Background: Pegylated Interferon-alpha (PEG-IFNa) improves hematologic parameters and reduces mutant allele fraction in patients with myeloproliferative neoplasms (MPNs); however, widespread clinical use is limited by frequent discontinuation due to side effects. Identifying biomarkers of response to PEG-IFNa therapy might identify patient subsets most likely to derive benefit from this therapy. Serum pro-inflammatory cytokine levels are increased in MPN patients and associated with adverse clinical outcomes. The effect of PEG-IFNa therapy on cytokine levels in MPN patients and correlation with clinical response has not been elucidated. We hypothesized that identification of cytokine signatures could predict for clinical/molecular response to PEG-IFNa in MPNs.
Methods: Pre- and post-treatment serum samples were collected from available patients enrolled on the Myeloproliferative Disorders Research Consortium (MPD-RC) 111/112 trials-multicenter studies assessing efficacy of PEG-IFNa in patients with high-risk polycythemia vera/essential thrombocythemia either up front or in the setting of hydroxyurea failure/intolerance. Serum was prepared using the Millipore MAP Human 41-Cytokine Magnet Bead System (Millipore; Cat:HCYTOMAG-60K) and run on the Luminex FLEXMAP 3D platform. Data was analyzed using Luminex xPonent 4.3 software. Correlative mutational data on 156 genes implicated in myeloid malignancies was also available for all patients. Age-matched serum from healthy individuals (N=6) was obtained from AllCellsTM for use as wild-type (WT) controls. Baseline cytokine samples were compared between groups of interest by use of Wilcoxon rank-sum test. Change from baseline in cytokines for each group were compared to the null hypothesis of zero change. P values <0.05 were considered statistically significant.
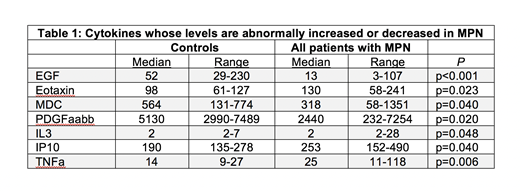
Results: Pre- and post-treatment serum samples were available on 29 MPN patients from the MPN-RC 111/112 cohorts. 21 patients were classified as complete responders (CRs) vs. 8 non-responders (NRs) using European LeukemiaNet criteria. Baseline cytokine levels were generally increased in both CRs and NRs compared to WT, including significant increases in Eotaxin (p=0.023), IL3 (p=0.048), IP10 (p=0.04), and TNFa (p=0.006) (Table 1), as well as a non-significant trend increase in IL8 (p=0.076). Notably, other cytokines were decreased at baseline in comparison to WT, including EGF (p<0.001), as well as MDC (p=0.040) and PDGFaabb (p=0.02), in line with previous studies. Compared to CRs, NRs displayed significantly lower levels of GRO (p=0.020), MDC (p=0.003), and PDGFaabb (p=0.026) at baseline along with elevated levels of IP10 (p=0.028) suggesting pre-treatment levels of these cytokines might predict response to therapy. Comparison of pre- and post- treatment samples revealed a general increase in median cytokine levels in CRs, with significant changes in GM-CSF (p=0.012), IP10 (p<0.001), MCP1 (p=0.015) and IFNa itself (p=0.010). Notably, median cytokine levels in NRs post-treatment, including IFNa, were relatively unchanged from baseline despite on-going PEG-IFNa therapy. Additional pre-treatment serum samples, as well as correlative analysis of cytokine changes with JAK-STAT driver mutant allele fraction and mutational profiling are underway and will be presented at the meeting.
Conclusions: Preliminary findings suggest individual cytokines are affected by PEG-IFNa in MPNs. Furthermore, baseline levels of GRO, MDC, PDGFaabb, and IP10 correlate with response to PEG-IFNa. These data may identify MPN patients most likely to respond to PEG-IFNa therapy and those for whom this therapy may be most appropriate. Further analysis with a larger cohort of treatment-naïve patients is underway to validate these findings and identify additional molecular and cytokine signatures of response to PEG-IFNa therapy.
Yacoub:Agios: Speakers Bureau; Hylapharm: Equity Ownership; Dynavax: Equity Ownership; Cara: Equity Ownership; Ardelyx: Equity Ownership; Incyte: Consultancy, Honoraria, Speakers Bureau; Seattle Genetics: Honoraria, Speakers Bureau; Novartis: Consultancy, Speakers Bureau. Mascarenhas:Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Research Funding; Roche: Consultancy, Research Funding; Merck: Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; CTI Biopharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Research Funding; Promedior: Research Funding; Merus: Research Funding; Pharmaessentia: Consultancy, Membership on an entity's Board of Directors or advisory committees. Levine:Lilly: Honoraria; Qiagen: Membership on an entity's Board of Directors or advisory committees; Imago Biosciences: Membership on an entity's Board of Directors or advisory committees; Loxo: Membership on an entity's Board of Directors or advisory committees; Prelude Therapeutics: Research Funding; Isoplexis: Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Research Funding; Gilead: Consultancy; Amgen: Honoraria; C4 Therapeutics: Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Research Funding; Novartis: Consultancy. Hoffman:Merus: Research Funding. Rampal:Constellation, Incyte, and Stemline Therapeutics: Research Funding; Agios, Apexx, Blueprint Medicines, Celgene, Constellation, and Jazz: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal