Cellular immunotherapy is a rapidly progressing field in multiple myeloma (MM). Multiple clinical trials have reported impressive efficacy of B cell maturation antigen (BCMA) directed chimeric antigen receptor cell therapy (BCMA CART) in MM. While trials demonstrated an overall response rate of 70-90% in patients with relapsed/refractory MM, the durable response rate is around 30%. Most patients lose their CART cells and the disease relapses within the first year, suggesting an inhibition by the MM tumor microenvironment (TME). Therefore strategies to overcome this inhibition would represent a major advance in CART cell therapy for MM. Cancer associated fibroblasts (CAFs) within the TME play a critical role in promoting tumor growth and in the generation of an immunosuppressive microenvironment. We hypothesized that CAFs from bone marrows of patients with MM (MM-CAFs) inhibit BCMA CART cells and contribute to their failure and that targeting both the malignant plasma cells and CAFs can overcome this resistance.
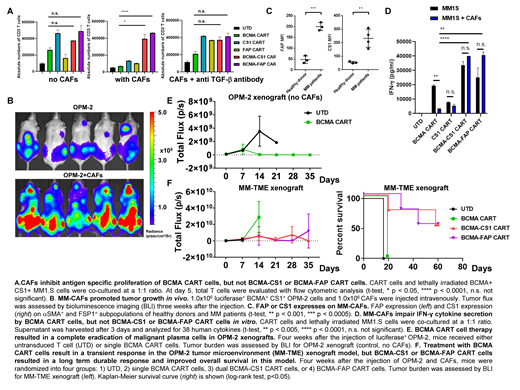
To test this hypothesis, we isolated MM-CAFs and studied their interaction with BCMA CART cells generated from normal donors (41BB costimulated, lentivirally transduced). Our initial findings suggest that MM-CAFs inhibit BCMA CART cell antigen specific proliferation in the presence of the BCMA+ MM cell line OPM2, and this inhibition is predominantly mediated through the secretion of TGF-β (Fig A). MM-CAFs also promoted MM tumor growth in an MM-TME xenograft model established in the laboratory (Fig B). Here, immunocompromised NOD-SCID-γ-/- (NSG) mice were engrafted with 1x106 luciferase+ BCMA+ OPM2, in combination with either 1x106 CAFs or vehicle control intraveneously (IV). Subsequent tumor burden was monitored by bioluminescent imaging of these mice. The presence of CAFs in this model significantly accelerated MM progression (Fig B).
Based on these findings, we aimed to develop CART cell therapy targeting both malignant MM cells and their CAFs and to determine whether this strategy can reverse MM-CAF induced CART cell inhibition. To identify targets for these CART cells, we first verified the expression of Fibroblast Associated Protein (FAP), an established CAF target, on MM-CAFs. Flow cytometric analysis of MM-CAFs showed significantly higher expression of FAP, compared to fibroblasts derived from normal bone marrow (Fig C). In addition, our screening flow cytometric analysis identified CS1 as another protein overexpressed by MM-CAFs (Fig C).
We therefore designed and generated FAP CART cells (41BB costimulated, lentivirally transduced) and CS1 CART cells (CD28 costimulated, lentivirally transduced). We also generated dual CART cells for both BCMA-FAP CART cells and BCMA-CS1 CART cells. These dual CART cells were generated through the dual transduction of two lentiviral vectors during CART manufacturing.
Next, we evaluated the impact of CAFs on effector functions of BCMA CART cells compared to dual targeting CART cells. When CART cells were stimulated with the BCMA+ MM cell line MM1S, in the presence of MM-CAFs, the antigen specific proliferation of BCMA CART cells, but not the dual targeting CART cells was significantly inhibited (Fig A). Similarly, in the presence of MM-CAFs, production of key effector cytokines by BCMA CART cells, but not the dual CART cells was reduced (Fig D).
Finally, to verify the significance of our laboratory findings, we investigated the impact of CAFs on CART cell functions in vivo. First, using OPM2 xenografts, treatment with BCMA CART cells were able to completely eradicate MM (Fig E). However, to determine the effect of targeting CAFs, we used our MM-TME model. Here, NSG mice were engrafted with the luciferase+ MM cell line OPM2, along with MM-CAFs, as described in Fig 1B. Mice were then imaged for engraftment and randomized to treatment with 1) untransduced control T cells, 2) BCMA CART cells, 3) BCMA-FAP CART cells, or 4) BCMA-CS1 CART cells. A lower dose (1x106 IV) of CART cell was used to induce relapse post BCMA CART cells. Treatment with BCMA CART cells led to a transient antitumor activity in this MM-TME model (mice died within 2 weeks), while dual targeting CART cells resulted in durable remissions and long term survival of these mice (Fig F).
In summary, we demonstrate for the first time that dual targeting both malignant plasma cells and the CAFs within the TME is a novel strategy to overcome resistance to CART cell therapy in multiple myeloma.
Sakemura:Humanigen: Patents & Royalties. Cox:Humanigen: Patents & Royalties. Parikh:Janssen: Research Funding; Pharmacyclics: Honoraria, Research Funding; MorphoSys: Research Funding; AbbVie: Honoraria, Research Funding; Acerta Pharma: Research Funding; Ascentage Pharma: Research Funding; Genentech: Honoraria; AstraZeneca: Honoraria, Research Funding. Kay:Celgene: Other: Data Safety Monitoring Board; Infinity Pharmaceuticals: Other: DSMB; MorphoSys: Other: Data Safety Monitoring Board; Agios: Other: DSMB. Kenderian:Lentigen: Research Funding; Kite/Gilead: Research Funding; Humanigen: Other: Scientific advisory board , Patents & Royalties, Research Funding; Tolero: Research Funding; Novartis: Patents & Royalties, Research Funding; Morphosys: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal