Key Points
A CD19/CD3 single-chain Fv-Fc bsAb mediated potent killing of CLL cells by autologous T cells in vitro and in vivo.
bsAb-mediated cytotoxicity was enhanced by prior therapy with ibrutinib and extended to ibrutinib-resistant disease.
Abstract
The Bruton tyrosine kinase inhibitor ibrutinib induces high rates of clinical response in chronic lymphocytic leukemia (CLL). However, there remains a need for adjunct treatments to deepen response and to overcome drug resistance. Blinatumomab, a CD19/CD3 bispecific antibody (bsAb) designed in the BiTE (bispecific T-cell engager) format, is approved by the US Food and Drug Administration for the treatment of relapsed or refractory B-cell precursor acute lymphoblastic leukemia. Because of its short half-life of 2.1 hours, blinatumomab requires continuous intravenous dosing for efficacy. We developed a novel CD19/CD3 bsAb in the single-chain Fv-Fc format (CD19/CD3-scFv-Fc) with a half-life of ∼5 days. In in vitro experiments, both CD19/CD3-scFv-Fc and blinatumomab induced >90% killing of CLL cells from treatment-naïve patients. Antileukemic activity was associated with increased autologous CD8 and CD4 T-cell proliferation, activation, and granzyme B expression. In the NOD/SCID/IL2Rγnull patient-derived xenograft mouse model, once-weekly treatment with CD19/CD3-scFv-Fc eliminated >98% of treatment-naïve CLL cells in blood and spleen. By contrast, blinatumomab failed to induce a response, even when administered daily. We next explored the activity of CD19/CD3-scFv-Fc in the context of ibrutinib treatment and ibrutinib resistance. CD19/CD3-scFv-Fc induced more rapid killing of CLL cells from ibrutinib-treated patients than those from treatment-naïve patients. CD19/CD3-scFv-Fc also demonstrated potent activity against CLL cells from patients with acquired ibrutinib-resistance harboring BTK and/or PLCG2 mutations in vitro and in vivo using patient-derived xenograft models. Taken together, these data support investigation of CD19/CD3 bsAb’s and other T cell-recruiting bsAb’s as immunotherapies for CLL, especially in combination with ibrutinib or as rescue therapy in ibrutinib-resistant disease.
Introduction
The Bruton tyrosine kinase (BTK) inhibitor ibrutinib achieves high response rates in patients with chronic lymphocytic leukemia (CLL) and is approved for all lines of therapy.1-4 However, it has limitations. Under current practice, patients remain on ibrutinib indefinitely for disease control. Still, some patients discontinue therapy because of adverse events, with 1 study reporting a cumulative incidence of nonrelapse discontinuation of 15.6% at 18 months.5 Most importantly, patients with high-risk disease are at risk of disease progression on single-agent ibrutinib. For example, previously treated patients with chromosome 17p deletion had an estimated progression-free survival of 48% after 30 months on ibrutinib.6 Outcomes for patients who progress on ibrutinib are poor. A first report found a median overall survival of 3.1 months after ibrutinib failure,7 and a more recent study reported a median survival of 17.6 months after relapse with progressive CLL and 3.5 months after relapse with Richter transformation.5 Although checkpoint inhibitors have shown promise for the treatment of Richter transformation, they appear less active against progressive CLL in this context.8 There thus remains a need for effective therapies to treat ibrutinib-resistant CLL and to deepen ibrutinib-induced responses.
Despite recent excitement surrounding immunotherapy, the immune dysregulation characteristic of CLL has impeded the successful translation of many of these approaches. In particular, CLL is associated with multiple defects in T cells.9,10 CLL patients have elevated CD8 and CD4 T-cell counts, increased expression of T-cell exhaustion markers, and impairments in T-cell immune synapse formation, proliferative capacity, and cytotoxic activity.11-13 Furthermore, CLL is associated with T helper 2 (Th2) rather than Th1 polarized cytokine responses, which are inferior at coordinating antitumor activity.13 Recent investigations suggest that ibrutinib improves the immune dysfunction associated with CLL. Niemann et al observed decreases in chronic T-cell activation and exhaustion,14 and Dubovsky et al demonstrated a shift from Th2 to Th1 polarization with ibrutinib.15 Ibrutinib has also been shown to decrease frequency of immunosuppressive regulatory T cells in CLL.16,17 These findings suggest that ibrutinib may optimize the CLL immune system for immunotherapy. In fact, 1 study demonstrated improved CD19-directed chimeric antigen receptor T (CAR-T) cell expansion and engraftment in CLL patients previously treated with ibrutinib.18 Ibrutinib also increased CAR-T cell efficacy in murine models of acute lymphoblastic leukemia (ALL) and CLL.18 Furthermore, a recent study found that CD19-directed CAR-T cells induced responses in CLL patients after ibrutinib failure.19
CD19/CD3 bispecific antibodies (bsAb’s) recruit T cells to form cytolytic synapses with CD19+ tumor cells. Blinatumomab, a CD19/CD3 bsAb designed in the 54.1-kDa bispecific T-cell engager (BiTE) format, is approved by the US Food and Drug Administration for the treatment of relapsed or refractory B-cell precursor ALL20 and has shown promise for use in non-Hodgkin lymphoma.21,22 Although blinatumomab has demonstrated in vitro activity against CLL,23,24 its in vivo activity against CLL is largely unknown. A major disadvantage of blinatumomab is its 2.1-hour half-life and consequent requirement for continuous intravenous dosing. As a result, recent efforts have focused on designing bsAb’s in formats that replicate human immunoglobulin structures, resulting in half-lives close to those of traditional monoclonal antibodies.25,26
We hypothesized that CD19/CD3 bsAb’s hold promise for immunotherapy of CLL and developed a CD19-targeting bsAb in the 100-kDa single-chain Fv-Fc format (CD19/CD3-scFv-Fc). Because ibrutinib has been shown to improve T-cell function in CLL,14,15,18 we also hypothesized that bsAb’s would work well as adjuncts with ibrutinib and effectively target ibrutinib-resistant disease. To test our hypotheses, we investigated the activity of CD19/CD3-scFv-Fc using CLL samples from treatment-naïve and ibrutinib-treated patients, including samples obtained at disease progression.27
Patients, materials, and methods
Patients and clinical data
Peripheral blood mononuclear cells (PBMCs) were obtained with informed consent from treatment-naïve CLL and small lymphocytic lymphoma patients (www.clinicaltrials.gov, study #NCT00923507) and from patients treated with single-agent ibrutinib (#NCT01500733). Studies were approved by the Institutional Review Board. The ibrutinib study enrolled patients with TP53 aberration and patients over 65 years of age. Patients received ibrutinib 420 mg once daily until disease progression or intolerable side effects. Samples from treatment-naïve and ibrutinib-treated patients used in studies reported here were selected based on availability of viably frozen PBMCs; specifically, after 12 (±0.7) months on ibrutinib or at the time of clinically defined CLL progression in ibrutinib-treated patients. Cancer cell fractions of BTK and PLCG2 mutations in progressing patients were determined using digital droplet polymerase chain reaction.27 Clinical and laboratory data were collected from electronic medical records.
bsAb’s
Blinatumomab was concentrated from leftover infusion ready commercial supply (Amgen) using Amicon Ultra Centrifugal filters (Millipore) to between 0.5 and 1 mg/mL and stored at −80°C in single-use aliquots. The bsAb’s CD19/CD3-scFv-Fc and HER2/CD3-scFv-Fc were cloned, expressed, and purified as described.28 Supplemental Figure 1 (available on the Blood Web site) depicts bsAb format, construction, and pharmacokinetic properties.
In vitro cell cultures
Cryopreserved PBMCs were thawed and plated at 3 × 106 cells per mL in RPMI 1640 (Gibco) containing 10% fetal bovine serum, 1% penicillin-streptomycin, and 50 μM β-mercaptoethanol (Sigma). Cells were cultured with HER2/CD3-scFv-Fc, CD19/CD3-scFv-Fc, or blinatumomab at 6.6 nM, based on initial titration experiments (supplemental Figure 2A). Untreated wells containing cells and medium alone were left as controls; 60 IU/mL of interleukin-2 (IL-2) was added to cultures 4 to 12 hours after initial plating. Cells were cultured at 37°C and harvested at specified time points for analysis of CLL viability, synapse formation, and T-cell count, activation, and granzyme B expression. For proliferation assays, CLL PBMCs were labeled with CellTrace CFSE (carboxyfluorescein diacetate succinimidyl ester) Cell Proliferation Kit (Invitrogen) prior to culture.
In vivo murine studies
Experiments using NOD/SCID/IL2Rγnull (NSG) mice (Jackson Laboratory, stock #5557) were approved by the Institutional Animal Care and Use Committee. Patient-derived CLL xenografts were established as previously described,29 but without busulfan conditioning. After injection of 5 × 107 PBMCs, CLL engraftment was confirmed on day 3 by peripheral blood flow cytometry. bsAb’s were then injected intraperitoneally. To preserve molar equivalency, CD19/CD3-scFv-Fc and HER2/CD3-scFv-Fc were dosed at 0.5 mg/kg; blinatumomab was dosed at 0.25 mg/kg. CLL tumor burden was assessed in the peripheral blood on days 10 and 17, and in the spleen on day 17. Two to 5 mice were injected with cells from each CLL patient in each treatment group.
Flow cytometry
Cells were stained with commercial antibodies (BD Biosciences): CD4 (RPA-T4), CD8 (HIT8a), CD20 (L27), CD5 (L17F12), CD19 (HIB19), CD45 (HI30), CD69 (FN50), CD25 (M-A251), and granzyme B (GB11). Cell viability was assessed using LIVE/DEAD fixable violet stain (Invitrogen). We used LSRFortessa for in vitro and FACSCanto II for in vivo experiments (BD Biosciences). Experiments were analyzed with FlowJo (Version 10, TreeStar). For CLL viability analysis, CLL cells were identified as CD8−CD4−/CD5+CD20+, and T cells as either CD8+ or CD4+ (supplemental Figure 2B). Specific killing was calculated as: (%Untreated CLL Viability − % Treated CLL Viability)/(% Untreated CLL Viability) × 100. Absolute live cell counts were determined using AccuCount particles (Spherotech). Effector T-cell to target CLL cell (E:T) ratios were determined based on live cell frequencies. T-cell proliferation was reported based on percent change in mean fluorescence intensity (MFI) of CFSE signal in CD8 or CD4 cells using the formula: (CFSE MFI Untreated − CFSE MFI Treated)/(CFSE MFI Untreated) × 100. T-cell activation was assessed based on percent of live CD8 or CD4 cells staining for both CD25 and CD69. Intracellular staining for granzyme B was performed using GolgiPlug and Fixation/Permeabilization Solution kit (BD Biosciences). Expression was reported as percent of live CD8 or CD4 cells staining for granzyme B.
Mouse spleens were processed to single cell suspensions using 70-μm cell strainers (Corning), and both blood and spleen samples were treated with ACK lysis buffer (Quality Biological) prior to staining. CLL cells were identified as CD45+/CD8−CD4−/CD5+CD19+; T cells were identified as CD45+/CD8+ or CD45+/CD4+ (supplemental Figure 3). Blood CLL burden was reported as cells per microliter of blood using AccuCount particles (Spherotech). Spleen CLL burden was reported as percentage of CLL cells among all nucleated cells.
Multispectral fluorescence imaging
T-cell and CLL cell synapse formations were analyzed using Amnis Imagestream Mark II. PBMCs from treatment-naïve CLL patients were cultured for 15 minutes with CD19/CD3-scFv-Fc or HER2/CD3-scFv-Fc. Cells were stained with antibodies against CD4 (RPA-T4), CD8 (HIT8a), and CD20 (L27) (BD Biosciences). Images (5000–10 000 cells per sample) were captured at ×60 magnification. Ideas Software version 6.2 was used for offline analysis. Conjugates were identified using a gating strategy to include cells with areas larger than 130 units and aspect ratios lower than 0.8. CD8 or CD4 cells were identified by a positive fluorescence gate. To determine the count of immune synapse events, an “erode” masking tool was used. In brief, individual erode masks were used on T cells (CD8 or CD4) and CLL cells. Boolean logic was then applied to create 1 unique mask identifying fluorescent pixel overlap between T cells and CLL cells. Images positive for the mask were considered synapse positive.
Statistical analysis
To compare differences in response by treatment group either repeated-measures 1-way analysis of variance with Tukey’s multiple comparisons test or nonparametric Friedman test with Dunn’s multiple comparisons test were used, based on whether assumptions of normality were satisfied according to the Shapiro-Wilk normality test. In experiments where only 2 conditions were compared, either paired Student t test, unpaired Student t test, Wilcoxon matched-pairs signed rank test, or Mann-Whitney U test was used depending on sample pairing and normality of data. To assess treatment effect across mice in in vivo experiments, an unpaired Student t test was applied, taking into account the random batch effect from xenograft source. Results are reported and/or displayed as mean and 95% confidence interval (CI) or median and interquartile range (IQR) based on normality. Statistics were done using JMP and Graphpad Prism software.
Results
CD19/CD3-scFv-Fc has favorable pharmacokinetic properties and induces synapse formation between autologous T cells and CLL cells
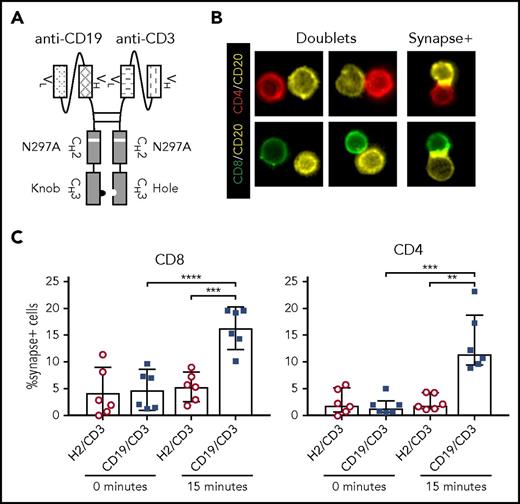
CD19/CD3-scFv-Fc was engineered by combining a human anti-human CD19 scFv with a humanized mouse anti-human CD3 scFv, along with a human immunoglobulin G1 Fc domain to enhance bsAb stability (Figure 1A; supplemental Figure 1). A nontargeting control bsAb, HER2/CD3-scFv-Fc, was made in the same format. The Fc domains were modified to promote dimerization of the 2 targeting moieties,30 and an N297A mutation was introduced to prevent Fc glycosylation and consequently Fc receptor and complement binding.31,32 The half-life of CD19/CD3-scFv-Fc was determined to be 121 (± 40) hours in mice, allowing for once-weekly dosing (supplemental Figure 1).
CD19/CD3-scFv-Fc promotes synapse formation between CLL cells and autologous T cells. CLL PBMCs from treatment-naïve patients were cultured with either CD19/CD3-scFv-Fc or HER2/CD3-scFv-Fc for 15 minutes. Cells were then stained with labels against CD8, CD4, and CD20, and frequency of CLL cell/T-cell synapses was measured using multispectral fluorescence imaging. (A) Schematic of CD19/CD3-scFv-Fc, illustrating the combination of human anti-human CD19 scFv and humanized mouse anti-human CD3 scFv via a mutated Fc domain of human immunoglobulin G1. The CH2 aglycosylation (N297A) and CH3 knob (S354C and T366W) and hole (Y349C, T366S, L368A, and Y407V) mutations in the Fc region are indicated. (B) Representative images of CLL cell/T-cell doublets, or cells captured in the same imaging frame not considered to be in synapse (doublets), and true CLL cell/T-cell synapses with overlapping CD20 and CD8 or CD4 signals (synapse+), at ×60 magnification. (C) Quantitative results depicting the percent of CD8 or CD4 T cells found in synapse with CLL cells by treatment condition. Mean and 95% CI are shown. Asterisks indicate statistical significance using paired Student t tests. **P < .01; ***P < .001; ****P < .0001. CD19/CD3, CD19/CD3-scFv-Fc; H2/CD3, HER2/CD3-scFv-Fc.
CD19/CD3-scFv-Fc promotes synapse formation between CLL cells and autologous T cells. CLL PBMCs from treatment-naïve patients were cultured with either CD19/CD3-scFv-Fc or HER2/CD3-scFv-Fc for 15 minutes. Cells were then stained with labels against CD8, CD4, and CD20, and frequency of CLL cell/T-cell synapses was measured using multispectral fluorescence imaging. (A) Schematic of CD19/CD3-scFv-Fc, illustrating the combination of human anti-human CD19 scFv and humanized mouse anti-human CD3 scFv via a mutated Fc domain of human immunoglobulin G1. The CH2 aglycosylation (N297A) and CH3 knob (S354C and T366W) and hole (Y349C, T366S, L368A, and Y407V) mutations in the Fc region are indicated. (B) Representative images of CLL cell/T-cell doublets, or cells captured in the same imaging frame not considered to be in synapse (doublets), and true CLL cell/T-cell synapses with overlapping CD20 and CD8 or CD4 signals (synapse+), at ×60 magnification. (C) Quantitative results depicting the percent of CD8 or CD4 T cells found in synapse with CLL cells by treatment condition. Mean and 95% CI are shown. Asterisks indicate statistical significance using paired Student t tests. **P < .01; ***P < .001; ****P < .0001. CD19/CD3, CD19/CD3-scFv-Fc; H2/CD3, HER2/CD3-scFv-Fc.
To demonstrate that CD19/CD3-scFv-Fc can concurrently engage T cells and CLL cells, we analyzed immune synapse formation in cultures of PBMCs from treatment-naïve patients using multispectral fluorescence imaging (Figure 1B). After 15 minutes of exposure to CD19/CD3-scFv-Fc, we observed specific synapse formation between CLL cells and a mean 16.3% (95% CI, 12.3-20.3) of CD8 T cells and 13.5% (7.8-19.2) of CD4 T cells. In contrast, when cultured with HER2/CD3-scFv-Fc, only 5.4% (2.6-8.1) of CD8 and 2.5% (1.0-4.0) of CD4 T cells formed synapses with CLL cells (P ≤ .003; Figure 1C), in line with the frequency of synapse formation at the start of experiments.
CD19/CD3 bsAb’s kill CLL cells and promote T-cell expansion
To assess the activity of CD19/CD3 bsAb’s against CLL cells, we cultured PBMCs from treatment-naïve CLL patients (supplemental Table 1) with CD19/CD3-scFv-Fc. For comparison, we included equimolar concentrations of blinatumomab, which also binds CD19, and our nontargeting control HER2/CD3-scFv-Fc. At the beginning of experiments, effector T-cell (CD8 and CD4) to target CLL cell (E:T) ratios ranged from 1:12 to 1:181, consistent with typical T- to B-cell ratios in CLL patients.
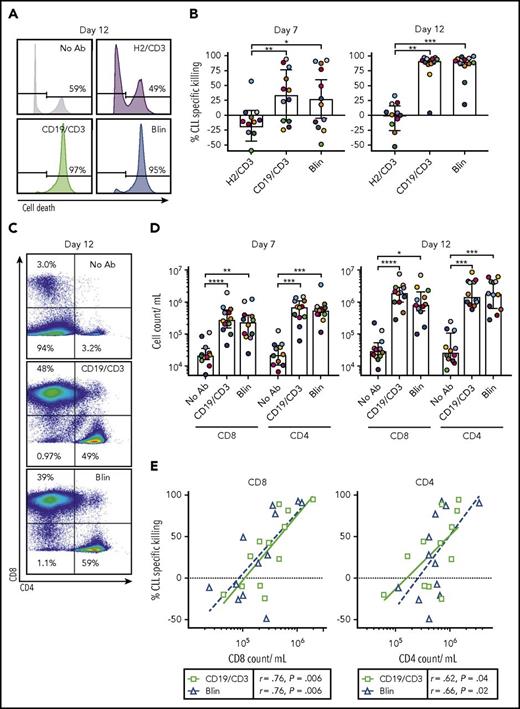
Analyzing treatment effects after 7 and 12 days using flow cytometry, we observed time-dependent killing of CLL cells in the presence of both CD19-targeting bsAb’s (Figure 2A-B). After 12 days, median CLL cell specific killing was 91.6% (IQR, 86.4-95.3) with CD19/CD3-scFv-Fc, and 90.4% (84.4-95.8) with blinatumomab. As expected, HER2/CD3-scFv-Fc did not induce CLL cell killing. Although no difference in antileukemic activity was observed between CD19/CD3-scFv-Fc and blinatumomab treatment groups (P > .99), both CD19-targeting bsAb’s demonstrated increased activity compared with HER2/CD3-scFv-Fc (P ≤ .0016).
CD19/CD3 bsAb’s induce CLL cell death and expansion of autologous T cells in vitro. PBMCs from treatment-naïve CLL patients were cultured with either CD19/CD3-scFv-Fc, blinatumomab, HER2/CD3-scFv-Fc, or medium alone at 6.6 nM (n = 12). Response to treatment was analyzed after 7 and 12 days by flow cytometry. (A) Representative histograms after 12 days of culture. Percent dead CLL cells (LIVE/DEAD positive) is indicated. (B) Percent CLL specific killing by treatment condition after 7 and 12 days in culture, calculated as: (%Untreated CLL Viability – % Treated CLL Viability)/(% Untreated CLL Viability) × 100. Untreated samples had average (range) CLL viability of 62% (35% to 82%) on day 7 and 64% (40% to 79%) on day 12. (C) Representative flow cytometry plots showing CD8 and CD4 staining in live cells after 12 days in culture. Frequencies of CD8+, CD4+, and CD8−/CD4− (composed mostly of CLL cells) populations are shown. (D) CD8 and CD4 cell counts after 7 and 12 days in culture. (E) Correlation of CLL specific killing by treatment condition with either CD8 or CD4 T-cell count after 7 days in culture by Spearman’s rank coefficient. Each dot represents 1 sample and treatment condition; color and shape indicate treatment. Color of data points in panels B and D represent patient-matched samples and correspond with colors in supplemental Table 1; median and IQRs are shown. Asterisks indicate statistical significance using Dunn’s multiple comparisons test. *P < .05; **P < .01; ***P < .001; ****P < .0001. Blin, blinatumomab; CD19/CD3, CD19/CD3-scFv-Fc; H2/CD3, HER2/CD3-scFv-Fc; No Ab, medium alone.
CD19/CD3 bsAb’s induce CLL cell death and expansion of autologous T cells in vitro. PBMCs from treatment-naïve CLL patients were cultured with either CD19/CD3-scFv-Fc, blinatumomab, HER2/CD3-scFv-Fc, or medium alone at 6.6 nM (n = 12). Response to treatment was analyzed after 7 and 12 days by flow cytometry. (A) Representative histograms after 12 days of culture. Percent dead CLL cells (LIVE/DEAD positive) is indicated. (B) Percent CLL specific killing by treatment condition after 7 and 12 days in culture, calculated as: (%Untreated CLL Viability – % Treated CLL Viability)/(% Untreated CLL Viability) × 100. Untreated samples had average (range) CLL viability of 62% (35% to 82%) on day 7 and 64% (40% to 79%) on day 12. (C) Representative flow cytometry plots showing CD8 and CD4 staining in live cells after 12 days in culture. Frequencies of CD8+, CD4+, and CD8−/CD4− (composed mostly of CLL cells) populations are shown. (D) CD8 and CD4 cell counts after 7 and 12 days in culture. (E) Correlation of CLL specific killing by treatment condition with either CD8 or CD4 T-cell count after 7 days in culture by Spearman’s rank coefficient. Each dot represents 1 sample and treatment condition; color and shape indicate treatment. Color of data points in panels B and D represent patient-matched samples and correspond with colors in supplemental Table 1; median and IQRs are shown. Asterisks indicate statistical significance using Dunn’s multiple comparisons test. *P < .05; **P < .01; ***P < .001; ****P < .0001. Blin, blinatumomab; CD19/CD3, CD19/CD3-scFv-Fc; H2/CD3, HER2/CD3-scFv-Fc; No Ab, medium alone.
Both CD8 and CD4 T-cell counts increased in a time-dependent manner in the presence of bsAb’s (Figure 2C-D), consistent with previous reports using blinatumomab.23 Compared with medium-only controls, CD19/CD3-scFv-Fc increased CD8 T-cell counts by a median 13.7-fold on day 7 (P < .0001) and 56.4-fold on day 12 (P < .0001); median increase with blinatumomab was 7.1-fold on day 7 (P = .002) and 31.6-fold on day 12 (P = .04). CD4 T-cell counts also increased with both CD19/CD3-scFv-Fc (median increase 29.0-fold, day 7; 51.8-fold, day 12) and blinatumomab (median increase 27.6-fold, day 7; 51.5-fold, day 12) (P ≤ .0005). Consistent with a T-cell-dependent mechanism of action, CLL cell killing with CD19/CD3-scFv-Fc and blinatumomab was tightly correlated with both CD8 and CD4 T-cell counts (Figure 2E).
CD19/CD3 bsAb’s drive autologous T-cell proliferation, activation, and granzyme B expression
Having observed an absolute increase in autologous T cells with bsAb’s, we sought to directly measure T-cell proliferation using CFSE dilution experiments. After 5 and 7 days, robust proliferation of both CD8 and CD4 T cells occurred in cultures treated with CD19/CD3-scFv-Fc or blinatumomab, whereas no proliferation occurred in untreated controls (Figure 3A-B).
CD19/CD3 bsAb’s potentiate autologous T-cell proliferation, activation, and granzyme B expression. CLL PBMCs from treatment-naïve patients were cultured with either CD19/CD3-scFv-Fc, blinatumomab, or medium alone. To assess T-cell proliferation, PBMCs were stained with CFSE prior to culture. (A) CFSE staining in CD8 T cells from 1 representative patient after 5 and 7 days in culture. (B) CD8 and CD4 T-cell proliferation, depicted as percent decrease in CFSE MFI from the medium-only condition (n = 6). (C) Representative flow cytometry contour plots of CD25 and CD69 staining in CD8 T cells after 7 days in culture. Percent CD25+/CD69+ cells are shown. (D) CD8 and CD4 T-cell activation reported as percent CD25+/CD69+ cells after 7 days (n = 7). (E) Representative flow cytometry contour plots showing granzyme B expression in CD8 T cells after 7 days in culture. Percent granzyme B+ cells are shown. (F) Granzyme B expression in CD8 and CD4 T cells, reported as percent granzyme B+ cells, after 7 days (n = 10). For panels B and D, significance using paired Student t tests and Tukey’s multiple comparison tests are shown, respectively, along with mean and 95% CIs. For panel F, significance using Dunn’s multiple comparisons test and median and IQRs are shown, because of nonnormal distribution of data. Colors of data points in (B,D,F) correspond to colors in supplemental Table 1. *P < .05; **P < .01; ***P < .001; ****P < .0001. Grnz, granzyme.
CD19/CD3 bsAb’s potentiate autologous T-cell proliferation, activation, and granzyme B expression. CLL PBMCs from treatment-naïve patients were cultured with either CD19/CD3-scFv-Fc, blinatumomab, or medium alone. To assess T-cell proliferation, PBMCs were stained with CFSE prior to culture. (A) CFSE staining in CD8 T cells from 1 representative patient after 5 and 7 days in culture. (B) CD8 and CD4 T-cell proliferation, depicted as percent decrease in CFSE MFI from the medium-only condition (n = 6). (C) Representative flow cytometry contour plots of CD25 and CD69 staining in CD8 T cells after 7 days in culture. Percent CD25+/CD69+ cells are shown. (D) CD8 and CD4 T-cell activation reported as percent CD25+/CD69+ cells after 7 days (n = 7). (E) Representative flow cytometry contour plots showing granzyme B expression in CD8 T cells after 7 days in culture. Percent granzyme B+ cells are shown. (F) Granzyme B expression in CD8 and CD4 T cells, reported as percent granzyme B+ cells, after 7 days (n = 10). For panels B and D, significance using paired Student t tests and Tukey’s multiple comparison tests are shown, respectively, along with mean and 95% CIs. For panel F, significance using Dunn’s multiple comparisons test and median and IQRs are shown, because of nonnormal distribution of data. Colors of data points in (B,D,F) correspond to colors in supplemental Table 1. *P < .05; **P < .01; ***P < .001; ****P < .0001. Grnz, granzyme.
We next measured T-cell activation, assessed as the percent CD25+/CD69+ T cells after 7 days in culture. CD19/CD3 bsAb’s induced significant CD8 T-cell activation, with mean CD25+/CD69+ CD8 T cells increasing from 8.3% (95% CI, 2.9-13.7) in medium-only conditions to 68.4% (51.3-85.4, P = .0006) and 77.5% (65.2-89.8, P < .0001) with CD19/CD3-scFv-Fc and blinatumomab, respectively (Figure 3C-D). The frequency of activated CD4 T cells also increased with both bsAb’s compared with medium-only conditions (P ≤ .004).
Granzyme B release from effector cells is important for the cytotoxic activity of bsAb’s.33 In medium-only cultures of treatment-naïve PBMCs, granzyme B expression was detected in CD8 T cells but was largely absent in CD4 T cells (Figure 3E-F). Granzyme B expression increased in both CD8 and CD4 T cells after 7 days with CD19/CD3-scFv-Fc compared with medium-only conditions (P ≤ .0035), with a median 99.0% (IQR, 97.6-99.7) of CD8 T cells and 88.4% (60.9-94.8) of CD4 T cells positive for granzyme B. Similarly, blinatumomab induced increases in granzyme B in both CD8 and CD4 T cells compared with medium-only controls (P ≤ .0035). Taken together, these data indicate that both CD19/CD3-scFv-Fc and blinatumomab effectively induce autologous T-cell proliferation, activation, and granzyme B expression in concert with CLL cell killing.
CD19/CD3-scFv-Fc eliminates CLL cells in vivo
To investigate the efficacy of CD19/CD3 bsAb’s against CLL in vivo, we chose a patient-derived xenograft model.29,34 This model recapitulates interactions between the patient’s CLL and T cells in an in vivo setting and relies exclusively on autologous T cells as effectors of antibody-dependent cytotoxicity. PBMCs from treatment-naïve patients were injected into NSG mice on day 0. Following confirmation of human cell engraftment on day 3, we dosed mice with either CD19/CD3-scFv-Fc, blinatumomab, or HER2/CD3-scFv-Fc (nontargeting control) once weekly for 2 weeks. A cohort of engrafted mice was left untreated as an additional control. Overall, the experiment included 51 mice injected with cells from 4 different patients (Figure 4). Consistent with our in vitro data, treatment with HER2/CD3-scFv-Fc had no antitumor effects (Figure 4B-C; compare no antibody control and HER2/CD3-scFv-Fc). In contrast, 1 injection of CD19/CD3-scFv-Fc reduced leukemic cell counts by >90% compared with HER2/CD3-scFv-Fc treated mice (Figure 4B), and 2 injections eliminated >99% of CLL cells in the blood and >98% of CLL cells in the spleen (P ≤ .003; Figure 4C-D). We observed inter- and intrapatient variability in cell engraftment, as expected in this model.29,34,35 However, efficacy of CD19/CD3-scFv-Fc was demonstrated consistently across all patients and mice tested. Meanwhile, blinatumomab administered once weekly had no antitumor effects.
CD19/CD3-scFv-Fc eliminates CLL cells in patient-derived xenografts with once-weekly dosing. PBMCs from 4 treatment-naïve patients with E:T cell ratios of 1:50 to 1:181 were injected into NSG mice on experimental day 0. Cell engraftment was confirmed on day 3 by flow cytometry. Mice were then treated once weekly with either HER2/CD3-scFv-Fc, CD19/CD3-scFv-Fc, or blinatumomab; an untreated group was left for controls. Two to 5 mice were included for each patient and treatment group (n = 4 patients, n = 51 mice). (A) Representative flow cytometry analysis from the peripheral blood of mice treated with either HER2/CD3-scFv-Fc or CD19/CD3-scFv-Fc at experimental days 3 (top, pretreatment) and 17 (bottom, posttreatment). Gates show CD19+/CD5+ CLL population of live, CD45+, CD8−/CD4− cells. (B) Mean CLL cell count in the peripheral blood over time by treatment group. Arrows denote bsAb injections. (C) CLL cell count in the peripheral blood at experimental day 17. (D) CLL cell count in the spleen at experimental day 17. For panels C and D, each dot represents 1 animal; color code denotes patient source of xenografted cells and correlates with colors in supplemental Table 1. Mean and 95% CI are shown. Asterisks denote significance using unpaired Student t tests, taking into account the random batch effect from xenograft source. *P < .05; **P < .01; ***P < .001; ****P < .0001.
CD19/CD3-scFv-Fc eliminates CLL cells in patient-derived xenografts with once-weekly dosing. PBMCs from 4 treatment-naïve patients with E:T cell ratios of 1:50 to 1:181 were injected into NSG mice on experimental day 0. Cell engraftment was confirmed on day 3 by flow cytometry. Mice were then treated once weekly with either HER2/CD3-scFv-Fc, CD19/CD3-scFv-Fc, or blinatumomab; an untreated group was left for controls. Two to 5 mice were included for each patient and treatment group (n = 4 patients, n = 51 mice). (A) Representative flow cytometry analysis from the peripheral blood of mice treated with either HER2/CD3-scFv-Fc or CD19/CD3-scFv-Fc at experimental days 3 (top, pretreatment) and 17 (bottom, posttreatment). Gates show CD19+/CD5+ CLL population of live, CD45+, CD8−/CD4− cells. (B) Mean CLL cell count in the peripheral blood over time by treatment group. Arrows denote bsAb injections. (C) CLL cell count in the peripheral blood at experimental day 17. (D) CLL cell count in the spleen at experimental day 17. For panels C and D, each dot represents 1 animal; color code denotes patient source of xenografted cells and correlates with colors in supplemental Table 1. Mean and 95% CI are shown. Asterisks denote significance using unpaired Student t tests, taking into account the random batch effect from xenograft source. *P < .05; **P < .01; ***P < .001; ****P < .0001.
The lack of activity of blinatumomab in our CLL xenograft model contrasts with data in an ALL model, where blinatumomab dosed daily for 5 days was effective against NALM-6 xenografts in mice.36 We therefore explored activity of blinatumomab dosed for 8 consecutive days (days 3-10), followed by 3 additional doses (days 12-14). Once again, blinatumomab treatment failed to reduce CLL burden, whereas weekly treatment with CD19/CD3-scFv-Fc eliminated >99% of tumor cells (supplemental Figure 4).
T cells from patients treated with ibrutinib mediate effective antibody-dependent cytotoxicity
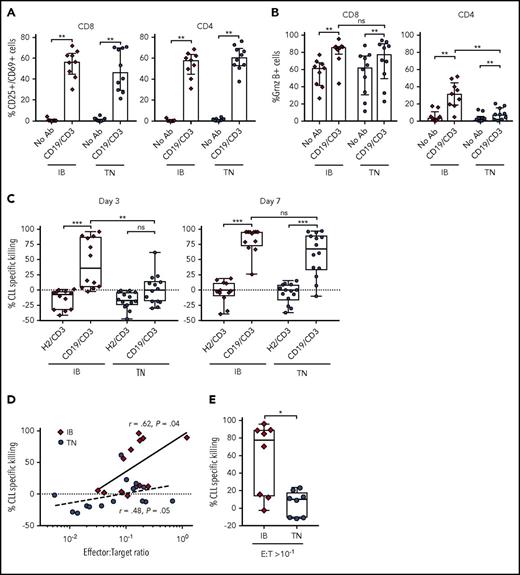
Ibrutinib is thought to ameliorate immune dysfunction in CLL patients14,16 and can enhance response to anti-CD19 CAR-T cell therapy.18 Conversely, ibrutinib is known to inhibit IL-2-inducible kinase15 and reduce cytokine release from T cells.37 The latter effects could dampen the T-cell response to bsAb’s. We therefore investigated the ability of CD19/CD3-scFv-Fc to activate T cells from patients treated with ibrutinib for 12 months (supplemental Table 2). Samples from treatment-naïve CLL patients were studied concurrently (supplemental Table 1). Compared with medium-only controls, CD19/CD3-scFv-Fc significantly increased the percentage of CD25+/CD69+ activated CD8 (median 56.6% vs 0.83%, P = .004) and CD4 (median 58.2% vs 0.47%, P = .004) T cells in samples from ibrutinib-treated patients after 48 hours (Figure 5A). A comparable increase in T-cell activation was observed in treatment-naïve patients.
CD19/CD3-scFv-Fc demonstrates activity against CLL cells from ibrutinib-treated patients. PBMCs from patients receiving ibrutinib for 12 months and from treatment-naïve CLL patients were cultured with CD19/CD3-scFv-Fc, HER2/CD3-scFv-Fc, or medium alone. Percent CD25+/CD69+ cells (A); percent granzyme B+ cells (B), both after 48 hours (n = 9, ibrutinib-treated; n = 10, treatment-naïve). (C) CLL specific killing by treatment group after 3 and 7 days, respectively (n = 12, ibrutinib-treated; n = 14, treatment-naïve). The average (range) viability of medium-only control samples from ibrutinib treated patients was 65% (48% to 85%) on day 3 and 68% (49% to 84%) on day 7, and from treatment-naïve patients 65% (46% to 86%) on day 3 and 68% (52% to 89%) on day 7. (D) Spearman’s correlation between initial E:T ratios in PBMC samples used and CLL cell specific killing by CD19/CD3-scFv-Fc after 3 days in culture. Solid and dotted lines show regressions for ibrutinib-treated and treatment-naïve samples, respectively. (E) CLL specific killing at 3 days for samples with initial E:T ratios of >1:10 (n = 8, ibrutinib-treated; n = 8, treatment-naïve). Median and IQR are shown. Asterisks in panels A-C denote significance using Wilcoxon matched-pairs signed rank test for comparisons within cohorts or Mann-Whitney U test for comparisons across cohorts. Mann-Whitney U test was used for panel E. *P < .05; **P < .01; ***P < .001; ****P < .0001; ns, P > .05. IB, ibrutinib-treated; TN, treatment naïve.
CD19/CD3-scFv-Fc demonstrates activity against CLL cells from ibrutinib-treated patients. PBMCs from patients receiving ibrutinib for 12 months and from treatment-naïve CLL patients were cultured with CD19/CD3-scFv-Fc, HER2/CD3-scFv-Fc, or medium alone. Percent CD25+/CD69+ cells (A); percent granzyme B+ cells (B), both after 48 hours (n = 9, ibrutinib-treated; n = 10, treatment-naïve). (C) CLL specific killing by treatment group after 3 and 7 days, respectively (n = 12, ibrutinib-treated; n = 14, treatment-naïve). The average (range) viability of medium-only control samples from ibrutinib treated patients was 65% (48% to 85%) on day 3 and 68% (49% to 84%) on day 7, and from treatment-naïve patients 65% (46% to 86%) on day 3 and 68% (52% to 89%) on day 7. (D) Spearman’s correlation between initial E:T ratios in PBMC samples used and CLL cell specific killing by CD19/CD3-scFv-Fc after 3 days in culture. Solid and dotted lines show regressions for ibrutinib-treated and treatment-naïve samples, respectively. (E) CLL specific killing at 3 days for samples with initial E:T ratios of >1:10 (n = 8, ibrutinib-treated; n = 8, treatment-naïve). Median and IQR are shown. Asterisks in panels A-C denote significance using Wilcoxon matched-pairs signed rank test for comparisons within cohorts or Mann-Whitney U test for comparisons across cohorts. Mann-Whitney U test was used for panel E. *P < .05; **P < .01; ***P < .001; ****P < .0001; ns, P > .05. IB, ibrutinib-treated; TN, treatment naïve.
CD19/CD3-scFv-Fc also significantly increased T-cell granzyme B expression (Figure 5B). Similar increases in the percentage of granzyme B–expressing CD8 T cells were seen in both ibrutinib-treated and treatment-naïve patients. Likewise, the proportion of granzyme B–expressing CD4 T cells increased with CD19/CD3-scFv-Fc for both ibrutinib-treated and treatment-naïve patients. However, CD4 T cells from ibrutinib-treated patients responded more strongly to CD19/CD3-scFv-Fc than cells from treatment-naïve patients (P = .002). Taken together, these data indicate that ibrutinib treatment does not diminish but may possibly enhance T-cell response to bsAb’s.
CD19/CD3-scFv-Fc demonstrates potent cytotoxic activity against ibrutinib-treated CLL cells
We next sought to assess the efficacy of CD19/CD3-scFv-Fc in PBMCs obtained from patients on ibrutinib. In ibrutinib-treated samples, CD19/CD3-scFv-Fc exposure was associated with significant CLL cell killing compared with HER2/CD3-scFv-Fc, with a median specific killing of 36.2% vs −7.5% after 3 days and 94.3% vs −1.6% after 7 days, respectively (P ≤ .0005; Figure 5C). In contrast, increased antibody-dependent killing of treatment-naïve CLL cells was only achieved after 7 days. The difference in CLL cell killing in samples from ibrutinib-treated compared with treatment-naïve patients after 3 days was statistically significant (median 36.2% vs 0.4%, P = .009). After 7 days, CLL killing by CD19/CD3-scFv-Fc increased in both cohorts, with a remaining trend toward greater killing in samples from ibrutinib-treated patients (median 94.3% vs 67.7%, P = .095). As expected, there was a positive correlation between E:T ratios and 72-hour CLL cell specific killing for both ibrutinib-treated (r = 0.62, P = .04) and treatment-naïve patients (r = 0.48, P = .05; Figure 5D). Overall, ibrutinib-treated patients had significantly higher E:T ratios than treatment-naïve patients (median 1:7 vs 1:14.6, P = .02). To determine if E:T ratio was the driving mechanism for this differential response, we directly compared samples from each cohort with relatively favorable E:T ratios of at least 1 T cell for every 10 CLL cells (Figure 5E). CLL specific killing remained significantly greater in the ibrutinib-treated cohort compared with the treatment-naïve cohort in this analysis (median 77.7% vs 10.0%, P = .02). Taken together, these data suggest that T cells from ibrutinib-treated patients are able to mount faster cytotoxic responses than T cells from ibrutinib-naïve patients.
CD19/CD3-scFv-Fc demonstrates activity against ibrutinib-resistant CLL
Ibrutinib-resistant CLL is often associated with mutations in BTK and/or PLCG2, and its treatment remains challenging.5,38,39 We thus explored the activity of CD19/CD3-scFv-Fc against CLL cells from 4 patients progressing with BTK and/or PLCG2 mutations 34 to 49 months after the start of ibrutinib (supplemental Table 2; supplemental Figure 5). CD19/CD3-scFv-Fc induced time- and antibody-dependent in vitro killing of ibrutinib-resistant CLL cells, eliminating >90% of tumor cells after 7 days (Figure 6A-B).
CD19/CD3-scFv-Fc eliminates ibrutinib-resistant CLL cells, both in vitro and in patient-derived xenografts in vivo. PBMCs from 4 CLL patients with acquired ibrutinib-resistance were treated with CD19/CD3-scFv-Fc or HER2/CD3-scFv-Fc, either in culture (A-B) or after transfer into NSG mice (C-E). (A) CLL cell specific killing in culture over time. Bold lines indicate mean killing of 4 ibrutinib-resistant samples by treatment group; dashed lines indicate killing for each sample. (B) In vitro CLL cell specific killing for each ibrutinib-resistant patient at the end of culture. Medium-only control samples had average (range) CLL viability of 68% (52% to 78%). Asterisks indicate results of a paired Student t test; mean and 95% CIs are shown. (C) Once-weekly treatment with CD19/CD3-scFv-Fc or HER2/CD3-scFv-Fc was initiated 3 days after PBMC transfer into NSG mice. CLL counts in the peripheral blood are shown, where dashed lines indicate mean CLL counts by treatment of each xenograft source used and bold lines indicate the mean of all mice in the same treatment group (n = 4 patients; n = 37 mice). (D) CLL counts in the peripheral blood at experimental day 10. (E) CLL counts in the spleen at experimental day 17. Dots in panels D-E represent 1 animal, colors in panels B and D-E indicate patient source of xenografted cells and correspond to colors in supplemental Table 2. Mean and 95% CI are shown. Asterisks denote significance using unpaired Student t tests, taking into account the random batch effect from xenograft source. *P < .05; **P < .01; ***P < .001; ****P < .0001.
CD19/CD3-scFv-Fc eliminates ibrutinib-resistant CLL cells, both in vitro and in patient-derived xenografts in vivo. PBMCs from 4 CLL patients with acquired ibrutinib-resistance were treated with CD19/CD3-scFv-Fc or HER2/CD3-scFv-Fc, either in culture (A-B) or after transfer into NSG mice (C-E). (A) CLL cell specific killing in culture over time. Bold lines indicate mean killing of 4 ibrutinib-resistant samples by treatment group; dashed lines indicate killing for each sample. (B) In vitro CLL cell specific killing for each ibrutinib-resistant patient at the end of culture. Medium-only control samples had average (range) CLL viability of 68% (52% to 78%). Asterisks indicate results of a paired Student t test; mean and 95% CIs are shown. (C) Once-weekly treatment with CD19/CD3-scFv-Fc or HER2/CD3-scFv-Fc was initiated 3 days after PBMC transfer into NSG mice. CLL counts in the peripheral blood are shown, where dashed lines indicate mean CLL counts by treatment of each xenograft source used and bold lines indicate the mean of all mice in the same treatment group (n = 4 patients; n = 37 mice). (D) CLL counts in the peripheral blood at experimental day 10. (E) CLL counts in the spleen at experimental day 17. Dots in panels D-E represent 1 animal, colors in panels B and D-E indicate patient source of xenografted cells and correspond to colors in supplemental Table 2. Mean and 95% CI are shown. Asterisks denote significance using unpaired Student t tests, taking into account the random batch effect from xenograft source. *P < .05; **P < .01; ***P < .001; ****P < .0001.
We next transferred PBMCs from the 4 ibrutinib-resistant patients into NSG mice (n = 37 mice). Following confirmation of engraftment 3 days after cell injection, we treated mice once weekly with either CD19/CD3-scFv-Fc or HER2/CD3-scFv-Fc. One dose of CD19/CD3-scFv-Fc reduced circulating CLL cells by >93% compared with HER2/CD3-scFv-Fc (range 93.2-99.6, P < .0001; Figure 6C-D). After 2 doses of CD19/CD3-scFv-Fc, mice engrafted with cells from 3 of 4 patients demonstrated >96.9% reduction in CLL cells in the spleen, and CLL cells from the fourth patient were reduced by 73.2% (Figure 6E). The overall decrease in the spleen CLL burden with CD19/CD3-scFv-Fc compared with HER2/CD3-scFv-Fc treatment was highly significant (P < .0001). Taken together, these data demonstrate the ability of CD19/CD3-scFv-Fc to eliminate ibrutinib-resistant CLL cells in vitro and in vivo.
Discussion
Our results assert the promise of bsAb’s for the treatment of CLL. CD19/CD3-scFv-Fc achieved CLL cell killing and promoted T-cell expansion and activation in vitro, comparable to the CD19/CD3 BiTE construct, blinatumomab. CD19/CD3-scFv-Fc also demonstrated impressive in vivo activity against treatment-naïve CLL cells in the patient-derived CLL xenograft mouse model, eliminating >99% of circulating tumor cells with once-weekly treatment. In contrast, blinatumomab had no anti-CLL activity in vivo, even when given once daily. Endowed with an Fc domain, CD19/CD3-scFv-Fc has an estimated half-life that is ∼60-fold longer than blinatumomab and is appropriate for once-weekly dosing. In contrast to commonly used therapeutic monoclonal antibodies, the Fc domain of CD19/CD3-scFv-Fc is aglycosylated, preventing Fc receptor binding or complement activation. As for other bsAb’s using similar designs, activity is dependent on T-cell recruitment.25,26
The lack of blinatumomab activity in our CLL xenograft model contrasts with data in an ALL model, where blinatumomab dosed daily for 5 days with concomitant adoptive transfer of healthy donor PBMCs was effective against NALM-6 xenografts in mice.36 However, there were notable differences between the models. First, the NALM-6 model introduced T cells to achieve a highly favorable E:T ratio of ∼100:1, and efficacy was lost when E:T ratio was reduced to 10:1.36 In contrast, our experiments relied on autologous T cells in patient PBMC samples, with starting E:T ratios ranging from 1:50 to 1:181. Second, the NALM-6 model used healthy donor T cells, which are known to have superior cytotoxic activity compared with T cells from CLL patients and can mount allogeneic reactions against tumor cells. Our model thus better recapitulates the immune microenvironment and E:T ratios encountered in CLL patients. However, as CLL xenografts do not lead to demise of the host animal, our model is not suitable for survival analysis.29,35,40 Nevertheless, the degree of antitumor response with CD19/CD3-scFv-Fc, reaching >98% with just 2 once-weekly injections, demonstrates the potency of the bsAb.
To date, CD19/CD3 bsAb’s have been primarily investigated for use in ALL and non-Hodgkin lymphoma.20-22 The clinical utility of bsAb’s in CLL remains largely unknown. Although ibrutinib is now established as an effective and well-tolerated treatment of CLL, there remains a need for new therapies. In particular, therapies that work effectively as adjuncts with ibrutinib could deepen responses, thereby shortening treatment duration and extending progression-free survival. Our results suggest that bsAb’s may be valuable partners for combination immunotherapy with ibrutinib. In samples from patients being treated with ibrutinib, CD19/CD3-scFv-Fc effectively recruited autologous T cells to kill CLL cells. Notably, bsAb-dependent killing of CLL cells was more rapid in samples from patients on ibrutinib than in samples from treatment-naïve patients. Although not fully understood, the increase in activity does not appear to be attributable merely to E:T ratios and suggests improvements in cytotoxic potential of T cells in patients on ibrutinib. Similar observations have been made with CAR-T cell therapies18 and may relate to improvements in the immune-microenvironment reported in CLL patients on ibrutinib.14,16
Disease progression on ibrutinib carries a poor prognosis,5,7,27 and treatments capable of eliminating ibrutinib-resistant disease are badly needed. Our results indicate that CD19/CD3-scFv-Fc is highly effective against CLL cells from patients with acquired ibrutinib resistance, both in vitro and in vivo. Encouragingly, we found activity of the bsAb against tumor cells from several patients carrying classic mutations in the 2 genes most commonly implicated in ibrutinib resistance, BTK and PLCG2.
Our results provide evidence of CD19/CD3-scFv-Fc activity against CLL and more broadly support the exploration of bsAb’s as a strategy to recruit T cells against CLL cells. We show the efficacy of this approach not only as immunotherapy in combination with ibrutinib, but also as rescue therapy in ibrutinib-resistant disease. In contrast to CAR-T cells, another T-cell redirection strategy that has demonstrated activity against CLL,18,19 bsAb’s are available “off-the-shelf” and are therefore not encumbered by the manufacturing requirements of cellular therapies. Furthermore, the improved pharmacokinetics of newer-generation bsAb’s facilitates broad clinical translation. We believe that our findings justify clinical investigation of T-cell-recruiting bsAb’s in combination with ibrutinib and for the treatment of ibrutinib-resistant disease.
Presented in abstract form at the 59th annual meeting of the American Society of Hematology, Atlanta, GA, 11 December 2017.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank their patients for participating and donating samples to make this research possible; Susan Soto and Pia Nierman for protocol support; Leigh Samsel from the National Heart, Lung, and Blood Institute Flow Cytometry Core Facility for advice and assistance; and Pharmacyclics for providing ibrutinib, research support, and comments on a draft of this manuscript.
This work was funded by the Intramural Research Program of the National Heart, Lung, and Blood Institute (A.W.) and the National Cancer Institute, National Institutes of Health (grant R01 CA181258) (C.R.). H.R.R. was supported by the National Institutes of Health Medical Research Scholars Program, a public-private partnership supported jointly by the National Institutes of Health and generous contributions to the Foundation for the National Institutes of Health from the Doris Duke Charitable Foundation, the American Association for Dental Research, the Colgate-Palmolive Company, Genentech, and alumni of student research programs and other individual supporters via contributions to the Foundation for the National Institutes of Health (https://fnih.org/what-we-do/current-education-and-training-programs/mrsp).
Authorship
Contribution: H.R.R., S.B., J.Q., S.E.M.H., C.R., and A.W. were responsible for concept and design; I.E.A. and C.S. were responsible for conduct of the clinical trial; H.R.R., J.Q., S.B., E.M.C., I.E.A., C.S., E.L.D., C.N., and C.U. performed acquisition of data; K.K. provided technical support; J.Q., N.S.S., and C.R. were responsible for provision of critical reagents; H.R.R., J.Q., S.B., E.M.C., S.E.M.H., C.R., A.W., C.N., and C.U. performed analysis and interpretation of data; all authors participated in writing, review, and/or revision of the manuscript; and A.W., S.E.M.H., S.B., and C.R. were responsible for study supervision.
Conflict-of-interest disclosure: A.W. received research funding from Pharmacyclics LLC, an AbbVie Company. The remaining authors declare no competing financial interests.
Correspondence: Adrian Wiestner, Hematology Branch, National Heart, Lung, and Blood Institute, National Institutes of Health, Building 10, CRC 3-5140, 10 Center Dr, Bethesda, MD 20892-1202; e-mail: wiestnera@mail.nih.gov.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal