INTRODUCTION: Acute myeloid leukemia with myelodysplastic related changes (AML-MRC) is a heterogeneous disorder defined by morphologic, cytogenetic and clinical features. Defining the optimal therapy of this subgroup of patients depending on disease features and characteristics is necessary.
METHODS:We evaluated all patients (pts) with AML-MRC diagnosed and treated at The University of Texas MD Anderson Cancer Center from 2013 to 2018. All cases where reviewed by two hematopathologists to establish diagnosis of AML-MRC following WHO 2017 criteria. Patients with therapy-related myeloid neoplasms were excluded. Sequencing data was obtained by use of a 81-gene targeted PCR-based next generation sequencing (NGS) platform. Previously described somatic mutations registered at the Catalogue of Somatic Mutations in Cancer (COSMIC: http://cancer.sanger.ac.uk/cosmic) were considered as potential driver mutations. The Kaplan-Meier product limit method was used to estimate survival outcomes for each clinical/demographic factor. Univariate Cox proportional hazards regression was used to identify any association with each of the variables and survival outcomes.
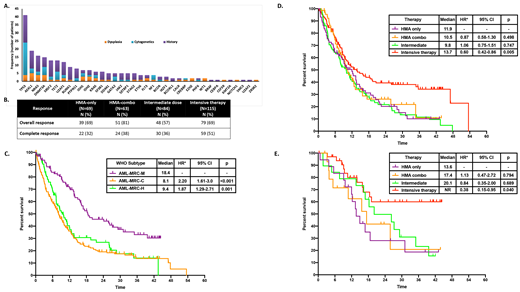
RESULTS:A total of 415 pts with AML-MRC where identified. Median age at diagnosis was 70 years (range 18-94). Diagnosis of AML-MRC was based on cytogenetic abnormalities (AML-MRC-C) in 214 (52%) pts, presence of >50% dysplasia in 3 lineages (AML-MRC-M) in 98 (24%) pts and due to history of prior myelodysplastic syndrome (MDS) or myelodysplastic/myeloproliferative neoplasm (MDS/MPN) in 103 (25%) pts (AML-MRC-H). Among pts with a prior history of MDS or MDS/MPN, 23 (22%) had received therapy with hypomethylating agents, 1 (1%) with lenalidomide and 3 (3%) with ruxolitinib and 1 with 7+3 (1%). Median bone marrow blast percentage on aspirate was 35% (range 1-97%). Among pts with AML-MRC-C the defining cytogenetic abnormality included: complex karyotype in 162 (76%), monosomy 5 or del(5q) in 14 (7%), monosomy 7 or del(7q) in 34 (16%), concurrent chromosome 5 or 7 abnormalities in 2 (1%), del(13q) in 1 (0.5%) and i(17) in 1 (0.5%) pt. Data on NGS was available in 95 pts. Identified mutations are shown in Figure 1A. Mutations in TP53where more commonly observed in pts with AML-MRC-C (p=0.001). In addition, mutations in RUNX1where more commonly observed in pts with AML-MRC-H (p=0.038). Prior publications (Lindsley et al Blood 2015) have shown that secondary-type mutations (ASXL1, BCOR, EZH2, SF3B1, SRSF2, STAG2, U2AF1and ZRSR2) identify a subset of pts with AML with worse than expected outcomes. Among evaluable pts, a total of 37 (39%) pts had secondary-type mutations. Mutations in ASXL1, BCOR, SF3B1, SRSF2, andU2AF1tended to appear in dominant clones. A total of 73 (18%) pts received therapy with single agent hypomethylating agents (HMA), 67 (16%) with HMA in combination with investigational drugs, 86 (21%) with intermediate dose chemotherapy and 119 (29%) with intensive chemotherapy. Response outcomes on evaluable pts are shown in Figure 1B. With a median follow up of 28.3 months (95% CI 25.7-30.9 months) the median overall survival (OS) was 10.5 months (95% CI 9.1-12.0 months). Pts with AML-MRC-M had significantly better outcomes than those with AML-MRC-H or AML-MRC-C (median OS 20.8 months vs 18.7 vs 16.9 months, p<0.001) (Figure 1C). Although a trend to worse survival was observed in pts with AML-MRC-H with prior therapy for the antecedent MDS or MDS/MPN this difference was not significant (median OS 5.9 vs 9.9 months, HR 1.5, 95% CI 0.8-2.9, p=0.216). Among pts with AML-MRC-C those with complex karyotype had significantly worse outcomes than those with other defining cytogenetic abnormalities (p<0.001). Pts treated with intensive chemotherapy had improved OS compared to those treated with other forms of therapy (p=0.002, Figure 1D). However, when evaluating outcomes by AML-MRC subtype, use of intensive therapy was only associated with improved survival on AML-MRC-M (p=0.05, Figure 1E) but not in AML-MRC-C or AML-MRC-H. No significant differences in OS were observed in pts with secondary-type mutations (p=0.568) but, among pts with AML-MRC-M presence of these mutations was associated with a trend to worse OS (median OS 3.8 months vs NR, p=0.228).
CONCLUSION:AML-MRC is a heterogeneous group of AML with diverse mutational abnormalities and outcomes. Selection of therapy should be based on cytogenetic abnormalities and AML-MRC subtype.
Sasaki:Otsuka: Honoraria; Pfizer: Consultancy. Ravandi:Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Macrogenix: Consultancy, Research Funding; Cyclacel LTD: Research Funding; Xencor: Consultancy, Research Funding; Menarini Ricerche: Research Funding; Selvita: Research Funding. Cortes:Merus: Consultancy, Honoraria, Research Funding; Immunogen: Consultancy, Honoraria, Research Funding; Sun Pharma: Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Research Funding; Forma Therapeutics: Consultancy, Honoraria, Research Funding; BiolineRx: Consultancy; Biopath Holdings: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Astellas Pharma: Consultancy, Honoraria, Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding. Daver:Otsuka: Consultancy; Sunesis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Immunogen: Consultancy, Research Funding; BMS: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Astellas: Consultancy; Celgene: Consultancy; Astellas: Consultancy; Daiichi Sankyo: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Jazz: Consultancy; Jazz: Consultancy; Genentech: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Forty-Seven: Consultancy; Abbvie: Consultancy, Research Funding; Celgene: Consultancy; Servier: Research Funding; Hanmi Pharm Co., Ltd.: Research Funding; Hanmi Pharm Co., Ltd.: Research Funding; Glycomimetics: Research Funding; Otsuka: Consultancy; NOHLA: Research Funding; Incyte: Consultancy, Research Funding; Servier: Research Funding; Incyte: Consultancy, Research Funding; Karyopharm: Consultancy, Research Funding; Agios: Consultancy; Pfizer: Consultancy, Research Funding; Karyopharm: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Glycomimetics: Research Funding; Agios: Consultancy; Forty-Seven: Consultancy; Immunogen: Consultancy, Research Funding; NOHLA: Research Funding; Sunesis: Consultancy, Research Funding. Takahashi:Symbio Pharmaceuticals: Consultancy. DiNardo:medimmune: Honoraria; jazz: Honoraria; abbvie: Consultancy, Honoraria; daiichi sankyo: Honoraria; celgene: Consultancy, Honoraria; syros: Honoraria; notable labs: Membership on an entity's Board of Directors or advisory committees; agios: Consultancy, Honoraria. Jabbour:Cyclacel LTD: Research Funding; AbbVie: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Adaptive: Consultancy, Research Funding. Borthakur:Incyte: Research Funding; AbbVie: Research Funding; PTC Therapeutics: Consultancy; NKarta: Consultancy; BioLine Rx: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Cyclacel: Research Funding; GSK: Research Funding; Janssen: Research Funding; BMS: Research Funding; AstraZeneca: Research Funding; Bayer Healthcare AG: Research Funding; Agensys: Research Funding; Oncoceutics: Research Funding; Novartis: Research Funding; Xbiotech USA: Research Funding; Eisai: Research Funding; Tetralogic Pharmaceuticals: Research Funding; Strategia Therapeutics: Research Funding; Polaris: Research Funding; Arvinas: Research Funding; Merck: Research Funding; Cantargia AB: Research Funding; FTC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Argenx: Membership on an entity's Board of Directors or advisory committees; BioTheryX: Membership on an entity's Board of Directors or advisory committees; Eli Lilly and Co.: Research Funding; Oncoceutics, Inc.: Research Funding. Konopleva:Genentech: Honoraria, Research Funding; F. Hoffman La-Roche: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria; Cellectis: Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Eli Lilly: Research Funding; Calithera: Research Funding; Forty-Seven: Consultancy, Honoraria; Stemline Therapeutics: Consultancy, Honoraria, Research Funding; Ascentage: Research Funding; Kisoji: Consultancy, Honoraria; Reata Pharmaceuticals: Equity Ownership, Patents & Royalties; Ablynx: Research Funding; Astra Zeneca: Research Funding; Agios: Research Funding. Bueso-Ramos:Incyte: Consultancy. Kantarjian:Astex: Research Funding; Daiichi-Sankyo: Research Funding; Pfizer: Honoraria, Research Funding; Agios: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Jazz Pharma: Research Funding; Immunogen: Research Funding; AbbVie: Honoraria, Research Funding; Takeda: Honoraria; Ariad: Research Funding; BMS: Research Funding; Novartis: Research Funding; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; Cyclacel: Research Funding. Garcia-Manero:Amphivena: Consultancy, Research Funding; Helsinn: Research Funding; Novartis: Research Funding; AbbVie: Research Funding; Celgene: Consultancy, Research Funding; Astex: Consultancy, Research Funding; Onconova: Research Funding; H3 Biomedicine: Research Funding; Merck: Research Funding. Kadia:Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Jazz: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Research Funding; Bioline RX: Research Funding; BMS: Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal