Key Points
Selinexor is an oral XPO1 inhibitor with antimyeloma activity.
The RP2D is 45 mg/m2 (80 mg) selinexor plus 20 mg dexamethasone given twice weekly.
Abstract
Novel therapies are needed for patients with relapsed or refractory multiple myeloma (MM). We conducted a multicenter, phase 1 study in advanced hematological malignancies to assess the safety, efficacy, and recommended phase 2 dose (RP2D) of oral selinexor, a selective inhibitor of the nuclear export protein XPO1. In the dose-escalation phase, 25 patients with heavily pretreated MM (22) or Waldenstrom macroglobulinemia (3) were administered selinexor (3-60 mg/m2) in 8 or 10 doses per 28-day cycle. In the dose-expansion phase, 59 patients with MM received selinexor at 45 or 60 mg/m2 with 20 mg dexamethasone, twice weekly in 28-day cycles, or selinexor (40 or 60 mg flat dose) without corticosteroids in 21-day cycles. The most common nonhematologic adverse events (AEs) were nausea (75%), fatigue (70%), anorexia (64%), vomiting (43%), weight loss (32%), and diarrhea (32%), which were primarily grade 1 or 2. The most common grade 3 or 4 AEs were hematologic, particularly thrombocytopenia (45%). Single-agent selinexor showed modest efficacy with an objective response rate (ORR) of 4% and clinical benefit rate of 21%. In contrast, the addition of dexamethasone increased the ORR with all responses of ≥partial response occurring in the 45 mg/m2 selinexor plus 20 mg dexamethasone twice weekly cohort (ORR = 50%). Furthermore, 46% of all patients showed a reduction in MM markers from baseline. Based on these findings, we conclude that selinexor in combination with dexamethasone is active in heavily pretreated MM and propose a RP2D of 45 mg/m2 (80 mg) plus 20 mg dexamethasone given twice weekly. This trial was registered at clinicaltrials.gov as #NCT01607892.
Introduction
Despite significant improvements in the overall survival of patients with multiple myeloma (MM), the disease remains incurable and accounts for >80 000 annual deaths worldwide.1 Treatment options are limited for patients with MM whose disease has relapsed or is refractory to standard immunomodulatory drugs (IMiDs) and proteasome inhibitors (PIs).2 Although new monoclonal antibodies and small molecule therapeutics have shown activity in MM, none is curative and nearly all patients will succumb to their disease, highlighting the need for effective agents with novel mechanisms of action.3-7
Exportin 1 (XPO1, also called CRM1) is 1 of 8 known mammalian karyopherins responsible for the nuclear export of >200 cargo proteins, including nearly all tumor suppressor proteins (TSPs).8 In MM and other cancers, XPO1 is overexpressed, leading to enhanced transport of TSPs out of the nucleus and allowing cancer cells to evade genome surveillance and cell-cycle regulation.9-11 In addition, XPO1 in complex with the mRNA cap-binding protein eIF4E, transports multiple oncoprotein mRNAs (eg, c-Myc, cyclin D1, MDM2) to the cytoplasm, promoting their efficient synthesis on cytoplasmic ribosomes.12-14 Using genome-scale RNA interference, XPO1 was identified as a selective vulnerability in MM.9 In patients with MM, higher XPO1 levels correlate with poorer event-free and overall survival, as well as higher numbers of bone lytic lesions.15 These novel pleiotropic effects make XPO1 a unique target for therapeutic intervention.
Selinexor (KPT-330) is a first-in-class orally bioavailable selective inhibitor of nuclear export (SINE) compound that specifically blocks XPO1 by forming a slowly reversible covalent bond at cysteine-528 in the cargo binding groove of XPO1.16 By inhibiting XPO1, selinexor forces the nuclear retention and functional activation of TSPs and prevents the translation of oncoprotein mRNAs.9,15 This leads to selective induction of apoptosis in malignant cells, but largely sparing normal cells.17 SINE compounds showed single-agent activity in the Vk*MYC transgenic mouse model, which has accurately predicted clinical activity of anti-MM drugs.9,18 Similarly, SINE compounds demonstrated single-agent activity in MM xenograft studies and markedly reduced MM-associated osteopenia observed in these models.15 Tumor biopsies from these models showed the expected nuclear localization of TSPs, reduction of Myc protein, and induction of cleaved caspase 3.15
Glucocorticoids (GCs), such as dexamethasone and prednisone, are a backbone of MM therapy, but resistance to GCs develops over time. GCs mediate their anti-inflammatory effects by binding to the glucocorticoid receptor (GR) in the cytoplasm. The GC-GR complex undergoes a conformational change, becomes hyperphosphorylated, and translocates into the nucleus, where it regulates gene expression.19 Although selinexor alone has minimal effect on the location or transcriptional activity of the unphosphorylated GR, the nuclear retention of phosphorylated GR in the presence of dexamethasone is markedly enhanced by selinexor.19,20 Similarly, selinexor alone had no effect on the transcriptional activity of the GR; however, in the presence of dexamethasone, increased both transcriptional activator and repressor functions of the GR compared with dexamethasone alone.21 Moreover, the combination of selinexor and dexamethasone showed marked synergy in MM xenograft models.20,21 These results suggest that, along with its single-agent anti-MM activity, selinexor could augment the activity of GC in the treatment of MM via a unique mechanism, including in MM cells with resistance to GCs.
Based on these preclinical results, we assessed the effects of selinexor alone and in combination with dexamethasone in patients with heavily pretreated, refractory MM or Waldenstrom macroglobulinemia (WM).
Methods
Study oversight and design
These results were part of a larger, multicenter, phase 1 dose-escalation study to assess the effects of oral selinexor in patients with advanced hematologic malignancies (n = 285). The primary objectives of the study were to determine the safety, tolerability, and recommended phase II dose (RP2D) of selinexor; the secondary objectives were to assess pharmacokinetics, pharmacodynamics, and efficacy. Here, we report on the 81 patients with MM and 3 with WM. The study protocol was approved by the institutional review board or an independent ethics committee at each participating center and is in accordance with the Declaration of Helsinki, International Conference on Harmonization-Good Clinical Practice, and local laws. All patients provided written informed consent before study start.
Patients
The study enrolled patients ≥18 years of age with confirmed progressive disease, Eastern Cooperative Oncology Group (ECOG) performance status of ≤1, and adequate hepatic (total bilirubin <2 times the upper limit of normal [ULN], aspartate aminotransferase <2.5 times ULN, and alanine aminotransferase <2.5 times ULN), renal (creatinine clearance ≥30 mL/min), hematopoietic (white blood cell count ≥1500/mm3, absolute neutrophil count >800/mm3, platelet count ≥35 000/mm3, and hemoglobin ≥8 g/dL) and cardiac function. Patients with MM were required to have symptomatic measurable disease (serum M-protein ≥0.5 g/dL or for immunoglobulin A [IgA] MM by quantitative IgA or urine M-protein ≥200 mg/day or serum free light chain ≥10 mg/dL) and had previously received ≥3 lines of therapy including at least 1 alkylating agent, an IMiD, a PI, and a steroid. Patients with WM were eligible if they had received ≥2 prior regimens including a PI and a steroid. Patients were excluded if they received any anticancer therapy within 2 weeks of start of study drug (6 weeks for radio-immunotherapy) or had grade ≥2 peripheral neuropathy. A waiver was provided for 2 patients (1 who had an ECOG performance status of 2 at baseline; 1 patient with WM who did not receive a prior PI).
Selinexor administration, dose escalation, and expansion
Selinexor was administered orally in 4 week cycles starting at a dose of 3 mg/m2 based on extrapolation from preclinical toxicology studies in rats and monkeys. A dose escalation of 100% was used for cohorts 1 through 3, and 30% to 40% for cohorts 4 and above. Initially, 10 doses of selinexor were administered per cycle with a schedule of 3 times weekly (days 1, 3, and 5) for weeks 1 and 3, and 2 times weekly (days 1 and 3) for weeks 2 and 4. A lead-in week of 12 mg/m2 selinexor dosed on days 1, 3, and 5 before escalating selinexor to the target dose was investigated in an effort to improve tolerability, but was abandoned after 11 patients. Selinexor was then tested in several twice-weekly schedules (days 1, 2; 1, 3; and 1, 4) and also in a 3-weeks on/1-week off schedule in later cohorts. The safety and tolerability of selinexor dosed twice weekly with 20 mg dexamethasone was also evaluated. Dose escalation continued until at least 2 patients among a cohort of 3 to 6 patients experienced a dose-limiting toxicity (DLT) during the first treatment cycle. DLTs were evaluable only in patients in the dose-escalation phase meeting inclusion and exclusion criteria and were defined as any of the following occurring in the first 4 weeks: ≥3 missed doses because of drug-related toxicity, discontinuation of the patient because of a treatment-related adverse effect (AE), any grade ≥3 nonhematological AE except alopecia or electrolyte abnormalities correctable with supportive therapy, grade 4 neutropenia, febrile neutropenia, or grade ≥3 thrombocytopenia associated with bleeding.
Safety
All patients that received at least 1 dose of selinexor were considered evaluable for safety. Toxicities were assessed by the Investigator according to the National Cancer Institute Common Terminology Criteria for Adverse Events v4.03.
Pharmacokinetic assessment
In cycle 1, serial blood samples from 22 patients were obtained predose and at 0.5, 1, 2, 4, 8, 24, and 48 hours postdose on day 1, and predose and 0, 1, 2, 4, and 8 hours on days 15 or 17. Plasma concentrations of selinexor were measured at Tandem Labs (Durham, NC) using a validated liquid chromatography-tandem mass spectrometry method (Sciex API 4000). Pharmacokinetic parameters were calculated by standard noncompartmental analysis using PK Solutions Software (Summit Research Services, Montrose, CO).
Pharmacodynamics
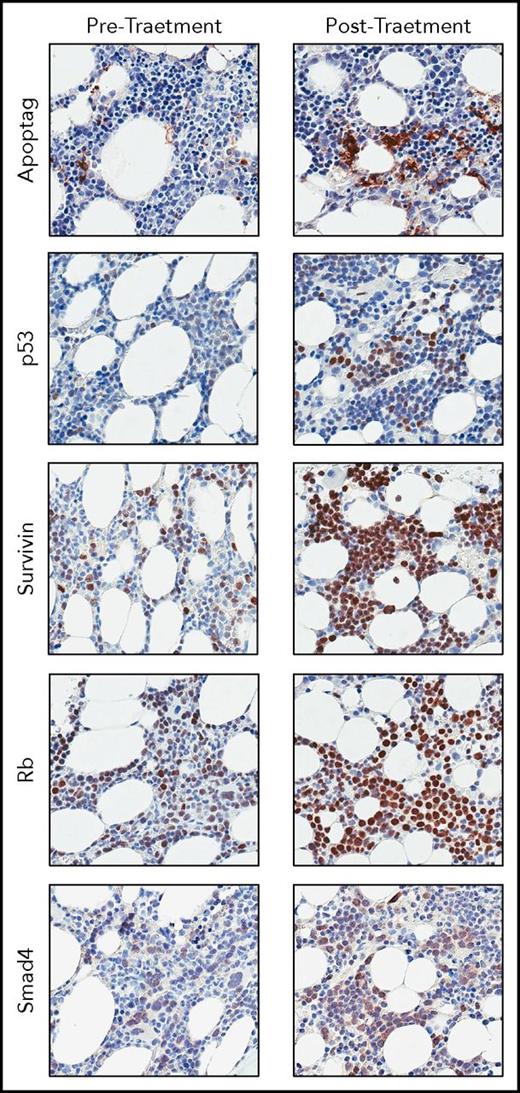
Immunohistochemical analyses were performed by CGI Laboratories (Rutherford, NH). Paired tumor biopsies were collected from 17 patients at baseline and during week 1 of cycle 2 and stained with antibodies against Apoptag (Millipore, S71003), p53 (Santa Cruz, sc-126), survivin (Abcam, ab76424), Rb (Abcam, ab181616), and Smad4 (Santa Cruz, sc-7966) using the Benchmark Ultra Stainer per the manufacturer’s instructions. Slides were scanned with the Aperio ScanScope AT Turbo at ×10 magnification.
Efficacy
Disease response was measured according to the International Myeloma Working Group Response Criteria for patients with MM or the VIth International Workshop on Response Criteria in WM.22,23 Overall response rate (ORR) was defined as complete response (CR), very good partial response (VGPR), and partial response (PR). Clinical benefit rate (CBR) was defined as CR, VGPR, PR, and minimal response (MR). All patients on study were included in the calculation of ORR and CBR based on intention-to-treat. Patients that did not complete 1 cycle (4 weeks) of selinexor, had evidence of clinical progression before withdrawal, or were in violation of the protocol were considered nonevaluable (NE) for best response.
Statistics
All patients were included in the description of baseline characteristics, adverse events, and best response. One-way analysis of variance was used to compare pharmacokinetic parameters at different doses in a subset of 22 patients that underwent pharmacokinetic evaluation and body weight changes between dose groups.
Results
Patient demographics
Between July 2012 and September 2015, 84 patients (81 MM and 3 WM) were enrolled in the study. A total of 57 patients received selinexor as a single agent, including 25 patients in dose escalation and 32 in dose expansion. Twenty-seven patients received selinexor (45 or 60 mg/m2) plus a low dose (20 mg) of dexamethasone. The median number of prior systemic therapies was 6. Twenty-three patients (27%) were previously treated with lenalidomide, bortezomib, carfilzomib, and pomalidomide, including 2 that had also received daratumumab. A full summary of patient baseline characteristics can be found in Table 1.
Patient baseline characteristics
| Total (n = 84) . | |
|---|---|
| Age | 62 (43-85) |
| ≥65 y | 31 (37%) |
| ≥75 y | 8 (10%) |
| Sex | |
| Male | 44 (52%) |
| Female | 40 (48%) |
| Ethnic origin | |
| White | 68 (81%) |
| Black or African American | 11 (13%) |
| Asian | 4 (5%) |
| East Indian | 1 (1%) |
| ECOG performance status | |
| 0 | 15 (18%) |
| 1 | 68 (81%) |
| 2 | 1 (1%) |
| Prior therapies | 6 (1-16) |
| 1 | 1 (1%) |
| 2-3 | 12 (14%) |
| 4-6 | 32 (38%) |
| 7-9 | 24 (29%) |
| ≥10 | 15 (18%) |
| Types of prior therapies | |
| Proteasome inhibitor | 83 (99%) |
| IMiD | 82 (98%) |
| Alkylating agent | 83 (99%) |
| CD38 antibody | 5 (6%) |
| Topoisomerase II inhibitor | 21 (25%) |
| Steroid | 84 (100%) |
| Stem cell transplant | 45 (54%) |
| Total (n = 84) . | |
|---|---|
| Age | 62 (43-85) |
| ≥65 y | 31 (37%) |
| ≥75 y | 8 (10%) |
| Sex | |
| Male | 44 (52%) |
| Female | 40 (48%) |
| Ethnic origin | |
| White | 68 (81%) |
| Black or African American | 11 (13%) |
| Asian | 4 (5%) |
| East Indian | 1 (1%) |
| ECOG performance status | |
| 0 | 15 (18%) |
| 1 | 68 (81%) |
| 2 | 1 (1%) |
| Prior therapies | 6 (1-16) |
| 1 | 1 (1%) |
| 2-3 | 12 (14%) |
| 4-6 | 32 (38%) |
| 7-9 | 24 (29%) |
| ≥10 | 15 (18%) |
| Types of prior therapies | |
| Proteasome inhibitor | 83 (99%) |
| IMiD | 82 (98%) |
| Alkylating agent | 83 (99%) |
| CD38 antibody | 5 (6%) |
| Topoisomerase II inhibitor | 21 (25%) |
| Steroid | 84 (100%) |
| Stem cell transplant | 45 (54%) |
Data are median (range), n (%) or n. Data include 3 patients with WM.
The median duration of treatment of all patients on study was 45 days (range, 2-774). Reasons for study discontinuation included progressive disease (n = 49), AEs (n = 26), or withdrawal of consent by patient’s decision (n = 9). All patients on study received at least 1 dose of selinexor and were therefore included in the safety and response assessments based on intention-to-treat. Of the 84 patients, 14 were considered NE for best response based on early consent withdrawal (6 for AEs, 2 for nonmedical reasons), protocol violation from concurrent antimyeloma treatment, primarily dexamethasone for antiemetic purposes (4 patients), or septic death during the first cycle deemed secondary to advanced myeloma and unrelated to selinexor (2 patients). Of the 2 septic deaths, 1 patient neutropenic at baseline died after only 3 doses of selinexor during week 1 of therapy. The second patient died after 4 doses of 45 mg/m2 selinexor given over the first 17 days on study.
Safety
Selinexor was tested at 12 dose levels (3 to 60 mg/m2) and in 6 different schedules (Table 2). Treatment-related AEs reported in ≥10% of patients are shown in Table 3 and by dose range in supplemental Table 1 on the Blood Web site. The most frequent nonhematologic AEs were nausea (75%), fatigue (70%), anorexia (64%), vomiting (43%), weight loss (32%), and diarrhea (32%), which were primarily grade 1 or 2 and reversible. When vomiting did occur, it was usually within the first 2 weeks of treatment and attenuated over time. Although lower grade AEs, nausea and fatigue were the primary reasons for withdrawal of consent in the early, dose-escalation phase of the trial. As the study progressed, improved management of these AEs resulted in a marked reduction of discontinuation because of gastrointestinal (GI) and constitutional AEs (supplemental Table 1). Supportive care for common treatment-related GI AEs included dose reductions, drug holidays, and/or prophylactic supportive care for anorexia and nausea (5-HT3 antagonists, D2 antagonists, antiemetic dose of dexamethasone [4 mg twice weekly], megestrol, and/or olanzapine) or for diarrhea (loperamide). The most common grade 3 or 4 toxicities were hematologic and included thrombocytopenia (45%), neutropenia (23%), and anemia (23%). Grade 3 hyponatremia was also frequently noted (22 patients; 26%), although most were asymptomatic, with only 2 patients reporting mental state changes (confusion) at the time of hyponatremia. Hyponatremia was easily corrected with sodium replacement.
Dose and treatment schedules
| Schedule . | Selinexor dose . | Selinexor doses per cycle . | Patients with MM or WM (n) . |
|---|---|---|---|
| Days 1, 3, and 5 of weeks 1 and 3; Days 1 and 3 of weeks 2 and 4 | 3 mg/m2 | 10 | 1 |
| 6 mg/m2 | 10 | 2 | |
| 12 mg/m2 | 10 | 2 | |
| Lead-in week, 12 mg/m2; Days 1, 3, and 5 of weeks 1 and 3; Days 1 and 3 of weeks 2 and 4 | 16.8 mg/m2 | 10 | 5 |
| 23 mg/m2 | 10 | 5 | |
| 30 mg/m2 | 10 | 1 | |
| Twice weekly (includes days 1 and 2, 1 and 3, 1 and 4) | 23 mg/m2 | 8 | 2 |
| 30 mg/m2 | 8 | 1 | |
| 35 mg/m2 | 8 | 14 | |
| 45 mg/m2 | 8 | 10 | |
| 45 mg/m2 (+20 mg dex) | 8 | 12 | |
| 55 mg/m2 | 8 | 1 | |
| 60 mg/m2 | 8 | 2 | |
| 60 mg/m2 (+20 mg dex) | 8 | 15 | |
| Days 1 and 3 for 3 weeks; 1-week holiday; no corticosteroids allowed | 40 mg | 6 | 10 |
| 60 mg | 6 | 1 | |
| Total | — | — | 84 |
| Schedule . | Selinexor dose . | Selinexor doses per cycle . | Patients with MM or WM (n) . |
|---|---|---|---|
| Days 1, 3, and 5 of weeks 1 and 3; Days 1 and 3 of weeks 2 and 4 | 3 mg/m2 | 10 | 1 |
| 6 mg/m2 | 10 | 2 | |
| 12 mg/m2 | 10 | 2 | |
| Lead-in week, 12 mg/m2; Days 1, 3, and 5 of weeks 1 and 3; Days 1 and 3 of weeks 2 and 4 | 16.8 mg/m2 | 10 | 5 |
| 23 mg/m2 | 10 | 5 | |
| 30 mg/m2 | 10 | 1 | |
| Twice weekly (includes days 1 and 2, 1 and 3, 1 and 4) | 23 mg/m2 | 8 | 2 |
| 30 mg/m2 | 8 | 1 | |
| 35 mg/m2 | 8 | 14 | |
| 45 mg/m2 | 8 | 10 | |
| 45 mg/m2 (+20 mg dex) | 8 | 12 | |
| 55 mg/m2 | 8 | 1 | |
| 60 mg/m2 | 8 | 2 | |
| 60 mg/m2 (+20 mg dex) | 8 | 15 | |
| Days 1 and 3 for 3 weeks; 1-week holiday; no corticosteroids allowed | 40 mg | 6 | 10 |
| 60 mg | 6 | 1 | |
| Total | — | — | 84 |
Selinexor dose level and schedule for the subset of patients with multiple myeloma (n = 81) or WM (n = 3) in the NCT01607892 study (n = 285). Dose escalation for the study followed a standard 3+3 design and included patients with a spectrum of hematological malignancies. Dose expansion cohorts were tested at 35, 45, 45, and 60 mg/m2 plus dexamethasone, as well as 40 and 60 mg flat dose without corticosteroids to better understand tolerability and efficacy.
Dex, dexamethasone.
Treatment-related AEs occurring in ≥10% of patients on study (n = 84)
| . | Any grade . | Grade 3 or 4 . |
|---|---|---|
| Nausea | 63 (75%) | 2 (2%) |
| Fatigue | 59 (70%) | 11 (13%) |
| Anorexia | 54 (64%) | 3 (4%) |
| Thrombocytopenia | 44 (52%) | 38 (45%) |
| Vomiting | 36 (43%) | 2 (2%) |
| Diarrhea | 27 (32%) | 4 (5%) |
| Weight loss | 27 (32%) | 1 (1%) |
| Anemia | 26 (31%) | 19 (23%) |
| Hyponatremia | 25 (30%) | 22 (26%) |
| Neutropenia | 23 (27%) | 19 (23%) |
| Dehydration | 22 (26%) | 4 (5%) |
| Blurred vision | 18 (21%) | — |
| Dysgeusia | 17 (20%) | — |
| Dyspnea | 11 (13%) | — |
| Leukopenia | 10 (12%) | 7 (8%) |
| Muscle weakness | 10 (12%) | 3 (4%) |
| Confusion | 10 (12%) | 2 (2%) |
| . | Any grade . | Grade 3 or 4 . |
|---|---|---|
| Nausea | 63 (75%) | 2 (2%) |
| Fatigue | 59 (70%) | 11 (13%) |
| Anorexia | 54 (64%) | 3 (4%) |
| Thrombocytopenia | 44 (52%) | 38 (45%) |
| Vomiting | 36 (43%) | 2 (2%) |
| Diarrhea | 27 (32%) | 4 (5%) |
| Weight loss | 27 (32%) | 1 (1%) |
| Anemia | 26 (31%) | 19 (23%) |
| Hyponatremia | 25 (30%) | 22 (26%) |
| Neutropenia | 23 (27%) | 19 (23%) |
| Dehydration | 22 (26%) | 4 (5%) |
| Blurred vision | 18 (21%) | — |
| Dysgeusia | 17 (20%) | — |
| Dyspnea | 11 (13%) | — |
| Leukopenia | 10 (12%) | 7 (8%) |
| Muscle weakness | 10 (12%) | 3 (4%) |
| Confusion | 10 (12%) | 2 (2%) |
One DLT was reported in a patient treated at the 16.8 mg/m2 (∼30 mg) dose of selinexor. This heavily pretreated patient had a baseline platelet count of 84 × 109/L, which progressed to grade 4 thrombocytopenia over the first cycle of treatment. The patient achieved an MR and, at the request of the physician, was allowed to continue treatment using platelet support as needed. The patient remained on study for 387 days with disease control, but the platelet count did not recover and the patient died of an intracranial hemorrhage sustained after a fall. Death was deemed possibly related to selinexor treatment.
There were 23 serious AEs (SAEs) considered by the investigator to be possibly related to study treatment. Fourteen of the SAEs occurred in patients treated with single-agent selinexor (61%) and 9 occurred in patients treated with selinexor plus dexamethasone (39%). In patients treated with single-agent selinexor, SAEs occurred at doses between 16.8 and 60 mg/m2 with no apparent relation to dose schedule. All SAEs resolved with hospital care except for the intracranial hemorrhage (described previously). A complete listing of treatment-related SAEs can be found in Table 4.
SAEs
| SAE term . | Starting dose . | Grade . |
|---|---|---|
| Intracranial hemorrhage after accidental fall | 16.8 mg/m2 | 5 |
| Febrile neutropenia | 16.8 mg/m2 | 3 |
| Blurred vision | 23 mg/m2 | 2 |
| Dehydration | 23 mg/m2 | 3 |
| ALT + AST + alkaline phosphatase + bilirubin increased | 35 mg/m2 | 3 |
| Febrile neutropenia | 45 mg/m2 | 3 |
| Fever | 45 mg/m2 | 3 |
| Hypoxia | 45 mg/m2 | 3 |
| Fever | 60 mg/m2 | 3 |
| Anemia | 45 mg/m2 + 40 mg dex | 3 |
| AST increased | 45 mg/m2 + 4 0mg dex | 4 |
| Fatigue | 45 mg/m2 + 40 mg dex | 3 |
| Dehydration | 60 mg/m2 + 40 mg dex | 3 |
| Delirium | 60 mg/m2 + 40 mg dex | 3 |
| Febrile neutropenia | 60 mg/m2 + 40 mg dex | 3 |
| Hyponatremia | 60 mg/m2 + 40 mg dex | 3 |
| Nausea | 60 mg/m2 + 40 mg dex | 3 |
| Vomiting | 60 mg/m2 + 40 mg dex | 3 |
| Acute kidney injury | 40 mg | 4 |
| Febrile neutropenia | 40 mg | 3 |
| Febrile neutropenia | 40 mg | 3 |
| Sepsis | 40 mg | 4 |
| Encephalopathy | 40 mg | 4 |
| SAE term . | Starting dose . | Grade . |
|---|---|---|
| Intracranial hemorrhage after accidental fall | 16.8 mg/m2 | 5 |
| Febrile neutropenia | 16.8 mg/m2 | 3 |
| Blurred vision | 23 mg/m2 | 2 |
| Dehydration | 23 mg/m2 | 3 |
| ALT + AST + alkaline phosphatase + bilirubin increased | 35 mg/m2 | 3 |
| Febrile neutropenia | 45 mg/m2 | 3 |
| Fever | 45 mg/m2 | 3 |
| Hypoxia | 45 mg/m2 | 3 |
| Fever | 60 mg/m2 | 3 |
| Anemia | 45 mg/m2 + 40 mg dex | 3 |
| AST increased | 45 mg/m2 + 4 0mg dex | 4 |
| Fatigue | 45 mg/m2 + 40 mg dex | 3 |
| Dehydration | 60 mg/m2 + 40 mg dex | 3 |
| Delirium | 60 mg/m2 + 40 mg dex | 3 |
| Febrile neutropenia | 60 mg/m2 + 40 mg dex | 3 |
| Hyponatremia | 60 mg/m2 + 40 mg dex | 3 |
| Nausea | 60 mg/m2 + 40 mg dex | 3 |
| Vomiting | 60 mg/m2 + 40 mg dex | 3 |
| Acute kidney injury | 40 mg | 4 |
| Febrile neutropenia | 40 mg | 3 |
| Febrile neutropenia | 40 mg | 3 |
| Sepsis | 40 mg | 4 |
| Encephalopathy | 40 mg | 4 |
SAEs occurring in the 81 patients with MM and 3 patients with WM in the NCT01607892 study. All SAEs resolved with hospital care with the exception of the intracranial hemorrhage after accidental fall, which was fatal.
ALT, alanine aminotransferase; AST, aspartate aminotransferase.
The maximum tolerated dose (MTD) was not identified in this study; however, based on a parallel phase 1 study of selinexor in patients with solid tumors, an MTD of 65 mg/m2 (∼110 mg) twice weekly was established across all cancer types.24 To better understand the safety profile and efficacy of selinexor in patients with MM and WM, 2 expansion cohorts were added to the study, which tested 45 mg/m2 selinexor plus 20 mg dexamethasone vs 60 mg/m2 selinexor plus 20 mg dexamethasone. In general, patients receiving dexamethasone appeared to have improved GI tolerance over selinexor monotherapy, trending toward less weight loss at 4 weeks than without dexamethasone. For example, median weight loss at the 45 mg/m2 dose of selinexor without dexamethasone was −6.6% (±5.9%) of baseline body weight vs −2.6% (±1.9%) with dexamethasone (P = .06). Of the 2 selinexor doses combined with dexamethasone, patients treated at 45 mg/m2 selinexor showed significantly less weight loss over the first 2 weeks on therapy compared with 60 mg/m2 selinexor (median loss, −1.9% ± 2.3% vs −5.0% ± 3.0%, respectively; P = .01). In addition, patients dosed at 45 mg/m2 selinexor plus 20 mg dexamethasone required fewer dose reductions and drug holidays, and received more of their target dose over the first cycle (average 82% of target dose for 45 mg/m2 selinexor plus 20 mg dexamethasone vs 58% of target dose for 60 mg/m2 selinexor plus 20 mg dexamethasone). Patients receiving twice-weekly 45 mg/m2 selinexor plus 20 mg dexamethasone remained on the study longer (median ,118 days; range, 14-391) than those receiving higher doses at 60 mg/m2 (median, 29 days; range, 2-108) (P = .04), suggesting improved tolerability of 45 mg/m2 selinexor plus 20 mg dexamethasone.
Pharmacokinetics
The pharmacokinetic parameters of selinexor were assessed in 22 patients receiving selinexor monotherapy and are summarized in supplemental Table 2. The mean clearance of drug from plasma, time of maximum concentration, and estimated volume of distribution were comparable at all dose levels (3-45 mg/m2). Maximum concentration and area under the curve increased dose proportionally (r2 on day 1 = 0.9093 and 0.9847, respectively) with no evidence of drug accumulation after multiple doses.
Pharmacodynamics
Tumor biopsies were obtained from 17 consenting patients at baseline and after 1 cycle of treatment and assessed for biochemical changes consistent with XPO1 inhibition. Consistent with preclinical findings and previously reported clinical studies, immunohistochemical staining showed a marked increase in the number of apoptotic cells (Apoptag) and nuclear localization of the XPO1 cargo proteins p53, survivin, Rb, and Smad4 after selinexor treatment compared with baseline (Figure 1).24-28
Representative staining of bone marrow aspirates collected from a patient at baseline and after 1 cycle of treatment with 45 mg/m2selinexor + 20 mg dexamethasone, twice weekly. The patient achieved a best response of PR while on study. Staining shows increased apoptosis (Apoptag) and nuclear accumulation of known TSP cargoes of XPO1 (p53, survivin, Rb, and Smad4).
Representative staining of bone marrow aspirates collected from a patient at baseline and after 1 cycle of treatment with 45 mg/m2selinexor + 20 mg dexamethasone, twice weekly. The patient achieved a best response of PR while on study. Staining shows increased apoptosis (Apoptag) and nuclear accumulation of known TSP cargoes of XPO1 (p53, survivin, Rb, and Smad4).
Efficacy
Of the 84 patients with MM or WM, the ORR was 10% (95% confidence interval, 0.05-0.18), which included 1 CR and 7 PRs (Table 5). The median time to response was 1 month (range, 1-3 months) and median duration of response was 5 months (range, 2-11 months). MR was observed in an additional 13 patients (15%) for a CBR of 25%. Time on study for the 21 patients achieving ≥MR is depicted in Figure 2. Five of the 13 patients with MR remained on study for >10 months (range, 10-14) and 1 patient with SD remained on study for >2 years. Thirty-nine of 84 patients (46%) had a quantifiable reduction in M-spike values from baseline (supplemental Figure 3).
Best overall response (n = 84)
| Treatment . | N . | CR . | PR . | MR . | SD . | PD . | NE . | ORR . | CBR . |
|---|---|---|---|---|---|---|---|---|---|
| Selinexor alone | 57 | — | 2 (4%) | 10 (18%) | 18 (32%) | 17 (30%) | 10 (18%) | 2 (4%) | 12 (21%) |
| Selinexor 45 mg/m2 + dex 20 mg | 12 | 1 (8%) | 5 (42%) | 1 (8%) | 3 (25%) | 1 (8%) | 1 (8%) | 6 (50%) | 7 (58%) |
| Selinexor 60 mg/m2 + dex 20 mg | 15 | — | — | 2 (13%) | 5 (33%) | 5 (33%) | 3 (20%) | 0 (0%) | 2 (13%) |
| All patients | 84 | 1 (1%) | 7 (8%) | 13 (15%) | 26 (31%) | 23 (27%) | 14 (17%) | 8 (10%) | 21 (25%) |
| Treatment . | N . | CR . | PR . | MR . | SD . | PD . | NE . | ORR . | CBR . |
|---|---|---|---|---|---|---|---|---|---|
| Selinexor alone | 57 | — | 2 (4%) | 10 (18%) | 18 (32%) | 17 (30%) | 10 (18%) | 2 (4%) | 12 (21%) |
| Selinexor 45 mg/m2 + dex 20 mg | 12 | 1 (8%) | 5 (42%) | 1 (8%) | 3 (25%) | 1 (8%) | 1 (8%) | 6 (50%) | 7 (58%) |
| Selinexor 60 mg/m2 + dex 20 mg | 15 | — | — | 2 (13%) | 5 (33%) | 5 (33%) | 3 (20%) | 0 (0%) | 2 (13%) |
| All patients | 84 | 1 (1%) | 7 (8%) | 13 (15%) | 26 (31%) | 23 (27%) | 14 (17%) | 8 (10%) | 21 (25%) |
Fourteen patients were considered NE based on withdrawal from study before disease assessment with no evidence of disease progression (n = 8), early death from infection deemed unrelated to treatment (n = 2), and protocol violation (received concomitant antimyeloma therapy, n = 4), but were considered as treatment failures by intent-to-treat.
N, number of all patients within each group (evaluable and nonevaluable); PD, progressive disease; SD, stable disease.
Swim lane plot showing time on study for the 21 patients achieving ≥MR. *Progressive disease; ×, withdrawal of consent. X-AEs include both drug-related and drug-unrelated AEs.
Swim lane plot showing time on study for the 21 patients achieving ≥MR. *Progressive disease; ×, withdrawal of consent. X-AEs include both drug-related and drug-unrelated AEs.
Twenty-seven patients received 45 mg/m2 (12 patients) or 60 mg/m2 (15 patients) selinexor plus 20 mg dexamethasone. The ORR for either dose in combination with dexamethasone was 22% vs 4% for single-agent selinexor (Table 5). All responses were observed in the 45 mg/m2 plus 20 mg dexamethasone group (50% ORR). Three of 8 patients (38%) that responded had MM that was previously treated with bortezomib, carfilzomib, lenalidomide, and pomalidomide (1 CR and 2 PR).
Discussion
Although several new agents have recently been added to the armamentarium of available therapies for relapsed myeloma, outcomes remain dismal, particularly in patients with heavily pretreated, refractory disease. In historical cohorts of patients refractory to both PIs and IMiDs (double-refractory), event-free survival of 5 months and overall survival of 9 months provide a benchmark for comparison of novel regimens.29 In this double-refractory setting, pomalidomide plus dexamethasone responses can be achieved in 28% of patients with a median PFS of 3.7 months, single-agent carfilzomib achieves an ORR of 23% with a median PFS of 3.7 months, and single-agent daratumumab attains responses of ∼29% with a PFS of 3.7 months.3,30,31 Although combinations of these agents may improve these outcomes, response durations remain <1 year, with ultimate refractoriness warranting an ongoing need for new therapies with unique mechanisms of action.32-34
In this study of the novel XPO1 inhibitor selinexor (with or without dexamethasone), tolerability and antitumor activity in heavily pretreated patients with MM and WM (median, 6 prior lines of therapy) was demonstrated. In the phase 1 portion of this study, single-agent selinexor tested at daily oral doses ranging from 3 to 60 mg/m2 given 1 to 3 times per week, did not identify the MTD. Only 1 patient with MM experienced a DLT (prolonged grade 4 thrombocytopenia); this patient continued on study at the physician’s request for >1 year. Cytopenias were common causes of grade ≥3 AEs, but were rarely complicated by bleeding or fever. In the setting of heavily pretreated, refractory MM/WM, asymptomatic cytopenias are commonplace events. The most frequent nonhematologic toxicities were fatigue or GI (nausea, anorexia, vomiting, weight loss); most likely centrally mediated, given the known ability of selinexor to penetrate the blood–brain barrier.35 Lower rates of these AEs have been reported with KPT-8602, a related SINE compound, which has substantially reduced central nervous system penetration.36 GI symptoms, though mostly lower grade, limited tolerability of selinexor and were the primary cause of study discontinuation from AEs in the early portion of the study. These discontinuations were not considered DLTs as predefined in our protocol. As the trial progressed, improved management of GI AEs with routine use of antiemetics, careful attention to patient’s body weight, and the use of appetite stimulants, as applicable, led to a reduction in consent withdrawals (supplemental Table 1). Although the MTD was not reached, collective safety data suggested improved tolerance with the 45 mg/m2 dose of selinexor, compared with 60 mg/m2. Because evaluation of pharmacokinetic parameters showed similar results using body surface area–based and fixed dosing, the RP2D was determined as a twice-weekly fixed dose of 80 mg selinexor, corresponding to 45 mg/m2, plus 20 mg dexamethasone. Furthermore, no accumulation of drug after multiple doses was observed and sustained XPO1 inhibition over several days could be achieved, supporting the use of a twice-weekly schedule. Hence, from a safety perspective, the optimal dose and schedule of selinexor was deemed as 80 mg (fixed dose) plus 20 mg dexamethasone twice weekly.
In the dose-escalation portion of the study, selinexor demonstrated modest single-agent activity, with PR achieved in 2 of 57 (4%) patients, and an additional 10 (18%) MR and 18 (32%) SD. In 6 patients achieving best responses of MR or SD, prolonged duration on therapy (up to 2 years) was observed, demonstrating that disease stabilization in this heavily pretreated population can be associated with clinical benefit. Given the known XPO1-dependent inactivation of the GR and the improved GI tolerance of selinexor with oral dexamethasone added, the combination of selinexor and low-dose dexamethasone was further evaluated. Selinexor at the 45 mg/m2 dose with dexamethasone 20 mg twice weekly led to overall responses (≥PR) in 6 of 12 (50%) patients (1 CR, 5 PR). Three of the overall responders were previously exposed to all approved PIs and IMiDs, attesting to the efficacy of this combination. Time to first response was rapid (at a median of 1 cycle), a critical parameter for patients with heavily pretreated, refractory disease. Although the higher dose of selinexor, 60 mg/m2, was tested in combination with dexamethasone, this regimen was not as well-tolerated, with shorter duration on treatment, resulting in notably inferior efficacy with a CBR of 13% (all MR). Therefore, from both a safety and efficacy perspective, we identified selinexor 45 mg/m2 (equivalent to a flat 80 mg dose) in combination with dexamethasone 20 mg twice weekly as a promising, and better tolerated, regimen for subsequent trials.
Given the activity of selinexor plus dexamethasone in heavily pretreated patients with MM, this combination is being tested in the phase 2 Selinexor Treatment of Refractory Myeloma study in quad- and penta-refractory MM with preliminary reports supporting activity and tolerability.37 In addition, based on strong preclinical results, studies of selinexor in combination with standard MM agents are underway (Selinexor and Backbone Treatments of Multiple Myeloma Patients [STOMP] trial). Striking synergy with bortezomib has been observed and a randomized phase 3 study (Bortezomib, Selinexor and Dexamethasone in Patients with Multiple Myeloma [BOSTON]) is underway, investigating bortezomib, selinexor, and dexamethasone, all given once weekly.38 In summary, selinexor is an oral agent with a completely novel mechanism of action and anti-MM activity in combination with dexamethasone that could provide a new option for patients suffering from this incurable disease.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients who participated in this trial and their families, the coinvestigators, nurses, and study coordinators at each of the sites.
This study was sponsored by Karyopharm Therapeutics.
Authorship
Contribution: C.C. performed research, interpreted data, and wrote the manuscript; D.S., M.G., M.J., C.C.H., N.G., R.B., M.M.-S., and J.G.B. performed research, enrolled patients, interpreted data, reviewed the manuscript; M.S. performed research, enrolled patients, interpreted data, and wrote the manuscript; L.S., S.T., and N.A. performed research, enrolled patients, interpreted data, and reviewed the manuscript; T.J.U. analyzed and interpreted data and wrote the manuscript. T.R. designed research, interpreted data, and reviewed the manuscript; T.H. collected data, interpreted data, and reviewed the manuscript; M.K. and S.S. designed research, contributed reagents, analyzed and interpreted data, and reviewed the manuscript; and D.R. performed research, enrolled patients, interpreted data, and reviewed the manuscript; and all authors reviewed the manuscript and vouch for the accuracy of the data.
Conflict-of-interest disclosure: R.B., M.M.-S., and C.C.H. received research funding from Karyopharm. M.S. is a consultant for Karyopharm. M.M.-S. has received honorarium and a travel stipend from Karyopharm. T.J.U., T.R., T.H., M.K., and S.S. are stockholders in Karyopharm. M.K. and S.S. are officers of Karyopharm. The remaining authors declare no competing financial interests.
Correspondence: Christine Chen, Division of Medical Oncology & Hematology, Princess Margaret Cancer Centre, 700 University Ave, OPG Suite 6-225, Toronto, ON M5M 2G9, Canada; e-mail: christine.chen@uhn.ca.