Abstract
Thrombomodulin (TM) is an integral component of a multimolecular system, localized primarily to the vascular endothelium, that integrates crucial biological processes and biochemical pathways, including those related to coagulation, innate immunity, inflammation, and cell proliferation. These are designed to protect the host from injury and promote healing. The “traditional” role of TM in hemostasis was determined with its discovery in the 1980s as a ligand for thrombin and a critical cofactor for the major natural anticoagulant protein C system and subsequently for thrombin-mediated activation of the thrombin activatable fibrinolysis inhibitor (also known as procarboxypeptidase B2). Studies in the past 2 decades are redefining TM as a molecule with many properties, exhibited via its multiple domains, through its interacting partners, complex regulated expression, and synthesis by cells other than the endothelium. In this report, we review some of the recently reported diverse properties of TM and how these may impact on our understanding of the pathogenesis of several diseases.
Introduction
Thrombomodulin (TM) is central to the normal function of all organs, as it is expressed by endothelial cells that line all vessels (arteries, veins, capillaries, and lymphatics).1-3 It is thus well positioned to participate in the regulation of coagulation, innate immunity, inflammation, and cell trafficking. TM is also synthesized by several cell types, in mice and humans, including hematopoietic progenitor cells, dendritic cells, urothelial cells, vascular smooth muscle cells, monocytes and macrophages, neutrophils, the syncytiotrophoblast of the placenta, corneal epithelial cells of the eye, keratinocytes, lung alveolar epithelium, mesothelial epithelium, and some tumor cells.4-13 In this review, we explore “traditional” and “nontraditional” roles of TM within and beyond the hemostatic system.
Structure
Mature human TM is a 557-amino-acid residue, type 1 transmembrane glycoprotein encoded by the intronless gene THBD. TM comprises 5 structural regions14,15 (Figure 1). The most N-terminal region comprises 2 modules, the first being a 155-amino-acid residue C-type lectin-like domain with homology to the C-type lectins16 of CD93 and CD248, both of which play roles in inflammation, innate immunity, and cancer.17,18 The lectin-like domain of TM contains 2 sites for N-linked glycosylation as well as calcium-binding and carbohydrate-recognition sites that participate in homotypic interactions that may regulate cell trafficking.19 The second hydrophobic 67-amino-acid module, C-terminal to the lectin-like module, is joined to a region comprising 6 epidermal growth factor-like (EGF-like) repeats, best characterized for their roles in coagulation and fibrinolysis. The EGF-like region contains 2 sites for N-linked glycosylation and 2 methionines that are sensitive to oxidation, one of which (between EGF4 and EGF5),20 when oxidized, abrogates its anticoagulant function. The third region of TM is serine-threonine rich and has sites for O-linked glycosylation and variable attachment of a chondroitin sulfate. The fourth region is a single-pass transmembrane stretch that connects to the 36-amino-acid residue cytoplasmic tail.
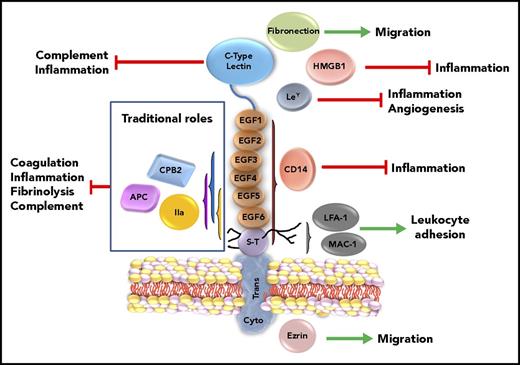
Schematic of TM structure with putative functional correlates. TM is represented by its 5 structural regions, with its traditional and nontraditional roles. Interactions of TM with thrombin that generate APC and CPB2 that dampen coagulation, inflammation, fibrinolysis, and complement are highlighted in the box on the left. The lectin-like domain of TM interferes with complement activation and inflammation. The lectin-like domain of TM also has several putative ligands, including fibronectin, HMGB1, and Ley, which are reported to impact migration, inflammation, and angiogenesis. The EGF-like region and serine-threonine–rich (S-T) region, via the chondroitin sulfate (shown as black lines from S-T regions), bind to CD14 and adhesion molecules LFA-1 and MAC-1, modulating responses to endotoxin and regulating leukocyte trafficking. Connected to the ectodomain via the transmembrane region (Trans), the cytoplasmic region (Cyto) binds, in some cells, to the adaptor protein ezrin, which alters the actin cytoskeleton and modulates cell migration. EPCR and PF4 are not shown. IIa, thrombin.
Schematic of TM structure with putative functional correlates. TM is represented by its 5 structural regions, with its traditional and nontraditional roles. Interactions of TM with thrombin that generate APC and CPB2 that dampen coagulation, inflammation, fibrinolysis, and complement are highlighted in the box on the left. The lectin-like domain of TM interferes with complement activation and inflammation. The lectin-like domain of TM also has several putative ligands, including fibronectin, HMGB1, and Ley, which are reported to impact migration, inflammation, and angiogenesis. The EGF-like region and serine-threonine–rich (S-T) region, via the chondroitin sulfate (shown as black lines from S-T regions), bind to CD14 and adhesion molecules LFA-1 and MAC-1, modulating responses to endotoxin and regulating leukocyte trafficking. Connected to the ectodomain via the transmembrane region (Trans), the cytoplasmic region (Cyto) binds, in some cells, to the adaptor protein ezrin, which alters the actin cytoskeleton and modulates cell migration. EPCR and PF4 are not shown. IIa, thrombin.
Soluble fragments of the ectodomain of TM (defined by the entire molecule except the transmembrane and cytoplasmic regions) also circulate in the blood at concentrations of ∼3-50 ng/mL.21,22 These are generated via proteolytic cleavage of the integral membrane protein. The responsible enzymes are poorly characterized but likely include leukocyte-derived proteases (eg, elastase and cathepsins), metalloproteases,23,24 and the intramembranous serine protease rhomboid-like-2.25 One group has characterized the fragments in urine, similar to those in plasma, by N-terminal sequencing; most have either an intact region 1 and/or an intact EGF region.26 During disorders associated with vascular damage, including infections, sepsis, diabetes, disseminated intravascular coagulation (DIC), and inflammation, soluble TM levels may be increased.27 However, this is not consistent, probably reflecting varying degrees of clearance of different fragments. Studies on the utility of soluble TM as a predictive marker of coronary heart disease and atherosclerosis have been conflicting.28,29 However, as an independent marker of disease, soluble TM levels are probably not reliable. The physiologic roles of the different fragments are also not known but may be speculated upon, as isolated fragments, such as EGF4-EGF6, may retain some function30 (see below).
Traditional properties of TM
TM was discovered in the 1980s31 following recognition that the zymogen protein C (PC) was converted by thrombin to activated protein C (APC). Acceleration of the reaction in the presence of the vasculature led to a hunt for an endothelial-associated cofactor. TM was thus isolated and determined to accelerate thrombin-mediated PC activation >1000-fold relative to thrombin alone.32 Thrombin’s anion-binding exosite I binds to EGF5-6 of TM,33 while EGF4-6 is necessary for PC cleavage/activation34,35 (reviewed in Morser36 ) (Table 1). The chondroitin sulfate moiety provides a second site for thrombin binding, enhancing its affinity ∼10-fold.37,38 PC activation is further increased ∼5-20-fold by the transmembrane endothelial cell protein C receptor (EPCR).39 Catalytic activity is additionally increased ∼25-fold by platelet factor 4 (PF4) binding to the chondroitin sulfate,40 resulting in local accumulation of APC at an injury site where platelet activation/aggregation occurs.41
Structure–function correlates of TM
| Structure . | Residues . | Function/interaction . |
|---|---|---|
| Lectin-like domain | 1-154 | Inhibits neutrophil adhesion |
| Mediates cell–cell interactions via homotypic interactions | ||
| Binding site for HMGB1, Ley | ||
| Inhibits complement activation | ||
| Binding site for kringle 1-5 fragment of plasminogen and urokinase receptor | ||
| Binding site for fibronectin | ||
| Hydrophobic | 155-222 | — |
| EGF1-EGF2 | 223-304 | Binding site for HMGB1 required for its cleavage by thrombin |
| EGF3-EGF6 | 305-462 | Activation of proCPB2 |
| EGF4-EGF6 | 346-462 | Activation of PC |
| Thrombin neutralization by PCI | ||
| Mitogenic | ||
| EGF5-EGF6 | 387-462 | Thrombin-binding site (high affinity) |
| Inactivation of scu-PA (+CS) | ||
| Binding to GPCR15 on T cells | ||
| Anti-inflammatory (?) | ||
| EGF1-EGF6 | 223-462 | Mitogenic |
| Binding to CD14 | ||
| Ligand for EGFR1 | ||
| Chondroitin sulfate in serine-threonine–rich region | 463-495 | Thrombin-binding site (low affinity) |
| Enhances neutralization of single chain urokinase (scu-PA) | ||
| Enhances neutralization of thrombin by PCI and heparin-antithrombin | ||
| PF4 binding, which enhances activation of PC | ||
| Binding to CD14 | ||
| Binding to integrins, MAC-1, and LFA-1 | ||
| Transmembrane | 496-521 | Target for TM cleavage by rhomboid-like-2 serine protease |
| Cytoplasmic tail | 522-557 | Binding site for adaptor protein ezrin, which modulates the cytoskeleton |
| Site of threonine phosphorylation |
| Structure . | Residues . | Function/interaction . |
|---|---|---|
| Lectin-like domain | 1-154 | Inhibits neutrophil adhesion |
| Mediates cell–cell interactions via homotypic interactions | ||
| Binding site for HMGB1, Ley | ||
| Inhibits complement activation | ||
| Binding site for kringle 1-5 fragment of plasminogen and urokinase receptor | ||
| Binding site for fibronectin | ||
| Hydrophobic | 155-222 | — |
| EGF1-EGF2 | 223-304 | Binding site for HMGB1 required for its cleavage by thrombin |
| EGF3-EGF6 | 305-462 | Activation of proCPB2 |
| EGF4-EGF6 | 346-462 | Activation of PC |
| Thrombin neutralization by PCI | ||
| Mitogenic | ||
| EGF5-EGF6 | 387-462 | Thrombin-binding site (high affinity) |
| Inactivation of scu-PA (+CS) | ||
| Binding to GPCR15 on T cells | ||
| Anti-inflammatory (?) | ||
| EGF1-EGF6 | 223-462 | Mitogenic |
| Binding to CD14 | ||
| Ligand for EGFR1 | ||
| Chondroitin sulfate in serine-threonine–rich region | 463-495 | Thrombin-binding site (low affinity) |
| Enhances neutralization of single chain urokinase (scu-PA) | ||
| Enhances neutralization of thrombin by PCI and heparin-antithrombin | ||
| PF4 binding, which enhances activation of PC | ||
| Binding to CD14 | ||
| Binding to integrins, MAC-1, and LFA-1 | ||
| Transmembrane | 496-521 | Target for TM cleavage by rhomboid-like-2 serine protease |
| Cytoplasmic tail | 522-557 | Binding site for adaptor protein ezrin, which modulates the cytoskeleton |
| Site of threonine phosphorylation |
CS, chondroitin sulfate; PCI, protein C inhibitor; scu-PA, single chain urokinase.
When complexed with TM, the substrate specificity of thrombin switches from procoagulant to anticoagulant. Thrombin-TM is unable to cleave fibrinogen, activate factor V or factor XIII, or cleave protease activated receptors −1 or −3 (PAR-1, PAR-3) on endothelial cells or platelets.42 Conversely, thrombin-TM efficiently converts PC to APC, which in complex with protein S, specifically proteolyses factors Va and VIIIa. APC thereby downregulates further thrombin generation, a mechanism that underlies the physiologic relevance of TM as a cofactor in a major natural anticoagulant system. The relevance of the PC-TM mechanism is evident by the increased risk of thrombosis in individuals with impaired function of PC or protein S,43 and in those with factor VLeiden.44
Thrombin-TM also accelerates activation of the plasma procarboxypeptidase B (proCPB2), which catalyzes removal of C-terminal basic amino acids from its substrates. ProCPB2 (also known as TAFI) is encoded by the CPB2 gene.45 The cofactor activity of TM for thrombin-mediated proCPB2 activation resides in EGF3-EGF6. This overlaps, but does not compete with, the TM structure required for PC activation.46 Interestingly, although oxidation of the methionine at position 388 between EGF4 and EGF5 reduces PC activation, it does not affect proCPB2 activation,20,47 indicating differential regulation during inflammation. Activated proCPB2 (CPB2) inhibits fibrinolysis by removing lysine residues from partially degraded fibrin, thereby interfering with binding of plasminogen and tissue-type plasminogen activator and their integration into the clot. This reduces fibrinolysis, as well as generation of plasmin by prourokinase.48,49 Other substrates of CPB2 include bradykinin, osteopontin, anaphylatoxins C3a, C5a, and chemerin, extending its properties beyond hemostasis.50,51 Elevated CPB2 levels correlate with an increased risk of chronic thromboembolic pulmonary hypertension, and in mouse models, reducing CPB2 ameliorates thrombotic disease.52
APC reveals nontraditional roles for TM
A link between the PC system and sepsis was confirmed in the 1980s when nonhuman primates, infused with Escherichia coli, were protected from its coagulopathic and organ-damaging effects by APC- and PC-blocking antibodies.53 Dependent on TM for its generation, APC has profound anti-inflammatory and cytoprotective effects.54 These are in part achieved by interfering with nuclear translocation of NF-κB and activation of AP-1, which also dampen expression of cell-surface leukocyte adhesion molecules and leukocyte-endothelial interactions. EPCR, expressed by endothelial cells, monocytes, neutrophils eosinophils, and dendritic cells,55 facilitates the anti-inflammatory effects of APC. APC binds to EPCR and can cleave PAR-1 and PAR-356 and/or induce β-arrestin-2–biased PAR1 signaling that is independent of the protease cleavage site.57 In either case, a transcriptional response is triggered that results in protection of the vascular endothelium from apoptosis while promoting barrier function. APC also inhibits neutrophil activation and neutrophil extracellular trap release,58 and it cleaves and detoxifies histones, a major proinflammatory and prothrombotic component of neutrophil extracellular traps.59
Structures of TM provide novel nontraditional functional insights
The lectin-like domain
Mice lacking the lectin-like domain of TM are more sensitive to endotoxin-induced lung damage and death, myocardial and lung ischemia-reperfusion injury,60,61 and inflammatory arthritis.62 This domain, soluble or part of the intact molecule, is anti-inflammatory and cytoprotective, attenuating MAPK pathways and NF-κB translocation and interfering with neutrophil adhesion to endothelial cells,60 partly by suppressing ICAM-1 and VCAM-1. Soluble lectin-like domain protects against ischemia-reperfusion injury and inflammation in several mouse models by APC-independent mechanisms.
Several groups have reported that the lectin-like domain of TM has binding partners that participate in modulating innate immunity. The physiologic relevance of these interactions is difficult to confirm, partly because yeast-expression systems that generate recombinant TM modified by sugars foreign to mammalian systems were often used. With that note of caution, but strong evidence that the lectin-like domain does regulate the inflammatory response, we herein present some examples.
HMGB1 is a nuclear chromatin-binding protein that is released by damaged or necrotic cells, whereupon it engages TLR4 and the receptor for advanced glycation endproducts (RAGE), triggering proinflammatory signaling pathways, generation of reactive oxygen species and NF-κB activation. Like TM, TLR4 and RAGE are widely expressed on the endothelium and thus well-positioned for cross talk with TM. When released in response to injury, HMGB1 becomes sequestered by the lectin-like domain of TM, unable to interact with RAGE,63 and susceptible to degradation by thrombin, possibly via additional binding to EGF1-EGF2.64 Several in vivo preclinical studies (eg, sepsis, myocardial ischemia, and stroke) have been performed in which benefit has been achieved by administration of recombinant forms of TM that encompass the lectin-like domain that is coincident with suppression of plasma levels of HMGB1.65-67
Lewisy (Ley) is a carbohydrate moiety that decorates glycoproteins, providing them with functions relevant to leukocyte trafficking. Ley is increased in inflamed endothelial cells, where it facilitates leukocyte recruitment, adhesion, rolling, and transmigration. In vitro and in vivo studies indicate that the lectin-like domain of TM binds to Ley, reducing leukocyte adhesion/transmigration and protecting mice from vascular injury, atherosclerosis, and peritonitis (Figure 2). The relationship between Ley and HMGB1 in the context of TM has not been examined, although one might expect them to cooperate. In contrast to the protective properties of endothelial TM, monocytes expressing TM adhere to Ley-decorated endothelium, triggering p38 MAPK phosphorylation, thereby activating β2-integrin, which mediates firm monocyte-endothelial interactions.68 Mice that lack myeloid TM exhibit reduced macrophage infiltration and neointima formation after arterial injury.
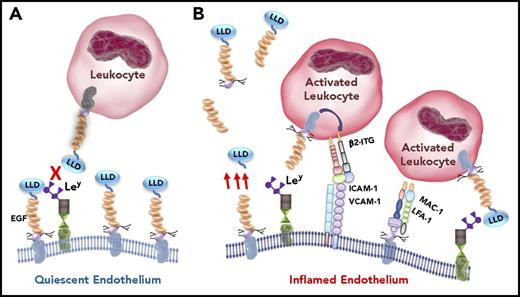
A postulated paradigm of TM–leukocyte interactions. Under quiescent conditions (A), endothelial TM is intact. Its lectin-like domain binds to whatever Ley is exposed by endothelial glycoproteins, blocking Ley interactions with TM expressed by circulating leukocytes, thereby preventing leukocyte adhesion. During inflammatory conditions (B), endothelial cells become activated. TM expression is reduced, and various fragments of the ectodomain of TM are released (only a couple are shown schematically) into the circulation (red arrows) by proteolytic cleavage of the membrane-bound form. Remaining EGF4-EGF5 is oxidized, diminishing the generation of APC (not shown). ICAM-1, VCAM-1, and Ley expression on glycoproteins is increased on the endothelial cell surface. Ley binds to the lectin-like domain of TM expressed by activated leukocytes, which induces activation of leukocyte β2-integrins (β2-ITG). These bind to endothelial cell adhesion molecules ICAM-1 and VCAM-1, enabling firm leukocyte adhesion and transmigration. Exposed by proteolysis of the intact protein, the serine-threonine–rich region of TM provides a binding site, likely via the chondroitin sulfate, for leukocyte-expressed adhesion molecules LFA-1 and MAC-1, further promoting leukocyte-endothelial cell attachment and inflammation. Therapeutic recombinant TM comprising the entire ectodomain, or the lectin-like domain alone, or the EGF-like region plus the serine-threonine rich region (TMD23), exhibit anti-inflammatory properties by blocking leukocyte-endothelial interactions. LLD, lectin-like domain.
A postulated paradigm of TM–leukocyte interactions. Under quiescent conditions (A), endothelial TM is intact. Its lectin-like domain binds to whatever Ley is exposed by endothelial glycoproteins, blocking Ley interactions with TM expressed by circulating leukocytes, thereby preventing leukocyte adhesion. During inflammatory conditions (B), endothelial cells become activated. TM expression is reduced, and various fragments of the ectodomain of TM are released (only a couple are shown schematically) into the circulation (red arrows) by proteolytic cleavage of the membrane-bound form. Remaining EGF4-EGF5 is oxidized, diminishing the generation of APC (not shown). ICAM-1, VCAM-1, and Ley expression on glycoproteins is increased on the endothelial cell surface. Ley binds to the lectin-like domain of TM expressed by activated leukocytes, which induces activation of leukocyte β2-integrins (β2-ITG). These bind to endothelial cell adhesion molecules ICAM-1 and VCAM-1, enabling firm leukocyte adhesion and transmigration. Exposed by proteolysis of the intact protein, the serine-threonine–rich region of TM provides a binding site, likely via the chondroitin sulfate, for leukocyte-expressed adhesion molecules LFA-1 and MAC-1, further promoting leukocyte-endothelial cell attachment and inflammation. Therapeutic recombinant TM comprising the entire ectodomain, or the lectin-like domain alone, or the EGF-like region plus the serine-threonine rich region (TMD23), exhibit anti-inflammatory properties by blocking leukocyte-endothelial interactions. LLD, lectin-like domain.
Ley is also expressed by lipopolysaccharides (LPS) of E coli and Klebsiella pneumoniae. Recombinant lectin-like domain of TM binds to these gram negative bacteria, inducing their agglutination and rapid clearance by macrophages. Recombinant TM also interferes with LPS binding to its coreceptor, CD14, probably via the serine-threonine–rich region, reducing proinflammatory reactions,69 with reduced nitric oxide, activation/phosphorylation of MAPKs, and NF-κB signaling70 (Figure 3). In E coli–induced pyelonephritis, mice lacking the lectin-like domain accumulated a larger bacterial load in the bladder and kidney.70 The differential function of monocyte TM is again evident in that it interacts via Ley/LPS with CD14 and the TLR4/myeloid differentiation factor-2 complex, triggering a proinflammatory response to LPS. Thus, mice deficient in myeloid TM are protected from K pneumoniae–induced sepsis.71
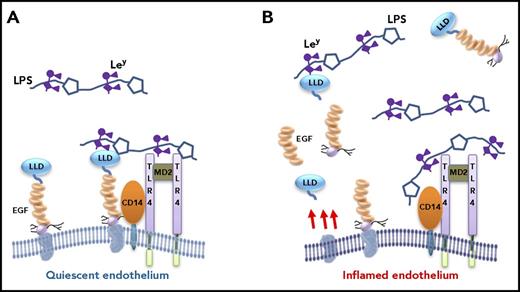
Postulated role of TM in modulating response to endotoxin/sepsis. LPS from gram-negative bacteria bind to the coreceptor CD14 of monocytes (not depicted) and endothelial cells, triggering proinflammatory intracellular signaling pathways via activation of the TLR4-MD2 complex. In the early stages of infection/inflammation (A), when the endothelium is unperturbed and in a quiescent state, TM interferes with LPS-CD14 interactions in 2 ways: (1) the lectin-like domain of TM binds to LPS via Ley, and (2) the serine-threonine–rich region of TM binds to CD14. Both would predictably block LPS-CD14 interactions, thereby interfering with the proinflammatory response. As the balance tips, and inflammation persists and progresses, the endothelial cells become inflamed and activated (B). TM expression decreases, soluble fragments of TM are released via proteolysis (only some of which are represented), and the TM-dependent protective mechanisms on the endothelium are reduced or lost. Expression of adhesion molecules and Ley on the endothelial cells increase (not depicted), activated leukocytes adhere (Figure 2), and the inflammatory response escalates. Again, therapeutic recombinant TM comprising the entire ectodomain, or the lectin-like domain alone, or the EGF-like region plus the serine-threonine–rich region (TMD23) will likely be of benefit, in part, by blocking LPS-CD14 interactions. LLD, lectin-like domain.
Postulated role of TM in modulating response to endotoxin/sepsis. LPS from gram-negative bacteria bind to the coreceptor CD14 of monocytes (not depicted) and endothelial cells, triggering proinflammatory intracellular signaling pathways via activation of the TLR4-MD2 complex. In the early stages of infection/inflammation (A), when the endothelium is unperturbed and in a quiescent state, TM interferes with LPS-CD14 interactions in 2 ways: (1) the lectin-like domain of TM binds to LPS via Ley, and (2) the serine-threonine–rich region of TM binds to CD14. Both would predictably block LPS-CD14 interactions, thereby interfering with the proinflammatory response. As the balance tips, and inflammation persists and progresses, the endothelial cells become inflamed and activated (B). TM expression decreases, soluble fragments of TM are released via proteolysis (only some of which are represented), and the TM-dependent protective mechanisms on the endothelium are reduced or lost. Expression of adhesion molecules and Ley on the endothelial cells increase (not depicted), activated leukocytes adhere (Figure 2), and the inflammatory response escalates. Again, therapeutic recombinant TM comprising the entire ectodomain, or the lectin-like domain alone, or the EGF-like region plus the serine-threonine–rich region (TMD23) will likely be of benefit, in part, by blocking LPS-CD14 interactions. LLD, lectin-like domain.
The lectin-like domain of TM is claimed also to be a receptor for the kringle 1-5 fragment of plasminogen, regulating its angiogenic property via internalization and degradation.72 Fibronectin also reportedly binds to the lectin-like domain, promoting cell migration, tube formation, and leukocyte adhesion.73 These interactions require further study to establish their relevance.
In models of inflammatory arthritis, Shiga toxin–induced hemolytic anemia, and diabetic nephropathy, mice lacking the lectin-like domain exhibit increased complement activation.62,74,75 Although not limited to the lectin-like domain, 2 mechanisms have been described by which TM regulates complement. The first is via thrombin-TM generation of CPB2 (dependent on EGF3-EGF6), which in turn inactivates C3a and C5a.50 In the second, TM is a cofactor for factor I/factor H inactivation of C3b, preventing assembly of the C3 convertase.76 Mutations of THBD confer an increased risk of developing the complement mediated thrombotic microangiopathy, atypical hemolytic uremic syndrome.76 Atypical hemolytic uremic syndrome is likely triggered by an infectious/inflammatory event that suppresses TM expression, which in combination with a THBD mutation sets off an escalating cascade of complement and coagulation activation with endothelial damage and organ failure.
EGF-like repeats and the serine-threonine domain
TMD23 encompasses the EGF-like and serine-threonine–rich regions. Yeast-expressed recombinant TMD23 exhibits angiogenic and mitogenic effects in an APC-independent manner by increasing expression of matrix metalloproteinase 9 (MMP-9) and plasminogen activators in rat corneal implant77 and hindlimb ischemia neovascularization models. This recombinant TMD23 is also a ligand for endothelial expressed proangiogenic fibroblast growth factor receptor 1, inducing its activation/phosphorylation.78 These effects of soluble TMD23 are in contrast to the antiangiogenic properties of soluble lectin-like domain that interferes with Ley-induced chemotaxis and tube formation in vitro and tumor angiogenesis in vivo.79 The apparent disparities may be related to differences in glycosylation between yeast and mammalian expression systems. Indeed, TMD23 derived from a mammalian cell line did not exhibit angiogenic properties in a model of keratitis.80
Recombinant TMD23 also binds to soluble and membrane-bound CD14 expressed by monocytes/macrophages and endothelial cells and thus blocks the interaction between membrane-bound TM and CD14, reducing release of tumor necrosis factor α and interleukin 6 (IL-6) and increasing the survival of mice exposed to LPS.81 Within TMD23 (soluble or membrane bound), the serine-threonine region is the predominant anti-inflammatory structure, which is interesting for 2 reasons: (1) this region contains a second thrombin-binding site (the chondroitin sulfate) of lower affinity than EGF5-EGF6,37 and (2) it is a binding site (probably via the chondroitin sulfate) for integrins LFA-1 and MAC-182 that are expressed by mononuclear leukocytes. Integrin-TM interactions may promote leukocyte adhesion to the endothelium during inflammatory disorders. We speculate that in the setting of vascular injury/inflammation, the lectin-like and EGF-like domains are variably removed from membrane-bound TM by proteolytic cleavage while retaining the serine-threonine fragment for the recruitment of inflammatory leukocytes to the site of injury.82 Indeed, as noted before, multiple soluble TM fragments of varying lengths can be identified at low levels in plasma and urine, and these may retain function23,24 (Figure 3). Interestingly, APC also binds to β-integrins, blocking neutrophil adhesion to the endothelium.83 With endothelial damage and proteolytic loss of the EGF-like repeats and/or cytokine induced suppression of TM, APC generation would be lost, allowing recruitment of inflammatory cells.
Histones H3 and H4 bind to both PC and TM, interfering with activation of PC and enhancing thrombin generation in vitro.59 Recombinant TM (the entire ectodomain) protects mice from histone-induced thrombocytopenia, thrombosis, and lethality, interfering with histone binding to endothelial TM. This effect is largely independent of degradation of the histones by APC but due to blocking of histone interactions with endothelial TM. It is not known where on TM histones interact, although likely candidates are EGF4-EGF6 and/or the lectin-like domain.84 Again, with respect to TM, the relationships among histones, HMGB1, and Ley has not been examined.
Cytoplasmic domain
Although mice lacking the cytoplasmic domain of TM appear normal, recent studies suggest that a threonine within this structure may be phosphorylated following direct binding to the adaptor protein, ezrin, which in turn modulates the actin cytoskeleton.85 TM knockdown causes morphologic changes in the cells, accelerating their migration. Indeed, in several epithelial cell tumors, reduced TM expression disrupts epithelial morphology and favors cell migration and metastasis. In keratinocytes, which also express TM, such an interaction has a minor impact on wound healing in a mouse model.86 This role of TM has not been evaluated in endothelial cells, where another member of the ezrin family, moesin, is more prominent.
TM plays traditional and nontraditional roles in disease
In the following section, we describe the putative roles of TM in several diseases. This is not a comprehensive list, but it serves to illustrate that TM, via traditional and nontraditional pathways, has multiple effects on disease pathogenesis and/or progression.
TM and malignancy
Numerous preclinical and clinical studies support the notion that TM has tumor-suppressor properties, dampening cell proliferation, invasion, and metastasis.87-89 Indeed, TM expression by tumor cells and the tumor endothelium often directly correlates with a better prognosis, evident in cancers of the bladder, breast, colon/rectum, prostate, lung, and oral epithelium (reviewed in Hanly et al90 ). Several mechanisms have been proposed.
Epithelial-mesenchymal transition (EMT) plays an important role in the initiation of cancer and metastasis, as the epithelial cells transform from a healthy, quiescent phenotype to an aggressive, mesenchymal-like phenotype.91 Reversal of EMT is believed to be a major mechanism by which TM exerts antitumor properties; ie, mesenchymal markers expressed by tumor cells (eg, N-cadherin, vimentin, fibronectin, Twist, and Snail) are suppressed, whereas epithelial markers such as E-cadherin are upregulated.88,92-94 These changes normalize the cell’s proliferative and migratory properties and may increase sensitivity to chemotherapy.94 The structure of TM responsible for inducing EMT has not been delineated. The EGF-containing and serine-threonine–rich regions could play a role, as TMD23 accelerates corneal epithelial cell proliferation, migration, differentiation, and wound healing in mice.11 The lectin-like domain has also been implicated.87,89
The antitumor effects of TM also include activation of PC and proCPB2. Thus, in a mouse model of pancreatic cancer, recombinant TM interfered with tumor growth by inhibiting NF-κB and thrombin-induced PAR-1 activation.95 In a breast cancer model, TM-dependent generation of CPB2 reduced plasmin generation and extracellular matrix proteolysis, dampening cell invasion and metastasis.96 In spite of the preceding promising findings, few human cancer trials of soluble TM have been performed, and these primarily assessed effects on DIC.97
Atherosclerosis
In atherosclerosis, TM localizes to macrophages and smooth muscle cells of the intima and media, as well as vasa vasorum endothelial cells and associated atheroma.98 TM is downregulated on endothelial cells that overly atherosclerotic lesions, findings consistent with local increases in thrombosis and inflammation.99 Antiatherogenic, anti-inflammatory, and endothelial-protective agents, such as fenofibrate and statins, upregulate endothelial TM by antagonizing NF-κB, JNK, and p38 pathways.100 The role of TM in vascular smooth muscle cells and macrophages of atheromatous lesions has not been defined. However, it is possible that it actually escalates damage, since monocyte TM promotes adhesion to activated endothelium and smooth muscle cell TM may contribute to vascular calcification and apoptosis.101
Administration of yeast-expressed recombinant TMD23 reduced macrophage infiltration and atherosclerosis in ApoE−/− mice, and neointima formation in a carotid artery injury model.102 Interpretation of these findings is challenged by the high mannose-type sugars generally attached with Pichia pastoris–expressed proteins. Nonetheless, the effects were believed to be mediated by TM blocking thrombin-mediated PAR-1 activation and downstream proinflammatory events. Recent studies reveal that yeast-expressed recombinant soluble TM (the entire ectodomain) also activates endothelial expressed fibroblast growth factor receptor 1, which in turn activates phosphatidylinositol 3-kinase/Akt/mTOR signaling pathways that suppress endothelial autophagy and apoptosis and thus protects against atherosclerosis.103 Confirmation of these observations must follow with native forms of TM.
In addition to EMT in cancer, it is intriguing to consider that TM might also attenuate endothelial-mesenchymal transition. Endothelial-mesenchymal transition is increasingly recognized as being critical in the progression of cardiovascular diseases, where endothelial cells acquire a fibroblast-like morphology. This contributes to vascular calcification, atherosclerosis, pulmonary arterial hypertension, system sclerosis, and organ fibrosis.104
Transplantation-associated vasculopathies
Administration of soluble TM has benefit in transplantation-associated thrombotic microangiopathy, hematopoietic stem cell transplantation–associated coagulopathy and sinusoidal obstructive syndrome (also known as veno-occlusive disease), and steroid-refractory graft-versus-host disease (GVHD).105,106 The etiologies of these vary but have in common endothelial damage, a hypercoagulable state, and exaggerated cytokine production. The presence of specific single-nucleotide polymorphisms in the THBD gene were predictive of GVHD-related mortality, suggesting that alterations in TM may render the endothelium more vulnerable and leaky in response to stresses associated with these disorders.107 There are likely several mechanisms by which TM is protective. TM may enhance APC generation, which signals via PAR-2/PAR-3 to expand regulatory T cells, mitigating the disease in mice.108 In addition, recombinant EGF5 has recently been reported by one group to induce anti-inflammatory effects by binding to G-protein–coupled receptor 15 on T cells, increasing regulatory T cells, reducing IL-6 release, and hampering bone marrow–derived dendritic cells from inducing an alloreaction.109 Interestingly, calcineurin inhibitors are frequently used in the treatment of GVHD and may induce capillary leak by inducing phosphorylation of Src/vascular-endothelial (VE)-cadherin and translocation of VE-cadherin from the cell surface. The same group suggested that EGF4-EGF5 of TM may counteract that by interfering with Src-triggered VE-cadherin internalization and decreasing NF-κB activation and cytokine production.110,111 Validation of such intriguing observations with recombinant TM that is amenable for human use could be beneficial for these challenging clinical situations.
The complex case of myeloid TM and its role in aortic aneurysms
In an abdominal aortic aneurysm model in mice, TM was mainly expressed by infiltrating macrophages and vascular smooth muscle cells. Genetic ablation of THBD in myeloid cells, attenuated aortic dilatation and elastin destruction and reduced macrophage accumulation, cytokine release, MMP-9 expression, and generation of reactive oxygen species.7 Mice in which TM was deleted only in smooth muscle cells responded the same as wild-type mice. TM-deficient monocytes/macrophages released lower amounts of cytokines and exhibited reduced adhesion to endothelial cells. These findings, in which membrane-bound TM in monocytes enhanced release of proinflammatory mediators, monocyte adhesion, and oxidative stress, highlight the distinctly different properties of monocyte vs endothelial TM. How does one explain these differences? The answer remains a mystery, but these might be attributed to cell- or context-dependent changes in posttranslational modification, resulting in altered functional interactions with partner proteins.
Diabetic nephropathy
In mouse models, TM-dependent formation of APC protected against diabetic nephropathy by preventing glucose-induced apoptosis of glomerular endothelial cells and podocytes.112 The lectin-like domain of TM also participates; diabetic mice lacking the lectin-like domain of TM develop worse nephropathy, with increased complement deposition on the glomerular endothelium and podocytes.75 Recombinant lectin-like domain ameliorates the nephropathy, reducing albuminuria, fibrosis, and sclerosis.113 Fewer macrophages are recruited into the kidneys, HMGB1 release and VEGF expression are dampened, and renal tubular cell and glomerular endothelial apoptosis is ameliorated. Overall, the lectin-like domain of TM interferes with complement activation and blocks activation of inflammasome components by endothelial cells.113 Thus, TM attenuates NF-κB/NLRP3 signaling, reduces IL-1β and HMGB1 release, and enhances NRF2 antioxidant activity.113,114 The paradigm of dysfunction in the inflammasome is increasingly being recognized as underlying vascular disease, and evidence suggests that TM participates in its regulation.
TM and organ ischemia-reperfusion injury
In preclinical studies, soluble TM (particularly the lectin-like domain) protects the heart, lung, and kidney from ischemia-reperfusion injury by APC-dependent and APC-independent mechanisms.61,66,115 Microvascular leukocyte rolling and attachment is reduced, and endothelial barrier function is improved. In liver ischemia-reperfusion injury, recombinant TM (entire ectodomain or lectin-like domain) protects mice by reducing release of inflammatory cytokines and HMGB1 and interfering with HMGB1-TLR4 interactions.116 Similarly, in a cardiopulmonary bypass model, recombinant TM (ectodomain) protected rodents from acute lung injury by attenuating inflammation, pulmonary congestion, alveolar hemorrhage, neutrophil accumulation, and edema. Serum levels of HMGB1, tumor necrosis factor α, and IL-6 were also reduced compared with sham-treated controls.117
Preeclampsia
TM expression in the syncytiotrophoblast of the placenta raised questions as to its role in preeclampsia. Preeclampsia features reduced angiogenesis, with elevated soluble Flt-1 and endoglin, endothelial dysfunction, placental cellular apoptosis, hypertension, fibrin deposition, inflammation, and complement activation.118 TM levels in the syncytiotrophoblast are reduced in preeclampsia and correlate inversely with soluble Flt-1.9 TM messenger RNA levels correlate directly with villous/placental cell survival and expression of active MMP-2 and MMP-9 which are important for trophoblast invasion. THBD single-nucleotide polymorphisms have not been linked to preeclampsia.119 However, in preclinical models of preeclampsia, recombinant TM (ectodomain) significantly improved uteroplacental blood flow and oxygenation in the placenta and fetal brain and decreased fetal death, albeit to a nonsignificant extent.120 These findings are promising and support follow-up studies with different forms of soluble TM.
Pulmonary disease
Idiopathic pulmonary fibrosis is an inadequately treated, chronic, progressive respiratory disorder with interstitial fibrosis and inflammation. Acute exacerbations feature endothelial damage, elevated cytokines, and coagulopathy. In a small number of patients, soluble TM prolonged 3-month survival.121 In 2 mouse models of idiopathic pulmonary fibrosis, recombinant TM suppressed HMGB1, interferon-γ, transforming growth factor β1, apoptosis of lung epithelial cells, and lung fibrosis.122 These favorable findings are reminiscent of the effects of TM on EMT in cancer.
TM also dampens the inflammatory response in a murine model of bronchial asthma123 by modulating the immunostimulatory properties of dendritic cells. In allergic asthma, dendritic cells phagocytose environmental allergens, which determines whether the host response will be one of tolerance or immunogenic/allergic. Soluble TM or endothelial surface TM induces TM expression by dendritic cells, causing them to become tolerogenic. The lectin-like domain appears most responsible for this function, possibly by sequestering HMGB1. However, it is likely that the more traditional TM-related pathways also contribute, as both APC and CPB2 exhibit protective properties in asthma,124,125 a disease in which fibrin deposition contributes to the pathology.
Summary
The discovery of TM as a cofactor for thrombin-mediated activation of PC coincided with other investigators’ reports that the vascular endothelium is a dynamic organ with a repertoire of delicately regulated mechanisms to mount an immune, inflammatory and procoagulant response to injury.126 That a single molecule, such as TM, should possess multiple properties that integrate several apparently distinct biological systems is reasonable, given the importance of a coordinated response to numerous stresses. We have presented a summary of the diverse properties of TM beyond its traditional role in activating PC and proCPB2, in conjunction with a view of its potential role in selected diseases. Numerous questions are raised throughout the review. For example, of the multiple putative ligands for the lectin-like domain, which are most relevant, how do they interact, where do they bind, and what are their relative contributions in different clinical scenarios? What is the role of TM in different cells, such as hematopoietic stem cells? How does TM regulate EMT? Although there is no evidence yet that TM has efficacy in the treatment of patients with cancer or atherosclerosis or most other inflammatory disorders, soluble forms of TM have entered the clinic for sepsis and DIC.127,128 There is no doubt that the future will see targeted approaches129 to treat a range of inflammatory, immune, and proliferative disorders with tailored forms of TM, as well as approaches to strategically modify expression of TM to improve health.130
Acknowledgments
E.M.C. was supported by operating grants from the Canadian Institutes of Health Research, the Natural Sciences and Engineering Research Council of Canada, and the Canada Foundations for Innovation, holds a Tier 1 Canada Research Chair in Endothelial Cell Biology, and is an adjunct Scientist with the Canadian Blood Services.
Authorship
Contribution: E.M.C. and H.L. researched and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Edward M. Conway, Centre for Blood Research, 4306-2350 Health Sciences Mall, University of British Columbia, Vancouver, BC V6T 1Z3, Canada; e-mail: ed.conway@ubc.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal