Abstract
Protein C is a plasma serine protease zymogen whose active form, activated protein C (APC), exerts potent anticoagulant activity. In addition to its antithrombotic role as a plasma protease, pharmacologic APC is a pleiotropic protease that activates diverse homeostatic cell signaling pathways via multiple receptors on many cells. Engineering of APC by site-directed mutagenesis provided a signaling selective APC mutant with 3 Lys residues replaced by 3 Ala residues, 3K3A-APC, that lacks >90% anticoagulant activity but retains normal cell signaling activities. This 3K3A-APC mutant exerts multiple potent neuroprotective activities, which require the G-protein–coupled receptor, protease activated receptor 1. Potent neuroprotection in murine ischemic stroke models is linked to 3K3A-APC–induced signaling that arises due to APC’s cleavage in protease activated receptor 1 at a noncanonical Arg46 site. This cleavage causes biased signaling that provides a major explanation for APC’s in vivo mechanism of action for neuroprotective activities. 3K3A-APC appeared to be safe in ischemic stroke patients and reduced bleeding in the brain after tissue plasminogen activator therapy in a recent phase 2 clinical trial. Hence, it merits further clinical testing for its efficacy in ischemic stroke patients. Recent studies using human fetal neural stem and progenitor cells show that 3K3A-APC promotes neurogenesis in vitro as well as in vivo in the murine middle cerebral artery occlusion stroke model. These recent advances should encourage translational research centered on signaling selective APC’s for both single-agent therapies and multiagent combination therapies for ischemic stroke and other neuropathologies.
Introduction
In 2001, when Seligsohn and Lubetsky reviewed the current state of knowledge for genetic susceptibility to venous thrombosis, the hereditary deficiencies of antithrombin, protein C, and protein S plus the gain-of-function mutations in the factor V or prothrombin genes were central to the current understanding of hemostasis for white populations.1 The major recognized function of activated protein C (APC) was that of an anticoagulant plasma serine protease, which inactivates coagulation factors Va and VIIIa with significant roles for various lipid and protein cofactors (eg, protein S) (Figure 1A), thereby preventing venous thrombosis. There were also inklings that APC can provide anti-inflammatory activity derived from the inflammatory nature of neonatal purpura lesions with which severe protein C–deficient infants present2-5 and from the observation that APC reduced death in a nonhuman primate severe sepsis model.6 In 2001, it was generally considered that APC was indirectly anti-inflammatory because its anticoagulant action would reduce generation of proinflammatory thrombin. Then came seminal studies of cultured endothelial cells in which Joyce et al7 showed that APC evoked both anti-inflammatory and antiapoptotic activities and altered gene expression profiles, setting the stage for investigations of APC’s nonhemostatic functions and mechanisms.
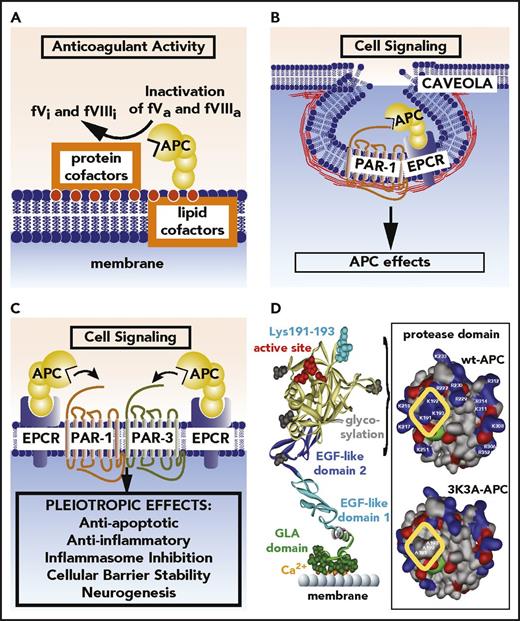
APC anticoagulant and cell signaling pathways and the structure of signaling-selective 3K3A-APC. (A) Anticoagulant activity of APC involves the proteolytic inactivation of factors Va and VIIIa on membrane surfaces containing phospholipids that are derived from cells, platelets, lipoproteins, or cellular microparticles. The irreversible inactivation of factors Va and VIIIa to yield inactive factors Vi and VIIIi by APC is accelerated by a variety of lipid and protein cofactors (eg, glucosyl ceramide, protein S, etc). (B) Beneficial direct effects of APC on cells require the EPCR and PAR1. One distinction between proinflammatory thrombin signaling and cytoprotective APC signaling is the localization of APC signaling in the caveolin-1–rich microdomains (caveolae). (C) Neuroprotective mechanisms for APC effects on cells may also involve other receptors including PAR-3. APC-initiated signaling effects on cells can include antiapoptotic activities, anti-inflammatory activities, inhibition of the inflammasome, stabilization of endothelial barrier functions including the BBB, and neurogenesis. (D) The polypeptide structure of APC comprises an N-terminal GLA domain (green) that binds to negatively charged lipids and EPCR, 2 EGF-like domains (blue), and the protease domain containing the active site triad of serine, histidine, and aspartic acid residues (red). Four glycosylation sites are indicated by gray-shaded moieties. Substrate selectivity of this protease is determined by interactions between the targeted substrates and the active site and also by multiple unique binding exosites on APC that vary for different substrates. The protease domain space–filled model (see insert) highlights in the yellow box 3 positively charged lysine (K) residues within the so-called 37 loop (KKK 191-193), which is an exosite for APC’s recognition of factors Va and VIIIa. Mutation of these 3 residues to alanine (3K3A-APC) reduces APC’s anticoagulant activity by >90%, but it does not affect its interactions with the cytoprotective substrates, PAR1, or its other known cell signaling receptors. Thus, 3K3A-APC is very signaling selective.
APC anticoagulant and cell signaling pathways and the structure of signaling-selective 3K3A-APC. (A) Anticoagulant activity of APC involves the proteolytic inactivation of factors Va and VIIIa on membrane surfaces containing phospholipids that are derived from cells, platelets, lipoproteins, or cellular microparticles. The irreversible inactivation of factors Va and VIIIa to yield inactive factors Vi and VIIIi by APC is accelerated by a variety of lipid and protein cofactors (eg, glucosyl ceramide, protein S, etc). (B) Beneficial direct effects of APC on cells require the EPCR and PAR1. One distinction between proinflammatory thrombin signaling and cytoprotective APC signaling is the localization of APC signaling in the caveolin-1–rich microdomains (caveolae). (C) Neuroprotective mechanisms for APC effects on cells may also involve other receptors including PAR-3. APC-initiated signaling effects on cells can include antiapoptotic activities, anti-inflammatory activities, inhibition of the inflammasome, stabilization of endothelial barrier functions including the BBB, and neurogenesis. (D) The polypeptide structure of APC comprises an N-terminal GLA domain (green) that binds to negatively charged lipids and EPCR, 2 EGF-like domains (blue), and the protease domain containing the active site triad of serine, histidine, and aspartic acid residues (red). Four glycosylation sites are indicated by gray-shaded moieties. Substrate selectivity of this protease is determined by interactions between the targeted substrates and the active site and also by multiple unique binding exosites on APC that vary for different substrates. The protease domain space–filled model (see insert) highlights in the yellow box 3 positively charged lysine (K) residues within the so-called 37 loop (KKK 191-193), which is an exosite for APC’s recognition of factors Va and VIIIa. Mutation of these 3 residues to alanine (3K3A-APC) reduces APC’s anticoagulant activity by >90%, but it does not affect its interactions with the cytoprotective substrates, PAR1, or its other known cell signaling receptors. Thus, 3K3A-APC is very signaling selective.
Mechanisms for APC’s nonhemostatic functions initially came from in vitro studies showing that protease activated receptor (PAR) 1,8,9 which has emerged as a major druggable target,10,11 was required for APC cell signaling effects.12-14 Convincingly, in vivo studies highlighted the requirement for PAR1 for APC’s neuroprotective actions.15,16 In 2015, an extensive review in this journal noted that PAR1 appeared to be required for APC’s remarkably diverse pharmacologic benefits in preclinical injury studies, including brain, coronary, and kidney ischemia-reperfusion injuries, sepsis, total body radiation, organ transplants, and wound healing, etc.17 Biased signaling mediated by PAR1 is thought to be central to APC’s benefits in many of those preclinical studies.17,18 Other cell receptors, especially endothelial cell protein C (EPCR) and PAR3 inter alia, may also significantly contribute to APC-initiated cell signaling.19-22 However, in this review, we focus on 1 type of APC’s nonhemostatic beneficial properties, namely neuroprotection, and on 1 receptor that mediates APC’s neuroprotective activities, namely PAR1.
APC
APC pathways
Although plasma protein C has been studied for decades,23-25 much remains to be learned about the pathways that are regulated by APC. Protein C is a serine protease zymogen whose normal plasma concentration is 70 nM, whereas very low levels of active APC (40 pM) are also present in circulating plasma. The physiologic mechanism for protein C activation involves proteolysis at Arg169 in EPCR-bound protein C by thrombomodulin-bound thrombin.
Two major distinct types of APC activities have been well defined as involving anticoagulant activity and cell signaling activities.17,23-25 For its anticoagulant effects, APC binds to phospholipid membranes, where it irreversibly inactivates factors Va and VIIIa by proteolysis at 1 or more Arg residues (Figure 1A); these inactivation reactions are greatly enhanced by physiologically important protein and lipid cofactors (eg, protein S, high-density lipoprotein, glucosylceramide, etc). Completely independently of its anticoagulant actions, APC can initiate cell signaling that can result in multiple distinct cytoprotective actions, including but not limited to antiapoptotic and anti-inflammatory activities, stabilization of endothelial barriers to prevent vascular leakage, extensive alterations in gene expression profiles, and neurogenesis (Figure 1B-C).17,23-25 Each of these actions may contribute to APC’s neuroprotective activities for different cell types (see “APC and neuroprotection”). These cytoprotective and neurogenerative activities most often, but not always, have been shown to involve PAR1 and EPCR; often other receptors are required, such as PAR3, sphingosine phosphate 1 receptor 1 (S1P1), the integrin Mac-1 or other β1 and β3 integrins, apolipoprotein E receptor 2, epidermal growth factor receptor, and Tie-2 (tunica intima endothelial receptor tyrosine kinase 2).17,25-32 In this review, we focus mainly on APC-induced signaling that involves PAR1, which is thought to occur in caveola, where EPCR-bound APC cleaves PAR1 (Figure 1B).11,12,26,33-35
The half-life of endogenous circulating APC in humans is 15 to 0 minutes and is dominantly determined by its irreversible reaction with the plasma serine protease inhibitors, alpha1-antitrypsin, protein C inhibitor, and alpha2-macroglobulin.
Engineering selectivity for APC activities
Detailed knowledge for structure-activity relationships of protein C was initially driven by the goal to understand the basis for protein C dysfunction in venous thrombosis patients who presented with protein C mutations: fundamentally, a major goal was to understand protein-protein interactions involving APC. However, once the cell signaling activities of APC were realized,7 we strove to engineer recombinant APC mutants, which selectively retained only its cell signaling activity or only its anticoagulant activity. This was based on the hypothesis that the substrates, factors Va and VIIIa, for APC’s anticoagulant actions differed greatly from the substrates for signaling; indeed, such is the case for PAR1 and PAR3 and APC’s receptors (EPCR, apoER2, Mac-1, Tie2) for its cell signaling actions. Signaling-selective APC mutants and an anticoagulant-selective APC mutant were generated by different laboratories.17,25,36-43 As depicted in the inset in Figure 1D, which shows the domain structure of APC, the replacement in APC of the 3 Lys residues by 3 Ala (residues 191-193), generating 3K3A-APC, provided the first signaling-selective mutant, which lost >90% of its anticoagulant activity while retaining normal cell signaling activities37 ; this mutant retains all the neuroprotective properties of wild-type (wt)-APC, and it has been translated to clinical trials for acute ischemic stroke21 (see “Ischemic stroke therapy: translation for 3K3A-APC”).
PAR1
PAR
The 4 PARs (PAR1-4) comprise a unique subset of the larger G-protein–coupled receptor (GPCR) family.8,9,44-48 Remarkably, PARs carry their own agonist ligand (Figure 2A-B), albeit encrypted until proteolytic activation. In contrast to the general external ligand-receptor binding mechanisms for GPCR activation, PARs instead rely on proteases to deencrypt their intramolecular ligand, a mechanism in which proteolysis generates a new N-terminus that acts as a tethered ligand with agonistic properties (Figure 2A).49 By sensing specific proteases, PARs translate and interpret the conditions of their immediate environment for endothelial cells, platelets, neurons, immune system cells, and other cells to induce activation of appropriate cell signaling pathways. Many different proteases, including coagulation factors (thrombin, APC, factor VIIa, factor Xa), metalloproteinases, kallikreins, and others, engage the 4 different PARs to varying extents with a diverse repertoire of functional outcomes.10,11,50 However, the efficient activation of PAR1 by thrombin, facilitated by the high affinity of thrombin for a hirudin-like sequence present in PAR1 (and PAR3), helped to define PAR1 as the “thrombin receptor.”51
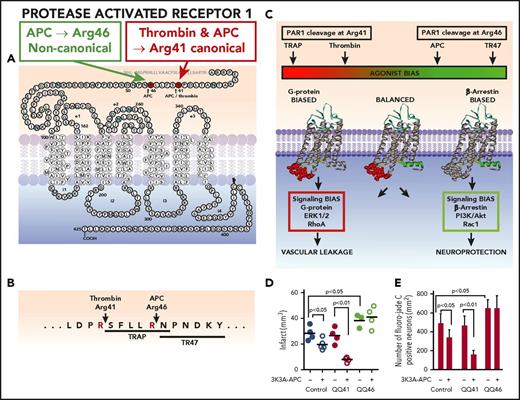
APC causes biased signaling via PAR1 cleavage at Arg46. PAR1 is a 7-transmembrane GPCR receptor capable of many conformational states. (A) The extracellular N-terminus contains the intramolecular ligands, which become exposed after proteolysis by APC or thrombin. PAR1 cleavage by thrombin at Arg41 exposes the canonical N-terminal tethered agonist that begins with residue Ser42 (SFLLRN-), whereas noncanonical cleavage by APC at Arg46 results in a different N-terminal tethered agonist that begins with residue Asn47 (NPNDKY-). (B) Synthetic agonist peptides with the N-terminal tethered-ligand sequences beginning with residue 42 (TRAP) or residue 47 (TR47) cause thrombin-like or APC-like effects on cells, respectively. (C) Activation of PAR1 by thrombin or TRAP stabilizes PAR1 conformers, whose intracellular loops provide surfaces that are highly favorable for interactions with G proteins, resulting in G-protein–dependent signaling. These PAR1 conformers are termed “G-protein biased.” In contrast, activation of PAR1 by APC or TR47 stabilizes different PAR1 conformers that preferentially interact with β-arrestin-2, resulting in β-arrestin-2–dependent signaling. Such PAR1 conformers are termed “β-arrestin biased.” Thus, the agonist bias is directly related to the cleavage site used to activate PAR1 because the cleavage determines which tethered ligand is exposed and which subsets of PAR1 conformations are stabilized. Biased agonism results in the induction of uniquely different signaling repertoires, such as the activation of ERK1/2 and RhoA, etc, resulting in vascular leakage by thrombin or the activation of PI3K, Akt, and Rac1, etc, by APC resulting in neuroprotection. (D-E) APC-mediated neuroprotection in ischemic stroke requires PAR1-dependent biased signaling due to cleavage at Arg46 in PAR1. To assess PAR1-biased signaling, studies employed homozygous QQ41-PAR1 mice, homozygous QQ46-PAR1 mice, and wt control mice. At 4 hours after a 60-minute MCAO, 3K3A-APC (0.04 mg/kg) or placebo was given IV, and then at 24 hours after occlusion, various parameters were measured. Treatment of mice with 3K3A-APC or vehicle is indicated by plus or negative signs under panels D and E. Data are shown for infarct volume (D) and for degenerating neurons as determined by Fluoro-Jade C stain (E) for each mouse group. For panels D and E, bars indicate mean ± standard deviation, n = 4-6 mice per group. (For details regarding panels D and E, see Sinha et al22 ).
APC causes biased signaling via PAR1 cleavage at Arg46. PAR1 is a 7-transmembrane GPCR receptor capable of many conformational states. (A) The extracellular N-terminus contains the intramolecular ligands, which become exposed after proteolysis by APC or thrombin. PAR1 cleavage by thrombin at Arg41 exposes the canonical N-terminal tethered agonist that begins with residue Ser42 (SFLLRN-), whereas noncanonical cleavage by APC at Arg46 results in a different N-terminal tethered agonist that begins with residue Asn47 (NPNDKY-). (B) Synthetic agonist peptides with the N-terminal tethered-ligand sequences beginning with residue 42 (TRAP) or residue 47 (TR47) cause thrombin-like or APC-like effects on cells, respectively. (C) Activation of PAR1 by thrombin or TRAP stabilizes PAR1 conformers, whose intracellular loops provide surfaces that are highly favorable for interactions with G proteins, resulting in G-protein–dependent signaling. These PAR1 conformers are termed “G-protein biased.” In contrast, activation of PAR1 by APC or TR47 stabilizes different PAR1 conformers that preferentially interact with β-arrestin-2, resulting in β-arrestin-2–dependent signaling. Such PAR1 conformers are termed “β-arrestin biased.” Thus, the agonist bias is directly related to the cleavage site used to activate PAR1 because the cleavage determines which tethered ligand is exposed and which subsets of PAR1 conformations are stabilized. Biased agonism results in the induction of uniquely different signaling repertoires, such as the activation of ERK1/2 and RhoA, etc, resulting in vascular leakage by thrombin or the activation of PI3K, Akt, and Rac1, etc, by APC resulting in neuroprotection. (D-E) APC-mediated neuroprotection in ischemic stroke requires PAR1-dependent biased signaling due to cleavage at Arg46 in PAR1. To assess PAR1-biased signaling, studies employed homozygous QQ41-PAR1 mice, homozygous QQ46-PAR1 mice, and wt control mice. At 4 hours after a 60-minute MCAO, 3K3A-APC (0.04 mg/kg) or placebo was given IV, and then at 24 hours after occlusion, various parameters were measured. Treatment of mice with 3K3A-APC or vehicle is indicated by plus or negative signs under panels D and E. Data are shown for infarct volume (D) and for degenerating neurons as determined by Fluoro-Jade C stain (E) for each mouse group. For panels D and E, bars indicate mean ± standard deviation, n = 4-6 mice per group. (For details regarding panels D and E, see Sinha et al22 ).
Proinflammatory effects and a loss of the endothelial barrier function with vascular leakage result from PAR1 activation by thrombin on endothelial cells. Surprisingly, these PAR1-dependent effects of thrombin contrast with PAR1-dependent signaling by APC that includes anti-inflammatory effects and enhancement of endothelial barrier function.18,33,52 These opposing effects on the same GPCR, PAR1, posed a conundrum of how APC could elicit cytoprotective signaling involving the thrombin receptor (ie, PAR1) and raised 2 fundamental questions: (1) How can PAR1 distinguish whether its activation occurred by thrombin or APC to mediate distinctly different signaling repertoires with opposite functional outcomes? (2) How can APC mediate physiologically relevant PAR1 signaling when PAR1 activation by thrombin, at least in vitro, is kinetically favored by several orders of magnitude compared with APC? One consideration for contemplating these questions is that there are differences in PAR1 internalization after activation by either thrombin or APC. Activation of PAR1 by thrombin results in rapid, agonist-induced internalization of PAR1 that terminates induction of signaling, whereas PAR1 agonist-induced internalization is not observed after activation by APC.53-56 This indicates that APC-activated PAR1 may accumulate on the cell membrane over time and induce continued signaling once a critical interactome is assembled and once certain cellular thresholds are overcome. Such temporal interpretation of APC-induced signaling is consistent with the delayed initiation of notable signaling events in cells and the general requirement of sustained APC signaling to initiate maximal biological functions on cells. Another consideration is that there is potentially a distinction between endogenously generated APC vs therapeutically administered, exogenous APC, as some evidence indicates that locally generated APC may have superior activity over exogenously added APC.57
PAR1 biased signaling
Answers to the question of how thrombin or APC can mediate distinctly different, even opposite PAR1 signaling repertoires came from considerations of receptor localization in the membrane and discovery of a novel APC cleavage site in PAR1, linked to biased GPCR signaling. Membrane localization studies showed that PAR1-dependent cytoprotective signaling by APC required colocalization of EPCR and PAR1 in caveolin-1–enriched cellular microdomains, caveolae (Figure 1B).33,34 The notion that APC-induced PAR1 signaling involved β-arrestin–mediated activation of signaling pathways rather that the traditional G proteins indicated that PAR1 was intrinsically capable of biased signaling, a well-known phenomenon of GPCRs that explains how different ligands can use the same receptor to selectively activate or inhibit specific signaling cascades with distinct biological functions.35,58-61 The discovery of noncanonical PAR1 activation due to cleavage at Arg46 by APC (Figure 2A), as opposed to the canonical PAR1 activation by thrombin’s cleavage at Arg41, provided the molecular mechanism for the generation of biased PAR1 ligands.18 Collectively, these advances summarized above induced a paradigm shift for the selectivity of PAR1 signaling repertoires and firmly established noncanonical PAR1 activation and biased signaling as the distinction between PAR1 activation by APC or thrombin.10,11,17
GPCRs, including PAR1, are now no longer considered simply as on-off switches, because they populate a spectrum of multiple conformations with potentially selective association to either G proteins (G-protein bias), β-arrestin (β-arrestin bias), or both (neutral bias), and different GPCR conformers induce different modes of cell signaling.61,62 Canonical PAR1 activation by thrombin at Arg41 generates the classical N-terminal sequence, a SFLLRN-tethered ligand that is strongly biased toward a G-protein signaling repertoire (Figure 2B).8,63 The noncanonical activation of PAR1 by APC at Arg46 generates a different N-terminal sequence, the NPNDKY-tethered ligand (also known as thrombin receptor peptide 47, TR47) that is a biased agonist selectively stabilizing PAR1 conformations that associate with β-arrestin and induce β-arrestin–dependent signaling cascades, but not the typical G-protein signaling (Figure 2C).18,35 Studies using peptides containing the different N-terminal sequences (eg, “thrombin receptor activating peptides” [TRAP] beginning with SFLLRN- or TR47 peptides beginning with NPNDKY-) trigger PAR1-dependent, different signaling actions resembling those triggered by the respective proteases, providing data supporting the biased signaling concept of PAR1.18 Biased agonism is dictated by the ligand, but biased signaling is a property of the receptor indicating that allosteric modulators might also contribute by selectively stabilizing certain subsets of PAR1 conformations, thereby introducing receptor bias to preferentially induce β-arrestin–dependent signaling cascades regardless of the agonist.64,65 This may provide an explanation for the neuroprotective effects of thrombin at very low concentrations, especially when protein C is bound to EPCR, a phenomenon sometimes referred to as “EPCR occupancy.”66-68
In vivo proof of concept for APC-initiated PAR1 biased signaling was recently presented in studies of stroke and sepsis using genetically modified mouse strains carrying R41Q or R46Q point mutations in PAR1.22 In the setting of transient distal MCAO stroke studies, 3K3A-APC significantly reduced infarct size for wt mice and QQ41-PAR1 mice but not for mice carrying QQ46-PAR1 (Figure 2D). In measurements of ischemia-induced neuronal apoptosis (Figure 2E), 3K3A-APC was antiapoptotic in wt and QQ41-PAR1 mice but not in QQ46-PAR1 mice. Thus, for 3K3A-APC’s neuroprotective actions in stroke, not only is PAR1 required but also Arg46 in PAR1 is specifically required, strongly supporting the concept that APC’s neuroprotection requires PAR1 biased signaling.
APC can induce noncanonical activation of PAR3 by cleavage at Arg41 as opposed to the canonical activation of PAR3 by thrombin’s cleavage at Lys38.69 Although PAR3 is considered to be a nonsignaling GPCR, its requirement for neuroprotective effects of APC in vivo indicates our incomplete understanding of PAR3 engagement in APC-mediated signaling.16,45,70 Emerging concepts in molecular mechanisms of GPCR signaling,50,58,71 such as GPCR dimerization bias, may provide some insights into how noncanonical PAR3 activation may contribute to APC-induced cytoprotective signaling as PAR3 is known to form heterodimers with PAR1.50,72 Alternatively, transactivation bias, such as the phosphorylation and activation of the receptor tyrosine kinase Tie2 by noncanonical PAR3 activation, may promote triggering of signaling nodes that are normally outside the realm of PARs.32,73,74
PAR1 cleavage by other proteases
Besides APC and thrombin, other proteases are known to cleave PAR1, including inter alia, elastase, cathepsin G, proteinase 3, kallikrein, granzymes A and B, matrix metalloproteinases 1, 2, 9, and 13, plasmin, and factors VIIa and Xa in EPCR-dependent reactions (see Flaumenhaft and De Ceunynck, 10 Hamilton and Trejo,11 Nieman,50 Isermann,75 Mohan Rao et al76 ). PAR1 cleavages by these proteases have been characterized to varying extents; such cleavages may either activate or inactivate PAR1 and/or may involve biased signaling. Cleavage of PAR1 at Asp39 by matrix metalloprotease 1 has been well studied, and some of its potential effects have been characterized in vitro.77 However, none of these proteases provides neuroprotection, the focus of this review; thus, their PAR1 cleavage activities are not further discussed here.
APC and neuroprotection
APC is neuroprotective for a variety of acute and chronic neuropathologies, including ischemic stroke, traumatic brain injury, chronic cerebral ischemia, amyotrophic lateral sclerosis, and multiple sclerosis, as previously reviewed.17,19-21,25,75,78-81 For these neuroprotective activities, APC’s cell signaling activities are central to the primary mechanism of action because many studies show that various APC receptors, especially PAR1, PAR3, and EPCR, are required, and signaling-selective APC’s are as active as neuroprotectants as wt-APC. Both in vitro and in vivo studies showed that APC beneficially affects all the cells of the neurovascular unit and neurons (Figure 3A). APC in blood readily accesses endothelial cells to stabilize the blood-brain barrier (BBB) and also can reach neurons due to EPCR-dependent transport of APC across the BBB.82 In later discussion, we highlight APC’s neuroprotective actions relevant to ischemic stroke and summarize the emerging translation of this knowledge to the clinic.
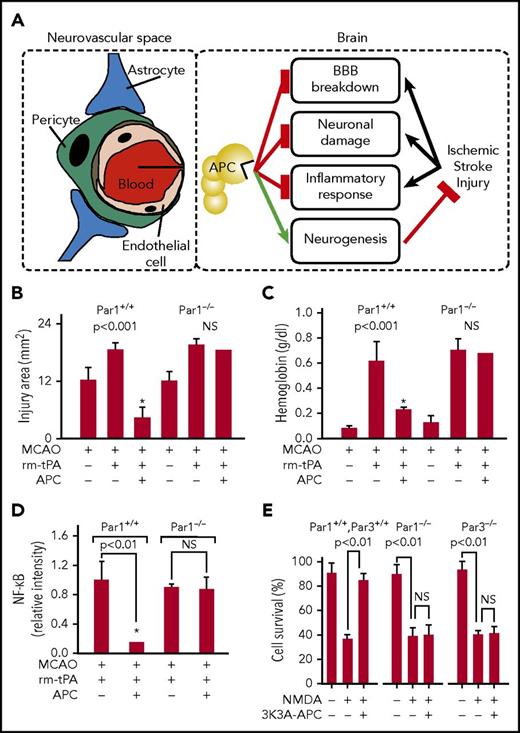
Neuroprotective effects of APC in the neurovascular space and in neurons. (A) APC can provide multiple neuroprotective effects in the neurovascular unit of the brain after ischemic stroke. APC inhibits the breakdown of the BBB, preventing extravasation of inflammatory cells. EPCR-mediated transfer of APC across the BBB permits APC to engage PAR1, PAR3, and EPCR directly on neurons, glia, and other cells in the brain to convey multiple cytoprotective activities and dampen neuronal damage. APC attenuates neuroinflammatory responses. Furthermore, APC promotes neurogenesis and vascular regeneration in the brain that directly contribute to repair and regeneration of the affected brain tissue after ischemic stroke (see Figure 4). Studies showing the requirement for PAR1 for APC’s neuroprotection are seen in panels B-D that show an assessment of brain damage after a 1-hour transient MCAO in wt (PAR1+/+) and knockout (PAR1−/−) mice treated with recombinant murine (rm)-tPA and recombinant murine wt-APC (0.2 mg/kg). Damage quantified at 24 hours after onset of ischemia was based on brain infarct volume (B), hemorrhage (C), and altered levels of NF-ĸB (D). Values are mean ± standard error of the mean (SEM), and n = 3-6 mice per group; * designates data for mice receiving both APC and rm-tPA (for details regarding panels B-D, see Cheng et al88 ). (E) Studies using cultured neuronal cells from wt mice (PAR1+/+, PAR3+/+) and PAR1−/− and PAR3−/− knockout mice treated with 3K3A-APC showed the requirement for both PAR1 and PAR3 for 3K3A-APC’s direct neuronal protection against N-methyl-d-aspartate–induced excitotoxic injury of neurons (E). Values are mean ± SEM, n = 5 mice per group. (For details regarding panel E, see Guo et al90 ).
Neuroprotective effects of APC in the neurovascular space and in neurons. (A) APC can provide multiple neuroprotective effects in the neurovascular unit of the brain after ischemic stroke. APC inhibits the breakdown of the BBB, preventing extravasation of inflammatory cells. EPCR-mediated transfer of APC across the BBB permits APC to engage PAR1, PAR3, and EPCR directly on neurons, glia, and other cells in the brain to convey multiple cytoprotective activities and dampen neuronal damage. APC attenuates neuroinflammatory responses. Furthermore, APC promotes neurogenesis and vascular regeneration in the brain that directly contribute to repair and regeneration of the affected brain tissue after ischemic stroke (see Figure 4). Studies showing the requirement for PAR1 for APC’s neuroprotection are seen in panels B-D that show an assessment of brain damage after a 1-hour transient MCAO in wt (PAR1+/+) and knockout (PAR1−/−) mice treated with recombinant murine (rm)-tPA and recombinant murine wt-APC (0.2 mg/kg). Damage quantified at 24 hours after onset of ischemia was based on brain infarct volume (B), hemorrhage (C), and altered levels of NF-ĸB (D). Values are mean ± standard error of the mean (SEM), and n = 3-6 mice per group; * designates data for mice receiving both APC and rm-tPA (for details regarding panels B-D, see Cheng et al88 ). (E) Studies using cultured neuronal cells from wt mice (PAR1+/+, PAR3+/+) and PAR1−/− and PAR3−/− knockout mice treated with 3K3A-APC showed the requirement for both PAR1 and PAR3 for 3K3A-APC’s direct neuronal protection against N-methyl-d-aspartate–induced excitotoxic injury of neurons (E). Values are mean ± SEM, n = 5 mice per group. (For details regarding panel E, see Guo et al90 ).
Ischemic stroke
Three clinical research studies from the 1990s stimulated us to pursue studies of the neuroprotective activity of APC. These included the finding that circulating APC was lower in stroke patients vs controls, that plasma protein C levels were inversely associated with ischemic stroke, and that during the short ischemic phase in the brain during routine carotid endarterectomy, brain-blood levels of APC were increased.83-85 In 2001, we demonstrated human APC’s neuroprotection in a murine ischemic stroke model, which employed middle cerebral artery occlusion (MCAO).86 Subsequently, we used recombinant murine APC87 or its signaling-selective variants to avoid cross-species artifacts in mechanistic studies.19,21
When human hypoxic brain endothelial cells were studied, recombinant human and murine wt-APC had remarkable antiapoptotic activity that was based on reducing p53, normalizing the proapoptotic Bax/Bcl-2 ratio, and lowering caspase-3 signaling.15 Moreover, these neuroprotective benefits of APC were observed using MCAO murine models, and data showed that APC’s neuroprotection in vitro and in vivo requires APC-initiated PAR1-dependent and PAR3-dependent cell signaling. To enable translation to the clinic for combined therapies using tissue plasminogen activator (tPA) and APC, we showed that APC protects the brain from tPA’s toxicity. For example, MCAO studies showed that APC reduced infarct volume (Figure 3B), tPA-induced bleeding (Figure 3C), and proinflammatory upregulation of NF-ĸB (Figure 3D) in wt mice but not in PAR1 null mice.19,88,89 Detailed studies showed that APC was directly neuronal protective in vitro and in vivo,16 and mechanistic studies showed that this neuronal protection requires PAR1, PAR3, and EPCR.16,19 For example, when cultured neurons from wt mice and PAR1 and PAR3 knockout mice (Figures 3E) were studied, as well as those 3 mice strains themselves, 3K3A-APC reduced N-methyl-d-aspartate–induced neuronal apoptosis.16,19,88-90 Thus, APC’s neuronal protective actions require PAR1 and PAR3. These results and data from recent studies of mice carrying the 46QQ-PAR1 point mutation strongly support the concept that APC-induced, PAR1-dependent biased signaling following Arg46 cleavage is central to APC’s in vivo neuroprotective benefits in this model of ischemic stroke.22
APC and neuroinflammation
Neuroinflammation is a major causal factor for ischemic stroke pathology. NLRP3 inflammasomes that generate caspase-1, which, in turn, generates the cytokines interleukin-1β and interleukin-18 help to mediate development of inflammation in ischemic stroke.91-93 Suppression of NLRP3 is reported to attenuate tPA-induced hemorrhagic transformation in a rat stroke model.94 We believe that the recent discovery that signaling selective 3K3A-APC thwarts NLRP3 inflammasome activation is likely a major factor contributing to APC’s neuronal anti-inflammatory actions.95 Consonant with this general point is the observation that APC suppresses the proinflammatory upregulation of NF-ĸB (Figure 3D), which is linked to inflammasome pathology.
Ischemic stroke therapy: translation for 3K3A-APC
No new drug has been approved since tPA for therapy for ischemic stroke.96 Efforts to translate the signaling-selective 3K3A-APC variant from preclinical successes to the clinic for therapy for ischemic stroke were based on its multiple, powerful neuroprotective actions summarized above and elsewhere19-21 and on the promise of reduced bleeding risk compared with wt-APC since >90% of anticoagulant activity is lost due to its 3 Lys-to-Ala mutations.37,38 Development of a new process for manufacturing 3K3A-APC for clinical studies enabled establishment of its safety and pharmacokinetic profiles in 2 animals.97 A successful phase 1 trial of 3K3A-APC in healthy adult human subjects established its safety and maximum tolerated dose (0.54 mg/kg) when it was administered IV 5 times every 12 hours,98 laying the groundwork for subsequent phase 2 studies in stroke patients.
The phase 2A NeuroNEXT trial NN104 (RHAPSODY) (see NCT02222714 at clinical trials.gov) was a dose-escalation safety trial for 3K3A-APC in stroke patients with a primary objective to evaluate the drug’s safety, and it included single doses and 4 multiple ascending doses of drug. Acute ischemic stroke patients were treated first with recanalization therapy (tPA and/or thrombectomy which mechanically removes thrombi from large arteries). Then, as in phase 1 studies, participants received up to 5 3K3A-APC infusions, each separated by 12 hours, beginning within 2 hours of recanalization therapy (tPA and/or thrombectomy). The RHAPSODY trial reported in January 2018 showed that 3K3A-APC in acute stroke patients was safe and that the maximum tolerated dose was 0.54 mg/kg.99 Hemorrhage incidence was reduced in 3K3A-APC–treated patients vs placebo-treated patients from 87% to 67% (P = .046). Thus, successful phase 1 and phase 2A trials have set the stage for further development of 3K3A-APC for therapy for acute ischemic stroke.
APC and regenerative activities
Among APC’s nonhemostatic activities, its recently discovered ability to promote murine and human neurogenesis is certainly a rather striking, and very promising, phenomenon. This activity has been characterized in vivo for murine neurons in the setting of transient MCAO and in vitro and in vivo for fetal human neural stem and progenitor cells (NSCs). The signaling selective 3K3A-APC variant not only reduces damage to neurons but also promotes growth and regeneration of neurons.70,100,101 This section summarizes the ability of APC to manifest regenerative activities.
Central nervous system (CNS) regenerative effects of APC and its cell-signaling analogs
In studies using a murine 1-hour MCAO injury followed by reperfusion, APC promoted postischemic endogenous neovascularization and neurogenesis.100 An APC multiple dosing regimen initiated 3 to 6 days after stroke enhanced cerebral perfusion in the ischemic border, inhibited BBB leakage of proteins, and increased the number of endothelial replicating cells by approximately fivefold, as determined within 7 days of stroke. Moreover, the APC multidosing regimen starting 3 to 6 days after an ischemic insult increased proliferation of neuronal progenitor cells in the subventricular zone by 40% to 50%, as well as migration of newly formed neuroblasts from that zone toward the ischemic border by approximately twofold. These effects of APC on neovascularization and neurogenesis required PAR1 and appeared to be independent of APC’s reduction of the infarction volume. That study was first to characterize neurogenesis and also to suggest a significant extension of the therapeutic window for APC intervention in the postischemic brain.100
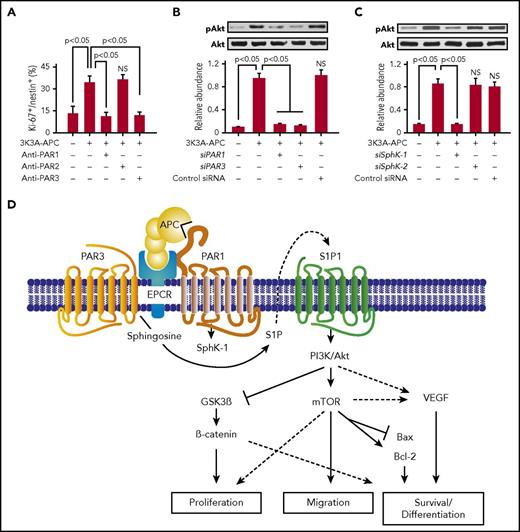
The discovery of murine neurogenesis in vivo led to in vitro studies of whether human 3K3A-APC can influence neuronal production from human resident progenitor cells.70 In 2013, studies of human fetal NSCs showed that 3K3A-APC stimulates human neuronal proliferation and differentiation. 3K3A-APC’s effects were comparable to those previously reported for fibroblast growth factor and brain-derived neurotrophic factor. Of note, 3K3A-APC’s effects included inhibition of astroglial differentiation and a modest antiapoptotic effect during neuronal production.70 Anti-PAR blocking antibodies (Figure 4A), small interfering RNA inhibition studies of PARs (Figure 4B), sphingosine 1 phosphate receptors (S1P1-5; not shown), and sphingosine kinases (SphKs) (Figure 4C) revealed that PAR1, PAR3, S1P1, and SphK-1 are required for the in vitro human fetal neurogenic effects of 3K3A-APC. In this setting, 3K3A-APC activated Akt, a downstream target of S1P1, and Akt activation was inhibited by silencing of S1P1, SphK-1, PAR1, and PAR3 (Figure 4B-C). Moreover, adenoviral transduction of human NSCs with a kinase defective Akt mutant abolished the effects of 3K3A-APC on NSCs, confirming a key role for Akt activation in 3K3A-APC–mediated proliferation, migration, and neuronal differentiation of human fetal NSCs.70 Therefore, these in vitro studies revealed the potential of APC-based therapy for structural repair of the human CNS through the actions of PAR1, PAR3, and S1P1 in resident NSCs (Figure 4D).
Regenerative activities of signaling-selective 3K3A-APC for human NSCs. (A-C) 3K3A-APC stimulates neuronal proliferation and differentiation from human embryo-derived NSCs in vitro. (A) Stimulation of human NSCs proliferation in culture by 3K3A-APC requires PAR1 and PAR3 but not PAR2. Quantification of proliferation was based on the percentage of Ki-67+/nestin+ cells in culture after 48 hours in the presence and absence of 3K3A-APC (2 nM) and/or PAR1-, PAR2-, and PAR3-specific cleavage site blocking antibodies. (B-C) Enhancement of neuronal proliferation and differentiation of NSCs by 3K3A-APC requires activation of Akt. Activation of the PI3K/Akt pathway by human recombinant 3K3A-APC (2 nM) in human NSCs requires PAR1 and PAR3 (B) and SphK-1 but not SphK-2 (C). Phosphorylation of Akt at Ser473 (pAkt) and total Akt was determined 3 hours after 3K3A-APC or vehicle treatment by western blot in whole-cell extracts of NSCs transfected with PAR1, PAR3, SphK-1, SphK-2, or control small interfering RNA. Intensity of pAkt signal was determined by scanning densitometry and normalized to total Akt. Data are shown as mean ± SEM, n = 3 independent cultures in triplicate. Statistical significance was determined by 1-way analysis of variance followed by Tukey’s post hoc test. NS, not significant. (For details regarding panels A-C, see Guo et al70 ). (D) Schematic overview of APC’s regenerative activities for human NPCs. Activation of the PI3K/Akt pathway in NSCs by 3K3A-APC requires PAR1, PAR3, S1P1, SphK-1, and EPCR. Through integration of signaling linked to multiple downstream effectors, activation of the PI3K/Akt signaling node by 3K3A-APC induces proliferation, migration, survival, and differentiation of NSCs. SphK-1, sphingosine kinase-1; SphK-2, sphingosine kinase-2.
Regenerative activities of signaling-selective 3K3A-APC for human NSCs. (A-C) 3K3A-APC stimulates neuronal proliferation and differentiation from human embryo-derived NSCs in vitro. (A) Stimulation of human NSCs proliferation in culture by 3K3A-APC requires PAR1 and PAR3 but not PAR2. Quantification of proliferation was based on the percentage of Ki-67+/nestin+ cells in culture after 48 hours in the presence and absence of 3K3A-APC (2 nM) and/or PAR1-, PAR2-, and PAR3-specific cleavage site blocking antibodies. (B-C) Enhancement of neuronal proliferation and differentiation of NSCs by 3K3A-APC requires activation of Akt. Activation of the PI3K/Akt pathway by human recombinant 3K3A-APC (2 nM) in human NSCs requires PAR1 and PAR3 (B) and SphK-1 but not SphK-2 (C). Phosphorylation of Akt at Ser473 (pAkt) and total Akt was determined 3 hours after 3K3A-APC or vehicle treatment by western blot in whole-cell extracts of NSCs transfected with PAR1, PAR3, SphK-1, SphK-2, or control small interfering RNA. Intensity of pAkt signal was determined by scanning densitometry and normalized to total Akt. Data are shown as mean ± SEM, n = 3 independent cultures in triplicate. Statistical significance was determined by 1-way analysis of variance followed by Tukey’s post hoc test. NS, not significant. (For details regarding panels A-C, see Guo et al70 ). (D) Schematic overview of APC’s regenerative activities for human NPCs. Activation of the PI3K/Akt pathway in NSCs by 3K3A-APC requires PAR1, PAR3, S1P1, SphK-1, and EPCR. Through integration of signaling linked to multiple downstream effectors, activation of the PI3K/Akt signaling node by 3K3A-APC induces proliferation, migration, survival, and differentiation of NSCs. SphK-1, sphingosine kinase-1; SphK-2, sphingosine kinase-2.
Potential of stem cells and APC combination therapy
The findings above stimulated a subsequent study that asked whether 3K3A-APC therapy combined with NSC delivery might be developed as an effective combination therapy for neuronal replacement and circuit repair after stroke.101 This study investigated the effects of late postischemic 3K3A-APC treatment on the production in vivo of neurons from transplanted human fetal NSCs,102 and the effects of this combination therapy on long-term neurological recovery and the restoration of disrupted neural circuitry in the postischemic mouse brain. Using the permanent distal MCAO model in mice, this study showed that late postischemic treatment of mice with 3K3A-APC administered 7 days after distal MCAO–induced stroke stimulated neuronal production by human NSCs transplanted into the mouse brain, promoted circuit restoration, and improved functional recovery.101 This study elegantly demonstrated the improved functional integration of grafted NSCs into the host neuronal circuitry as a result of 3K3A-APC treatment, suggesting 3K3A-APC may potentiate the integration and neurogenic activity of transplanted human NSCs in vivo in man. This combination therapy could be of interest not only in the repair of stroke-damaged neural circuits but also in regard to NSC delivery for various neurological disorders characterized by discrete neuron loss.
Therapy with human stem cells holds promise for the treatment of stroke and other CNS disorders.103,104 Stem cell therapy for stroke has been validated in multiple stroke models in rodents, large mammals, and primates.105-109 These studies have repeatedly suggested that grafted cells homing to the damaged brain regions can exert multiple beneficial effects such as neuron protection, anti-inflammation, neovascularization, and proneurogenesis, which all ultimately result in improved neurological outcomes.103,105,107 As noted above, the study of Wang et al provided direct evidence for functional integration of transplanted cells into the host neural circuits, which is accompanied by substantial improvement in sensory-motor performance after stroke.101 This suggests that this combination approach employing 3K3A-APC may potentially be used for late treatment of stroke in patients in ongoing phase 1 (NCT01151124) and phase 2 (NCT02117635) clinical trials that involve directly injecting manufactured NSCs into the brain of patients that remain moderately to severely disabled following an ischemic stroke. For example, the phase 1 PISCES trial found that a single intracerebral injection of up to 20 million NPCs did not have adverse events and was associated with improved neurological function.110 As the 3K3A-APC regimen for ischemic stroke (RHAPSODY) has proven to be safe in ischemic stroke patients,99 APC-based combination therapies combined with NSC transplantation in ongoing and future clinical trials may help regeneration of stroke-damaged CNS circuits.
Conclusions
APC initiates biased cell signaling via cleavage at Arg46 in PAR1 in combination with other cell receptors. This nonhemostatic property of signaling selective 3K3A-APC provides pharmacologic benefits that merit translation to the clinic.17 3K3A-APC exerts neuroprotective actions via multiple cells of the neurovascular unit and appears to be safe in ischemic stroke patients. This justifies further clinical testing for efficacy in ischemic stroke patients. The discovery that 3K3A-APC combined with human NSCs leads to enhanced neurogenesis should encourage translational research centered on 3K3A-APC not only for ischemic stroke but also for multiagent, combination therapies with NSCs for multiple neuropathologies.
Acknowledgments
The authors apologize to colleagues whose work was not cited due to limitations on text size and number of references.
This work was supported in part by grants from the National Institutes of Health (NIH), National Heart, Lung, and Blood Institute HL104165, HL142975, and HL130678 (L.O.M.), HL031950, HL052246, HL142975, and HL133728 (J.H.G.), and NIH, National Institute of Neurological Disorders and Stroke NS090904 (B.V.Z.).
Authorship
Contribution: J.H.G., B.V.Z., and L.O.M. wrote the paper, and all coauthors read and approved the paper.
Conflict-of-interest disclosure: B.V.Z. is a founder of ZZ Biotech LLC, a biotechnology company with a mission to develop APC and its functional mutants for the treatment of stroke and other neurological disorders. L.O.M. and J.H.G. are inventors for some uses of APC mutants, and J.H.G. is a consultant for ZZ Biotech LLC.
Correspondence: John H. Griffin, Department of Molecular Medicine, The Scripps Research Institute, 10550 N. Torrey Pines Rd, La Jolla CA 92037; e-mail: jgriffin@scripps.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal