INTRODUCTION: The outcome of patients (pts) with relapsed or refractory AML (R/R AML) or MDS after failing hypomethylating agents (HMA) is poor. CPX-351 is a liposomal formulation of cytarabine and daunorubicin, approved by the US Food and Drug Administration (FDA) for advanced secondary AML and AML post-MDS. GO is a humanized immunoglobulin G4 antibody directed against CD33 and conjugated to the DNA toxin calicheamicin, also approved by the FDA for the treatment of newly diagnosed or R/R CD33-positive AML. We have hypothesized that combination of CPX-351 and GO could induce superior antitumor efficacy compared to either agent alone for this pt population.
GOALS: To determine the safety and efficacy of CPX-351 in combination with GO in R/R AML and post-HMA failure HR-MDS.
METHODS: This is a single institution, pilot study (NCT03672539) enrolling pts with CD33 positive R/R AML, post-HMA failure High-Risk MDS (>10% blasts), and pts with newly diagnosed secondary AML after receiving HMA therapy. Pts received induction cycle CPX-351 (daunorubicin 44 mg/m2 and cytarabine 100 mg/m2) administered via intravenous (IV) infusion on days 1, 3 and 5. GO was administered at dose of 3 mg/m2 (always capped at one 4.5 mg vial) IV on day 1. Pts not attaining an IWG-defined complete remission (CR) or CR with incomplete count recovery (CRi) after 1 cycle, could receive a 2nd induction cycle of CPX-351 at the same dose, but only on days 1 and 3 with GO 3 mg/m2 on day 1. Pts attaining response could receive up to 2 consolidation cycles after a minimum of 4 weeks from the start of the last cycle with CPX-351 (daunorubicin 29 mg/m2 and cytarabine 65 mg/m2) IV on days 1 and 3, and GO at 3 mg/m2 on day 1. GO was only administered during the 2nd consolidation cycle if evidence of positive minimal residual disease (MRD). GO could also be administered as a single agent for maintenance treatment on day 1 every 6 weeks only in case of persistent detection of MRD.
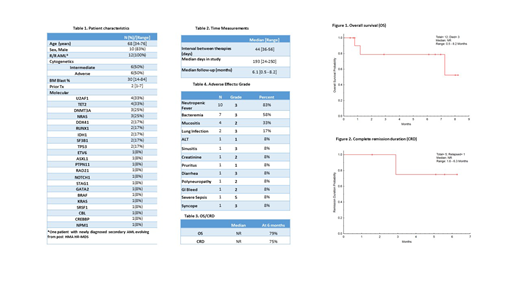
RESULTS: Twelve pts have been enrolled between November 2018 and July 2019. At the time of data cut off only 10 pts were evaluable for response. Two pts were still too early for assessment after receiving induction cycle. Pt characteristics are summarized on Table 1. Of 10 evaluable pts, 5 pts (50%) achieved an overall response of CR/CRi (4 CR, 1 CRi), including 2 pts with negative MRD at CR. Of note, one pt counted as a responder, had a bone marrow assessment (BMA) on day 21 with 1% blasts and flow cytometry not showing increased blasts but the sample was hypocellular. He was pancytopenic and suffering a grade 3 sinusitis suspicious for an invasive fungal infection. A repeated BMA on day 28 was aparticulate and insufficient for interpretation. Eventually his infection was controlled and his blood counts recovered on day 38 but he was transitioned to a lower intensity regimen given concerns of myelosuppression and infection reactivation. Another BMA upon peripheral blood counts recovery was not obtained. Among the other 4 responders, 1 pt relapsed after 2 consolidation cycles. One of 2 pts in CR with negative MRD was taken out of the study after 2 consolidations at treating physician discretion and the other one is under close protocol surveillance after completing 1 GO maintenance dose. Both are being considered for stem cell transplantation (SCT). The 4th pt is in CR with positive MRD and completed 4 GO maintenance doses. He is also being considered for SCT. Only 1 pt out of 5 not responding received 2 induction cycles and was refractory. Among responders, median time to ANC >0.5 x 109/L was 33 days (range 30-45) and Plt >50 x 109/L was 38 days (range 33-45). Median time to ANC >1 x 109/L was 34 days (range 31-45) and Plt >100 x 109/L was 43 days (range 38-53) after induction. With a median follow up of 6.1 months (mos) (Table 2), median OS has not been reached, and 6 mos OS is 79% (Figure 1). Median CR duration (CRD) has not been reached, with 75% CRD at 6 mos (Table 3, Figure 2). Adverse events regardless of causality are on Table 4. Three pts out of 12 (25%) have died, 2 of progressive disease and 1 of sepsis. Thirty-day mortality rate was 8% (n=1).
CONCLUSION: In this preliminary report CPX-351 and GO combination appears to be active with acceptable toxicities in this high-risk disease population. Treatment-related, grade 3-4 non-hematological toxicity have not been observed. There are concerns for significant myelosuppression and infectious complications. Safety and efficacy assessments are ongoing. Therapy may require dose adjustments.
Kadia:Celgene: Research Funding; Bioline RX: Research Funding; BMS: Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Jazz: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Research Funding. Faderl:Jazz Pharmaceuticals: Employment, Equity Ownership. Sasaki:Pfizer: Consultancy; Otsuka: Honoraria. Cortes:Bristol-Myers Squibb: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Honoraria, Research Funding; BiolineRx: Consultancy; Takeda: Consultancy, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Astellas Pharma: Consultancy, Honoraria, Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding; Sun Pharma: Research Funding; Biopath Holdings: Consultancy, Honoraria; Forma Therapeutics: Consultancy, Honoraria, Research Funding; Merus: Consultancy, Honoraria, Research Funding; Immunogen: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding. Daver:Pfizer: Consultancy, Research Funding; Agios: Consultancy; Genentech: Consultancy, Research Funding; Immunogen: Consultancy, Research Funding; Sunesis: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Otsuka: Consultancy; Celgene: Consultancy; Abbvie: Consultancy, Research Funding; Hanmi Pharm Co., Ltd.: Research Funding; Incyte: Consultancy, Research Funding; Glycomimetics: Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Jazz: Consultancy; Immunogen: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Incyte: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Karyopharm: Consultancy, Research Funding; Astellas: Consultancy; Astellas: Consultancy; Jazz: Consultancy; Servier: Research Funding; Sunesis: Consultancy, Research Funding; NOHLA: Research Funding; Otsuka: Consultancy; Hanmi Pharm Co., Ltd.: Research Funding; Novartis: Consultancy, Research Funding; Forty-Seven: Consultancy; Forty-Seven: Consultancy; NOHLA: Research Funding; Celgene: Consultancy; Glycomimetics: Research Funding; BMS: Consultancy, Research Funding; Karyopharm: Consultancy, Research Funding; Servier: Research Funding; Agios: Consultancy. DiNardo:agios: Consultancy, Honoraria; celgene: Consultancy, Honoraria; abbvie: Consultancy, Honoraria; jazz: Honoraria; medimmune: Honoraria; notable labs: Membership on an entity's Board of Directors or advisory committees; syros: Honoraria; daiichi sankyo: Honoraria. Jabbour:Takeda: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Adaptive: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Cyclacel LTD: Research Funding; Pfizer: Consultancy, Research Funding; Amgen: Consultancy, Research Funding. Borthakur:Xbiotech USA: Research Funding; Eli Lilly and Co.: Research Funding; Polaris: Research Funding; Eisai: Research Funding; AstraZeneca: Research Funding; BMS: Research Funding; BioLine Rx: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; NKarta: Consultancy; PTC Therapeutics: Consultancy; Arvinas: Research Funding; Merck: Research Funding; Cantargia AB: Research Funding; FTC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Argenx: Membership on an entity's Board of Directors or advisory committees; BioTheryX: Membership on an entity's Board of Directors or advisory committees; AbbVie: Research Funding; GSK: Research Funding; Cyclacel: Research Funding; Oncoceutics: Research Funding; Agensys: Research Funding; Incyte: Research Funding; Bayer Healthcare AG: Research Funding; Oncoceutics, Inc.: Research Funding; Novartis: Research Funding; Janssen: Research Funding; Strategia Therapeutics: Research Funding; Tetralogic Pharmaceuticals: Research Funding. Al Azzawi:Cyclacel LTD: Research Funding. Pemmaraju:sagerstrong: Research Funding; Daiichi-Sankyo: Research Funding; plexxikon: Research Funding; novartis: Consultancy, Research Funding; Stemline Therapeutics: Consultancy, Honoraria, Research Funding; cellectis: Research Funding; celgene: Consultancy, Honoraria; samus: Research Funding; abbvie: Consultancy, Honoraria, Research Funding; mustangbio: Consultancy, Research Funding; incyte: Consultancy, Research Funding; affymetrix: Research Funding. Konopleva:Ablynx: Research Funding; Reata Pharmaceuticals: Equity Ownership, Patents & Royalties; Kisoji: Consultancy, Honoraria; Ascentage: Research Funding; Stemline Therapeutics: Consultancy, Honoraria, Research Funding; Genentech: Honoraria, Research Funding; F. Hoffman La-Roche: Consultancy, Honoraria, Research Funding; Forty-Seven: Consultancy, Honoraria; Eli Lilly: Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria; Cellectis: Research Funding; Calithera: Research Funding; Astra Zeneca: Research Funding; Agios: Research Funding. Jain:Servier: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics, an AbbVie company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen Pharmaceuticals, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Genentech: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Research Funding; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; ADC Therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Research Funding; AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Cellectis: Research Funding; Verastem: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Precision Biosciences: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Garcia-Manero:Amphivena: Consultancy, Research Funding; Helsinn: Research Funding; Novartis: Research Funding; AbbVie: Research Funding; Celgene: Consultancy, Research Funding; Astex: Consultancy, Research Funding; Onconova: Research Funding; H3 Biomedicine: Research Funding; Merck: Research Funding. Andreeff:NIH/NCI: Research Funding; Center for Drug Research & Development: Membership on an entity's Board of Directors or advisory committees; Cancer UK: Membership on an entity's Board of Directors or advisory committees; NCI-CTEP: Membership on an entity's Board of Directors or advisory committees; German Research Council: Membership on an entity's Board of Directors or advisory committees; Leukemia Lymphoma Society: Membership on an entity's Board of Directors or advisory committees; NCI-RDCRN (Rare Disease Cliln Network): Membership on an entity's Board of Directors or advisory committees; CLL Foundation: Membership on an entity's Board of Directors or advisory committees; BiolineRx: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Consultancy; Daiichi Sankyo, Inc.: Consultancy, Patents & Royalties: Patents licensed, royalty bearing, Research Funding; CPRIT: Research Funding; Senti Bio: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Oncoceutics: Equity Ownership; Oncolyze: Equity Ownership; Breast Cancer Research Foundation: Research Funding; AstaZeneca: Consultancy; Amgen: Consultancy; 6 Dimensions Capital: Consultancy; Reata: Equity Ownership; Aptose: Equity Ownership; Celgene: Consultancy; Eutropics: Equity Ownership. Kantarjian:BMS: Research Funding; Jazz Pharma: Research Funding; Cyclacel: Research Funding; Takeda: Honoraria; Astex: Research Funding; Ariad: Research Funding; AbbVie: Honoraria, Research Funding; Agios: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Research Funding; Novartis: Research Funding; Daiichi-Sankyo: Research Funding; Immunogen: Research Funding. Ravandi:Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Xencor: Consultancy, Research Funding; Macrogenix: Consultancy, Research Funding; Selvita: Research Funding; Cyclacel LTD: Research Funding; Menarini Ricerche: Research Funding. Alvarado:Jazz Pharmaceuticals: Research Funding; Abbott: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal