INTRODUCTION:TP53 mutations are associated with adverse outcomes and shorter response to hypomethylating agents (HMA) in myelodysplastic syndromes (MDS). There is limited data evaluating the impact of the type, number, clonal size and patterns of TP53 mutations in response outcomes and prognosis.
METHODS: We evaluated all patients with newly diagnosed myelodysplastic syndromes (MDS) treated at The University of Texas MD Anderson Cancer Center (MDACC) from 2013 to 2018. Genomic DNA was extracted from whole bone marrow aspirate samples and was subject to 28, 53 or 81-gene targeted PCR-based sequencing using a next generation sequencing (NGS) platform. Response assessment was performed following 2006 IWG criteria. The Kaplan-Meier product limit method was used to estimate survival outcomes for each clinical/demographic factor. Univariate Cox proportional hazards regression was used to identify any association with each of the variables and survival outcomes.
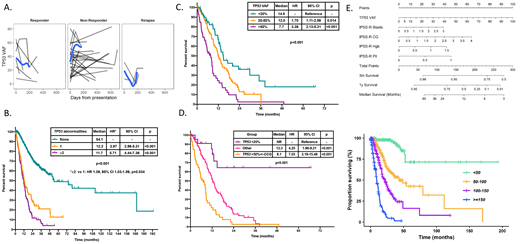
RESULTS: 938 patients were evaluated including 261 (28%) with detectable TP53 mutations of which 189 (72%) received therapy: chemotherapy-based in 5 (3%) patients, single agent hypomethylating agents in 116 (61%) and hypomethylating agent in combination with novel agents in 65 (34%). Most (n=175, 67%) patients had one TP53 mutation with 75 (29%), 10 (4%) and 1 (0.4%) having 2, 3 and 4, respectively. TP53 dynamics on 18 patients with multiple TP53 mutations and longitudinal sequencing suggested both could present within the same clone in 8 (44%) patients. Median variant allele frequency (VAF) of all TP53 mutations was 39% (range 1-94%). TP53 deletion was more frequent in patients with mutated TP53 (31.8% vs 2.2%, p<0.001). Sixty-three patients (24%) suffered transformation to acute myeloid leukemia with a median transformation-free survival (TFS) of 10.6 months (95% CI 8.8-12.3). By univariate analysis the number of TP53 mutations (HR 2.03, 95% CI 1.3-3.05, p<0.001), TP53 mutation VAF (HR 1.02 increase per 1% VAF increase, 95% CI 1.01-1.02, p<0.001), TP53 deletion (HR 2.10, 95% CI 1.38-3.19, p<0.001) and complex karyotype (HR 2.58, 95% CI 1.70-3.91, p<0.001) were predictors of shorter TFS. By multivariate analysis only TP53 mutation VAF remained an independent predictor of shorter TFS (HR 1.02 increase per 1% VAF increase, 95% CI 1.00-1.03, p=0.005). With a median follow-up of 21.9 months (95% CI 20.3-25.6 months), there were no significant differences in ORR (58% vs 63%, p=0.303) or CR (27% vs 22%, p=0.288) based on the presence of TP53 mutation. Presence of TP53 deletion was associated with lower ORR (OR 0.53, p=0.021). Lower TP53 VAF correlated with higher ORR. Presence of TP53 abnormalities was associated with shorter response duration (HR 2.9, 95% CI 1.64-5.13, p<0.001). Longitudinal sequencing was available in 64 patients. TP53 VAF decreased more among responders (p=0.022) with subsequent increase of VAF at the time of relapse (Figure 1A). Presence of ³2 TP53 abnormalities was associated with shorter survival (HR 1.39, 95% CI 1.03-1.89, p=0.034, Figure 1B). TP53 VAF was associated with worse prognosis (HR 1.02 per 1% VAF increase, 95% CI 1.01-1.03, p<0.001). Patients could be classified into three prognostic groups based on TP53 VAF (Figure 1C). Integration of TP53 VAF and karyotypic complexity identified prognostic subgroups (Figure 1D). We developed a multivariable Cox model including TP53 VAF and IPSS-R categories with a corrected concordance index of 0.81 demonstrating a strong model fit. This model was used to generate a nomogram for overall survival (Figure 1E).
CONCLUSION: This data suggests that the number and clonal size of TP53 mutations as well as other genomic events may help identify subgroups of patients with MDS with distinct prognosis and clinical outcomes.
Sasaki:Pfizer: Consultancy; Otsuka: Honoraria. Alvarado:Jazz Pharmaceuticals: Research Funding; Abbott: Honoraria. Kadia:Jazz: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; Bioline RX: Research Funding; Celgene: Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees. Ravandi:Selvita: Research Funding; Cyclacel LTD: Research Funding; Macrogenix: Consultancy, Research Funding; Xencor: Consultancy, Research Funding; Menarini Ricerche: Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Cortes:Astellas Pharma: Consultancy, Honoraria, Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding; Forma Therapeutics: Consultancy, Honoraria, Research Funding; Immunogen: Consultancy, Honoraria, Research Funding; Merus: Consultancy, Honoraria, Research Funding; Daiichi Sankyo: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Sun Pharma: Research Funding; Takeda: Consultancy, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Biopath Holdings: Consultancy, Honoraria; BiolineRx: Consultancy; Pfizer: Consultancy, Honoraria, Research Funding. Daver:Otsuka: Consultancy; Sunesis: Consultancy, Research Funding; Agios: Consultancy; Incyte: Consultancy, Research Funding; Karyopharm: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Hanmi Pharm Co., Ltd.: Research Funding; Glycomimetics: Research Funding; Servier: Research Funding; BMS: Consultancy, Research Funding; Jazz: Consultancy; Abbvie: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; NOHLA: Research Funding; Forty-Seven: Consultancy; Novartis: Consultancy, Research Funding; Astellas: Consultancy; Immunogen: Consultancy, Research Funding; Celgene: Consultancy; Pfizer: Consultancy, Research Funding. Takahashi:Symbio Pharmaceuticals: Consultancy. DiNardo:daiichi sankyo: Honoraria; agios: Consultancy, Honoraria; abbvie: Consultancy, Honoraria; celgene: Consultancy, Honoraria; notable labs: Membership on an entity's Board of Directors or advisory committees; syros: Honoraria; medimmune: Honoraria; jazz: Honoraria. Jabbour:Takeda: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Adaptive: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Cyclacel LTD: Research Funding; AbbVie: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding. Borthakur:Novartis: Research Funding; Merck: Research Funding; Incyte: Research Funding; Eli Lilly and Co.: Research Funding; Janssen: Research Funding; Cantargia AB: Research Funding; Eisai: Research Funding; Agensys: Research Funding; Oncoceutics: Research Funding; Bayer Healthcare AG: Research Funding; NKarta: Consultancy; BioLine Rx: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Xbiotech USA: Research Funding; Strategia Therapeutics: Research Funding; Tetralogic Pharmaceuticals: Research Funding; Argenx: Membership on an entity's Board of Directors or advisory committees; BioTheryX: Membership on an entity's Board of Directors or advisory committees; Oncoceutics, Inc.: Research Funding; Arvinas: Research Funding; Polaris: Research Funding; FTC Therapeutics: Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; GSK: Research Funding; Cyclacel: Research Funding; PTC Therapeutics: Consultancy; AbbVie: Research Funding; AstraZeneca: Research Funding. Pemmaraju:cellectis: Research Funding; Stemline Therapeutics: Consultancy, Honoraria, Research Funding; novartis: Consultancy, Research Funding; plexxikon: Research Funding; Daiichi-Sankyo: Research Funding; sagerstrong: Research Funding; affymetrix: Research Funding; incyte: Consultancy, Research Funding; mustangbio: Consultancy, Research Funding; abbvie: Consultancy, Honoraria, Research Funding; samus: Research Funding; celgene: Consultancy, Honoraria. Konopleva:Kisoji: Consultancy, Honoraria; Genentech: Honoraria, Research Funding; Reata Pharmaceuticals: Equity Ownership, Patents & Royalties; Ablynx: Research Funding; Astra Zeneca: Research Funding; Agios: Research Funding; Ascentage: Research Funding; Calithera: Research Funding; Stemline Therapeutics: Consultancy, Honoraria, Research Funding; Forty-Seven: Consultancy, Honoraria; Eli Lilly: Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Cellectis: Research Funding; Amgen: Consultancy, Honoraria; F. Hoffman La-Roche: Consultancy, Honoraria, Research Funding. Bueso-Ramos:Incyte: Consultancy. Andreeff:BiolineRx: Membership on an entity's Board of Directors or advisory committees; CLL Foundation: Membership on an entity's Board of Directors or advisory committees; NCI-RDCRN (Rare Disease Cliln Network): Membership on an entity's Board of Directors or advisory committees; Leukemia Lymphoma Society: Membership on an entity's Board of Directors or advisory committees; German Research Council: Membership on an entity's Board of Directors or advisory committees; NCI-CTEP: Membership on an entity's Board of Directors or advisory committees; Cancer UK: Membership on an entity's Board of Directors or advisory committees; Center for Drug Research & Development: Membership on an entity's Board of Directors or advisory committees; NIH/NCI: Research Funding; CPRIT: Research Funding; Breast Cancer Research Foundation: Research Funding; Oncolyze: Equity Ownership; Oncoceutics: Equity Ownership; Senti Bio: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Eutropics: Equity Ownership; Aptose: Equity Ownership; Reata: Equity Ownership; 6 Dimensions Capital: Consultancy; AstaZeneca: Consultancy; Daiichi Sankyo, Inc.: Consultancy, Patents & Royalties: Patents licensed, royalty bearing, Research Funding; Jazz Pharmaceuticals: Consultancy; Celgene: Consultancy; Amgen: Consultancy. Kantarjian:Ariad: Research Funding; Amgen: Honoraria, Research Funding; Takeda: Honoraria; Daiichi-Sankyo: Research Funding; Agios: Honoraria, Research Funding; Astex: Research Funding; Jazz Pharma: Research Funding; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; Cyclacel: Research Funding; Novartis: Research Funding; Immunogen: Research Funding; AbbVie: Honoraria, Research Funding; BMS: Research Funding; Pfizer: Honoraria, Research Funding. Garcia-Manero:Amphivena: Consultancy, Research Funding; Helsinn: Research Funding; Novartis: Research Funding; AbbVie: Research Funding; Celgene: Consultancy, Research Funding; Astex: Consultancy, Research Funding; Onconova: Research Funding; H3 Biomedicine: Research Funding; Merck: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal