Introduction: Chronic myelomonocytic leukemia (CMML), an aggressive myeloid malignancy, can be categorized into two subtypes, proliferative CMML (pCMML) and dysplastic (dCMML), based on a white blood cell (WBC) count of ≥ 13 x 109/L for the former (Arber et al. Blood 2016). While this WBC cut off is somewhat arbitrary, patients with pCMML have unique phenotypic features and a shorter survival. We carried out this study to assess the genomic, transcriptomic and epigenetic landscapes of these two CMML subtypes.
Methods: Peripheral blood (PB) and bone marrow (BM) mononuclear cells (MNC) were obtained from WHO-defined CMML patients. Next generation sequencing (NGS) using a 36-gene panel was performed on 350 patients with Illumina HiSeq4000 platform with median read depth of 400X. RNA sequencing (RNA-seq) was performed on 25 patients by bulk whole transcriptome sequencing using Illumina TruSeq. DNA immunoprecipitation sequencing (DIP-seq) was performed on 18 patients using 5-methylcystocine (5mC), 5-hydroxymethylcytosine (5hmC) and bridging monoclonal antibodies with subsequent paired-end sequencing using HiSeq4000. Chromatin immunoprecipitation sequencing (ChIP-seq) was performed on 30 patients with Illumina HiSeq2500 to a depth of 25 million for histone 3 lysine 4 monomethylation (H3K4me1) and histone 3 lysine 4 trimethylation (H3K4me3) and 50 million reads for histone 3 lysine 27 trimethylation (H3K27me3) and Input per sample.
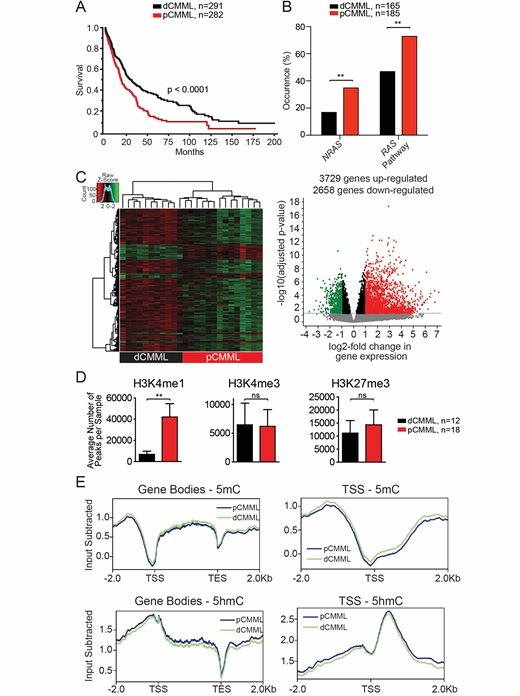
Results: Five hundred and seventy-three patients with WHO defined CMML were included; median age 71 years (range 18-95 years), 67% males. 282 patients had pCMML (49%), while 291 (51%) had dCMML. As pre-defined, patients with pCMML were more likely to have higher absolute monocyte counts (p<0.0001), circulating immature myeloid cells (p<0.0001), PB blasts (p<0.0001), and higher lactate dehydrogenase levels (p=0.03). At last follow up 234 (41%) deaths and 70 (20%) leukemic transformations were documented. The median OS for pCMML vs dCMML in this cohort was 19 months vs 30 months (p<0.0001, Figure 1A) and validated in an independent Austrian cohort (p=0.02).
Genomic profiling: NGS performed on 350 patients (BM MNC) revealed a higher frequency of NRAS (35 vs 17%, p=0.004), cumulative RAS pathway (NRAS, KRAS, CBL and PTPN11) (73 vs 47%, p=0.001), ASXL1 (p=0.003) and JAK2V617F (p=0.04) mutations in pCMML relative to dCMML (Figure 1B); while dCMML had a higher frequency of SF3B1 mutations (p=0.02). There were no differences in distribution of TET2 and SRSF2
Transcriptomic analysis: RNA-seq was performed on PB MNC from RAS pathway mutant pCMML patients (n=12) and RAS pathway wildtype dCMML patients (n=13). Unsupervised clustering analysis resulted in appropriate segregation revealing distinct expression profiles between disease subtypes (Figure 1C). Compared to dCMML, 3729 genes were significantly differentially upregulated and 2658 genes were differentially downregulated in pCMML. Among genes most highly upregulated were mitotic checkpoint kinases including AURBK, PLK1, PLK2, PLK4 and
Epigenetic profiling: ChIP-seq of PB and BM MNC from pCMML (n=18) and dCMML (n=12) patients and healthy, age-matched controls (n=10) revealed a global increase in H3K4me1, without significant differences in H3K4me3 or H3K27me3 occupancies (regardless of stratification by ASXL1 mutational status; 40% ASXL1mt in pCMML, 30% dCMML) in pCMML vs dCMML (Figure 1D). H3K4me1 occupancy was also increased in a sequence-specific manner at the transcription start sites of the aforementioned mitotic kinases (PLK1 and WEE1). DIP-seq was performed on PB MNC to assess global differences in 5-mC and 5-hmC levels, between pCMML (n=9) and dCMML (n=9), with no differences seen between the two subtypes (regardless of TET2 mutational status, 40% TET2mt in each subtype) (Figure 1E).
Conclusions: Despite the somewhat arbitrary WBC distinction between pCMML and dCMML, clear phenotypic, genetic, transcriptomic, epigenetic and survival differences exist between the two subtypes, providing strong biological rationale for this distinction. pCMML patients have a higher frequency of oncogenic RAS pathway mutations, a unique transcriptomic profile enriched in mitotic check point kinases and a unique chromatin configuration with global and sequence specific enrichment in H3K4me1, with no significant global differences in 5mC, 5hmC, or H3K4me3 and H3K27me3 occupancy.
Geissler:AOP: Honoraria; Pfizer: Honoraria; AstraZeneca: Honoraria; Novartis: Honoraria; Celgene: Honoraria; Roche: Honoraria; Abbvie: Honoraria; Ratiopharm: Honoraria; Amgen: Honoraria. Al-Kali:Astex Pharmaceuticals, Inc.: Research Funding. Patnaik:Stem Line Pharmaceuticals.: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal