
INTRODUCTION: A sizable proportion of elderly acute myeloid leukemia (AML) patients receive frontline hypomethylating agents (HMAs), namely azacitidine (AZA) and decitabine (DAC), as they are deemed unfit for intensive chemotherapy (ICT) by their treating physicians. A foreseeable high early death (ED) rate and lack of overall survival (OS) benefit under ICT are the main drivers for this decision. Several groups have published different predictive tools for ED or OS in elderly patients receiving ICT but, since ED in patients treated by HMAs is lower, the research activity has been restricted to OS in this population.
METHODS: 415 elderly AML patients (264 M, 152 F) aged 61-90 receiving frontline HMAs (AZA 297, DAC 118), either in daily practice or within clinical trials (AZA 27, DAC 17), with complete relevant clinical information (see Table I) were available from the PETHEMA epidemiologic AML registry (NCT02006004). We analyzed the predictive value for ED (8wk) of the prognostic factors for OS/ED in AML included in the Walter, MRC/LRF, ALFA and ALMA scoring systems, namely age, WBC count, performance status (PS), MRC 2010 cytogenetics, platelet count and secondary disease, as well as the type of HMA. The potential predictors were categorized following previous published models (Walter, MRC/LRF, ALFA, ALMA). Cumulative early death rate at 8 weeks was calculated by the life-time method and the relevant strata were tested for univariate significance by the Wilcoxon test. All significant covariates were included in a Cox multivariate regression model and those significant for death at 8wk were included in a new predictive tool (HMA-EDS). Patients were assigned randomly in a 1:1 ratio to a training cohort (TC) and a validation cohort (VC). The different scoring systems (Walter, MRC/LRF, ALFA, ALMA, HMA-EDS) were checked for their prognostic impact on ED. Finally the 95% CI for the expected death rate at 8wk for the different strata of the new model was calculated for the full patient series.
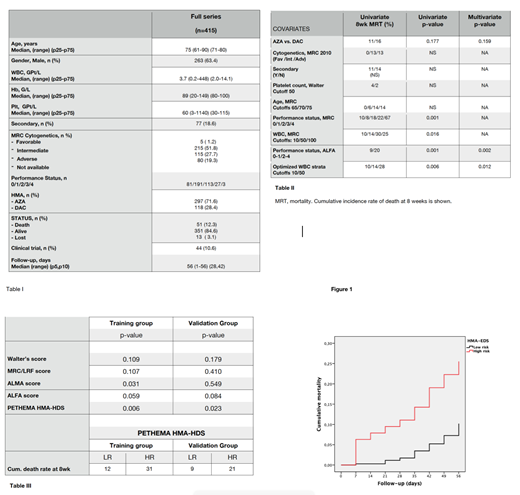
RESULTS: 51 patients out of 415 died and 13 were lost to follow-up before day 56 (cumulative ED rate at 8wk 13%, 95%CI 9-17%). Age, cytogenetics, secondary AML, platelet count and type of HMA were not significantly associated to ED. PS and WBC count strata confirmed their prognostic utility both in univariate and multivariate analysis (Table II). We developed the HMA-EDS by adding WBC (cutoffs 10 and 50, scores 1/2/3) and PS (0-1/2-4, scores 0/1) that classified patients in low-risk (score 1-2/ 84.6% of patients) and high risk (3-4/ 15.4% of patients) strata. When the prognostic utility for ED in the TC and the VC for the different scoring AML systems were checked, only HMA-EDS predicted ED in both cohorts (see Table III). The new EDS discriminates 2 different strata for ED at 8wk in unfit AML patients treated by HMA (see Figure 1 & Table III), namely a lower-risk group (ED rate 10%, 95% CI 6-14) and a high-risk group (ED rate 26%, 95% CI 14-38).
CONCLUSIONS: WBC count and PS are the main predictors for ED in unfit AML patients treated by HMAs. A new tool (HMA-EDS) discriminates two different risk groups and supersedes other previously published prognostic systems (Walter's, Wheatley's MRC/LRF, ALFA and ALMA) for this purpose. This score could be useful to select patients for front-line HMA or even HMAs-based combination therapies, given that several cycles are usually needed to achieve a clinical response. We suggest that other patient-related covariates such as geriatric assessment be checked in future studies.
Ramos:Daiichi Sankyo: Honoraria; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria; Abbvie: Honoraria. Fernandez:Incyte: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Teva: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Daiichi Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Karyopharm: Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal