Introduction: AML pts who relapse after allogeneic hematopoietic cell transplantation (HCT) face poor clinical outcomes and short overall survival (OS) (Schmid et al, Blood2012). The delivery of optimal salvage therapy is challenging as some pts do not tolerate high intensity regimens, and lower intensity therapies may not yield sufficient disease control. Favorable responses in older, treatment naïve AML pts with venetoclax (VEN) in combination with low-dose cytarabine (LDAC) or DNA methyltransferase inhibitor (DNMTi) led to its approval. Off-label use in relapsed/refractory AML is increasing (DiNardo et al, Am J Hematol2018; Aldoss et al, Haematologica2018). We retrospectively evaluated the overall response rate (ORR = CR+CRi+PR+MLFS) and report our clinical experience with VEN-based salvage in post-HCT relapsed AML.
Methods: After IRB approval, consecutive pts with post-HCT relapsed AML treated with VEN+LDAC or VEN+DNMTi from May 2018 to July 2019 were retrospectively analyzed. Selection of VEN partner and dosing were at the discretion of the treating physician based on institutional guidelines and published prescribing information. Responses were assigned based on the AML IWG criteria. The Kaplan-Meier method was used to describe OS. ORR and treatment complications were summarized via descriptive statistics.
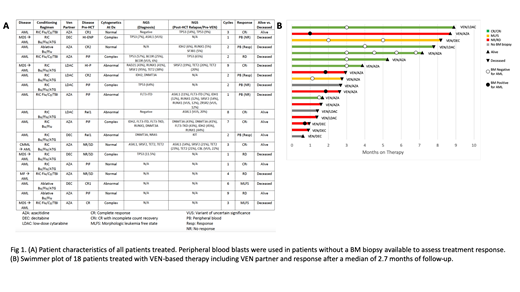
Results: 18 pts with post-HCT relapsed AML who received at least 1 cycle of VEN-based salvage chemotherapy were included. Median age at HCT was 64.5 years (range 34.5-73.7 years). Most pts were poor risk: 6/18 pts had an antecedent hematologic malignancy, 12/18 had an abnormal or complex karyotype (CK) prior to HCT, and 4/12 pts with CK also were TP53mut. 15/18 (83.3%) received reduced intensity conditioning and MUD was the predominant graft type (50%). All pts received PBSCs. Additional disease and response characteristics are reported in Fig 1A. Median time from HCT to relapse was 5.5 mos (range: 0.9 to 44.9 mos); 27.8% of pts relapsed within 100 days and 55.6% relapsed within 6 mos of HCT. At relapse, 1 patient had grade 2 aGVHD and 1 had severe, extensive cGVHD. No pts experienced a GVHD flare or progression during treatment. 14/18 (77.8%) of pts were receiving immunosuppressive therapy (IST) at relapse and received VEN concurrently with IST.
VEN-based salvage chemotherapy began shortly after confirmed relapse (range: 4-46 days); 4/18 pts received VEN with LDAC and 14/18 were treated with a DNMTi partner. 15/18 pts were evaluable for response. IWG responses were seen in 8 pts with an ORR of 53%. There were 0 CR, 6 CRi, 0 PR, 2 MLFS, 7 pts had progressive disease (4 by BM, 3 by PB, Fig 1B). 3/18 additional pts had a ≥50% reduction in circulating blasts indicating treatment effect but were non-evaluable given lack of surveillance BM biopsy. Pts received a median of 2.5 cycles (range 1-9). 15/18 pts had treatment held or delayed due to fever/infection (7), PB cytopenias (4), combination (3), or non-hematologic toxicity (1). 6/8 pts who achieved a CRi/MLFS had VEN dosing reduced or administered as a single-agent. One patient achieved CRi with dose interruptions lasting ≥3 mos, maintained this response, and subsequently cleared a TP53 mutation.
The majority of pts 13/18 (72.2%) experienced infectious complications during treatment: 7 developed bacterial pneumonia (4 associated with sepsis), 4 fungal penumonia, and 2 oral infections (both associated with sepsis). 8/18 pts had active infections at the time of death. Time from HCT or IST status did not appear to impact the frequency or severity of infectious complications. After a median of 2.7 mos of follow up (range 0.6-8.9 mos), the mOS after the start of VEN was 130 days. Consolidation with donor lymphocyte infusion or second HCT is planned for several pts.
Conclusions: Transplant related- and disease related-mortality are difficult to disentangle in post-HCT AML relapse making it challenging to ascertain the benefit of therapy. In this cohort, the majority of pts relapsed within 6 mos of HCT and were receving IST at the start of salvage therapy. In spite of this, VEN-based salvage induced meaningful responses. To convert these responses to long term survival benefit, as VEN-based salvage is more widely used in this setting, consideration of immunosuppression and previous marrow injury should inform alternative dosing regimens, careful monitoring for infectious complications in close follow-up, and broad spectrum antimicrobial prophylaxis.
Byrne:Karyopharm: Research Funding. Dholaria:Celgene: Honoraria. Ferrell:Incyte: Research Funding; Agios: Consultancy; Forma Therapeutics: Research Funding; Astex Pharmaceuticals: Research Funding. Jagasia:Kadmon: Consultancy; Incyte: Consultancy; Janssen: Research Funding. Strickland:Astellas Pharma: Consultancy; Sunesis Pharmaceuticals: Research Funding; AbbVie: Consultancy; Jazz: Consultancy; Kite: Consultancy; Pfizer: Consultancy. Savona:Sunesis: Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees; Boehringer Ingelheim: Patents & Royalties; Celgene Corporation: Membership on an entity's Board of Directors or advisory committees; Incyte Corporation: Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm Therapeutics: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Selvita: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Venetoclax use in relapsed/refractory AML
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal