lenalidomide doses.
Thalidomide, lenalidomide, and pomalidomide are effective and clinically approved therapies for multiple myeloma, subtypes of non-Hodgkin lymphoma, and myelodysplastic syndrome with chromosome 5q deletion. These drugs exert therapeutic properties by acting as molecular glue, bridging interactions between the CRL4CRBN ubiquitin ligase and disease-relevant proteins that are subsequently ubiquitinated and degraded by the proteasome. A set of Cys2-His2 (C2H2) zinc fingers have emerged as degron motifs mediating drug-dependent interactions with the CRL4CRBN ubiquitin ligase. We hypothesized that these small, modular, human polypeptide domains could be further engineered and repurposed as tags to induce drug-dependent depletion of engineered proteins. By systematically shuffling the subdomains of all known zinc finger degrons and functionally screening this hybrid zinc finger library, we engineered "super-degron" tags that are more efficiently degraded at lower drug concentrations than any C2H2 zinc finger in the human proteome.
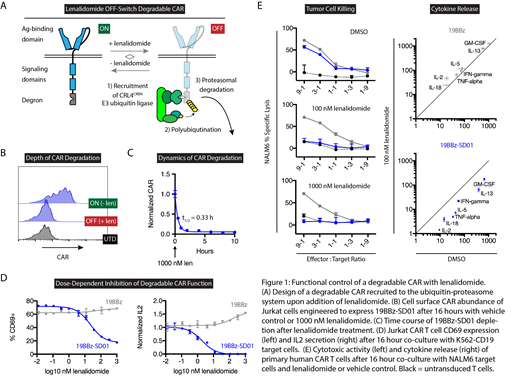
We then incorporated one of these super-degrons (SD01) into a second-generation CAR targeting CD19 (FMC63-4-1BB-CD3z), thereby constructing a degradable CAR (Figure 1A). In a Jurkat T cell model, addition of lenalidomide induced rapid and near-complete depletion of the degradable CAR (Figure 1B/C). When co-cultured with target cells expressing CD19, therapeutically relevant lenalidomide concentrations robustly inhibited T cell activation (Figure 1D). Indeed, the IC50 for inhibition of IL2 secretion and CD69 expression were 2 and 22 nM lenalidomide, respectively, whereas in myeloma patients the plasma concentration of lenalidomide following one 25 mg oral dose decays from ~1000 to 10 nM over the course of 24 hours (Connarn et al, CPDD, 2017). In primary T cells, 100 nM lenalidomide suppressed degradable CAR effector functions including tumor cell killing and cytokine release (Figure 1E). Using pomalidomide, which has a longer in vivo half-life, we demonstrated robust and reversible depletion of the degradable CAR in T cells engrafted in NSG mice. Together, these findings demonstrate reversible OFF-switch control of degradable CARs at clinically relevant lenalidomide concentrations. Experiments are underway to determine whether, in the context of tumor clearance in NSG mice, degradable CAR T cell effector functions can be paused and subsequently released with short-term administration of lenalidomide. Whereas the current management of CAR T cell hyperactivation syndromes consists of supportive care, tocilizumab, and/or high-dose corticosteroids, we propose that cytokine release and CAR-related encephalopathy syndromes may be more easily diagnosed and managed with degradable CARs. The super-degron tags presented here are generalizable and clinically suitable tools to achieve chemical genetic control of diverse genetically engineered cell therapies.
Jan:Broad Institute: Other: Contributor to a patent filing on this technology that is held by the Broad Institute.. Sievers:Broad Institute: Other: Contributor to a patent filing on this technology that is held by the Broad Institute.. Maus:Broad Institute: Other: Contributor to a patent filing on this technology that is held by the Broad Institute.. Ebert:Broad Institute: Other: Contributor to a patent filing on this technology that is held by the Broad Institute.; Deerfield: Research Funding; Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal