Generating effective immune responses in patients with chronic lymphocytic leukemia (CLL) without the concomitant toxicity of complications of graft-versus-host disease (GVHD) seen with allogeneic stem cell transplant (SCT) remains an elusive goal. In this issue of Blood, Robinson et al provide preclinical data demonstrating effective in vivo responses in a xenograft model of CLL using a novel CD19/CD3 bispecific antibody and that effective T-cell responses are augmented in samples obtained when patients have been treated with ibrutinib.1
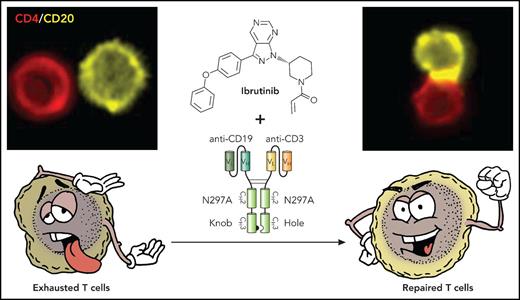
T cells in CLL exhibit features of exhaustion and functionally, both CD4 (shown in upper right panel) and CD8 (not shown) cells form ineffective immune synapses with CD20-expressing CLL cells. Treatment with ibrutinib repairs the observed T-cell defects so that when combined with the action of the bispecific antibody, we see repaired T cells with increased effector function and marked increase the ability of the T cells to form in immune synapses with the CLL cells (upper right panel) and induce leukemia cell killing. Professional illustration by Patrick Lane, ScEYEnce Studios.
T cells in CLL exhibit features of exhaustion and functionally, both CD4 (shown in upper right panel) and CD8 (not shown) cells form ineffective immune synapses with CD20-expressing CLL cells. Treatment with ibrutinib repairs the observed T-cell defects so that when combined with the action of the bispecific antibody, we see repaired T cells with increased effector function and marked increase the ability of the T cells to form in immune synapses with the CLL cells (upper right panel) and induce leukemia cell killing. Professional illustration by Patrick Lane, ScEYEnce Studios.
They suggest that the combination of a targeted agent such as ibrutinib in combination with an effective immunotherapy approach represents a potential advance in the goal of achieving durable responses in CLL, potentially without the need for continuous ongoing therapy (see figure).
The BTK inhibitor ibrutinib is now licensed for both relapsed and treatment-naïve patients with CLL.2,3 Whereas treatment with ibrutinib represents a major advance in the treatment of this incurable disease, this agent requires indefinite treatment of ongoing efficacy. There are therefore problems related to the high cost of long-term continuous therapy, compliance can be compromised by ongoing low-grade toxicity, and in many cases there is eventual emergence of resistance, particularly in the most high-risk patient population, including those with deletion of 17p and those with complex karyotype.
The only therapy that is offered with curative intent for the treatment of CLL is allogeneic SCT, and it is clear that the major mechanism for the success of this treatment approach is the graft-versus-leukemia effect mediated by donor T cells.4 However, SCT is associated with significant morbidity and mortality associated with GVHD and the need for immunosuppression leading to infectious complications. Generation of effective immune responses mediated by autologous T cells without the risk of GVHD remains the goal. Chimeric antigen receptor (CAR)-T cells represent a novel way to achieve this goal, but although CD19-targeted CAR-T cells are now approved for treatment of acute lymphoblastic leukemia and diffuse large B-cell lymphoma, responses in CLL have been more varied, and these products remains investigatory in CLL.5 A reason for this is likely the profound immune suppressive effect that CLL has on host immune responses and that CLL patients T cells exhibit phenotypic and functional characteristics of T-cell exhaustion.6
Although ibrutinib is an example of the class of “targeted therapy,” there is increasing evidence that ibrutinib also mediates alterations in T-cell function and is, at least in part, able to reverse the T-cell exhaustion phenotype typical of CLL.7 Notably, CAR-T cells exhibit increased efficacy when the T cells are obtained after ibrutinib treatment. It is therefore highly likely that the combined effects seen here result from increased effector and cytotoxic function of the ibrutinib-treated T cells brought together in effective synapse interactions with the target CLL cells by the bispecific antibody.
The work here is novel but, of course, is all preclinical, with the data presented being either in vitro or in xenograft mouse models. We are therefore still far from translating this work to patients. Blinatumomab is licensed for and highly effective for the treatment of acute lymphoblastic leukemia.8 There is no convincing data presented to explain why there should be such difference in activity in vivo between blinatumomab and the novel bispecific antibody construct when they work similarly in vitro. The experiments using samples from patients treated long term with ibrutinib remain difficult to interpret, and it is not clear if this is attributable to an effect of the ibrutinib on T cells or simply a reflection of taking samples when the CLL burden in the patients was much less. There is also no clear reason why ibrutinib-resistant cells should have been less sensitive to the bispecific antibody treatment as there is no suggestion that B-cell receptor–mediated signaling is in any way associated with its activity. We await with great interest the data that this novel bispecific antibody compound can be given safely to humans and the subsequent clinical trials in combination with ibrutinib to determine whether this combination can really transform, as hoped, once exhausted T cells into effective mediators of long-lasting disease control in CLL.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal