Key Points
Daratumumab conjugated with 64Cu efficiently binds to CD38 on myeloma cells and was mainly detected in the bones of mice.
PET/CT based on 64Cu-radiolabeled daratumumab displays a higher resolution and specificity for detecting myeloma than does 18F-FDG PET/CT.
Abstract
As a growing number of patients with multiple myeloma (MM) respond to upfront therapies while eventually relapsing in a time frame that is often unpredictable, attention has increasingly focused on developing novel diagnostic criteria to also account for disease dissemination. Positron emission tomography/computed tomography (PET/CT) is often used as a noninvasive monitoring strategy to assess cancer cell dissemination, but because the uptake of the currently used radiotracer 18fluorodeoxyglucose (18F-FDG) is a function of the metabolic activity of both malignant and nonmalignant cells, the results frequently lack sufficient specificity. Radiolabeled antibodies targeting MM tissue may detect disease irrespective of cell metabolism. Hence, we conjugated the clinically significant CD38-directed human antibody daratumumab (Darzalex [Dara]) to the DOTA chelator and labeled it with the positron-emitting radionuclide copper 64 (64Cu; 64Cu-DOTA-Dara). Here, we show that 64Cu-DOTA-Dara can efficiently bind CD38 on the surface of MM cells and was mainly detected in the bones associated with tumor in a MM murine model. We also show that PET/CT based on 64Cu-DOTA-Dara displays a higher resolution and specificity to detect MM cell dissemination than does 18F-FDG PET/CT and was even more sensitive than were bioluminescence signals. We therefore have supporting evidence for using 64Cu-DOTA-Dara as a novel imaging agent for MM.
Introduction
Prognostic information to stratify multiple myeloma (MM) patients into distinct risk-based treatments is currently based on specific genetic and cytogenetic abnormalities and clinical characteristics.1,2 However, the extensive heterogeneity in therapeutic response, even in patients sharing similar characteristics,1,2 emphasizes the need for more accurate prognostic tools.
Positron emission tomography/computed tomography using fluorodeoxyglucose-18 (18F-FDG PET/CT) imaging in newly diagnosed MM patients correlates with progression-free survival.3,4 18F-FDG PET/CT also predicts the progression rate from smoldering MM to active disease.5 Hence, most investigators now agree on the importance of using imaging as a prognostic criterion for MM.6-8
However, despite widespread use of 18F-FDG PET/CT, its ability to detect malignancies is dependent on metabolic activity. Lesions with a low metabolic rate may therefore be undetectable. In fact, low expression of hexokinase-2 in MM cells is associated with false-negative 18F-FDG PET/CT in MM.9 By binding to a tumor antigen rather than relying on the metabolic state of a malignancy, antibody-based approaches may allow imaging of cancer cells with low metabolic activity. In support, the chimeric fibril-reactive monoclonal antibody can be used in patients with light chain–associated amyloidosis to identify candidates for passive immunotherapy.10,11
Daratumumab (Dara), a human anti-CD38 immunoglobulin G1 (ĸ subclass) antibody against the highly expressed plasma cell receptor CD38, has shown particularly efficacious clinical activity12-16 and recently has been approved by the US Food and Drug Administration for the treatment of relapsed MM. Because almost all MM patients express CD38 on the surface of their cancer cells, we hypothesize that tracing MM cell dissemination by targeting CD38 could be a successful approach.
Study design
See the supplemental Methods section, available on the Blood Web site.
Results and discussion
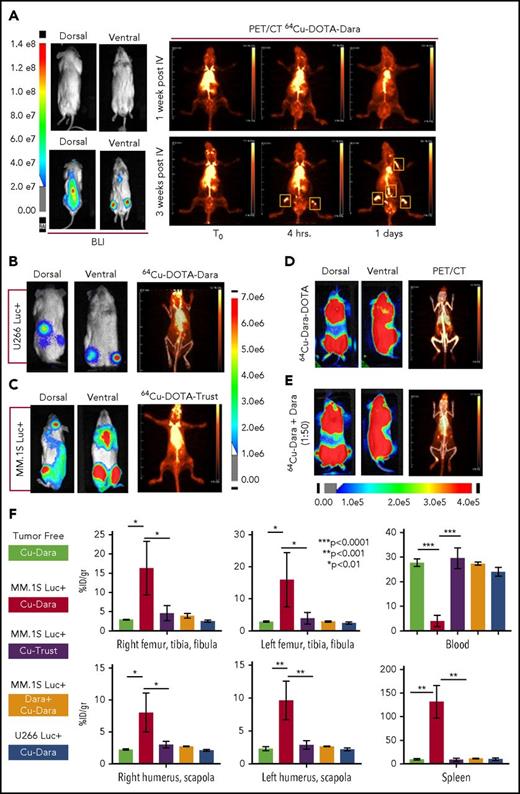
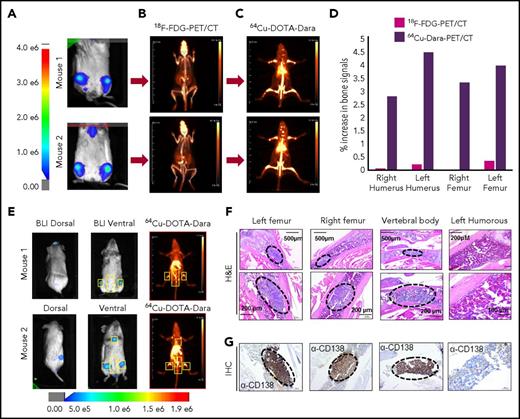
The conjugation of Dara to DOTA was successful, as was demonstrated by gel electrophoresis (supplemental Figure 1A-B); DOTA-Dara was labeled with copper 64 (64Cu) efficiently, with no evidence of aggregates (supplemental Figure 1C-D). The immunoreactivity of 64Cu-DOTA-Dara preparations was >95% (supplemental Figure 1E). 64Cu-DOTA-Dara was extremely stable up to 48 hours in saline solution and in mouse serum (supplemental Figure 2A-B). CD38-positive MM.1S green fluorescent protein/luciferase-positive (GFP+/Luc+) and CD38-negative U266 Luc+ MM cells,17 which express CD38 antigen at the same levels as do the respective parental cell lines (supplemental Figure 3A-B), were used to assess 64Cu-DOTA-Dara specificity in vivo. Mice were analyzed 7 and 21 days after MM.1S GFP+/Luc+ cell injection. Each animal was then injected with the radiolabeled antibody 24 hours after bioluminescence (BLI) assessment and imaged immediately (T0), at 4 hours and at days 1 and 2 after the injection (Figure 1A; supplemental Figure 4A). Both BLI and 64Cu-DOTA-Dara PET/CT imaging of the same mice suggested absence of MM bone engraftment in the mice imaged at day 7, but both methods detected signals at day 21 (Figure 1A). No positive bone signs were indicated in nonengrafted mice or in mice engrafted with U266 Luc+ cells (Figure 1B; supplemental Figure 4B). Lack of bone signaling was also observed in mice engrafted with MM.1S GFP+/Luc+ cells but imaged by a MM nonspecific radiolabeled antibody (64Cu-trastuzumab-DOTA [64Cu-trast-DOTA]) (Figure 1C), and when unlabeled Dara (cold) (50:1 excess) was used in combination with 64Cu-DOTA-Dara (Figure 1D-E; supplemental Figure 4C). Ex vivo biodistribution studies at 24 hours verified the trends observed through PET analysis (Figure 1F). 64Cu-DOTA-Dara was significantly detectable in MM.1S-engrafted mice (n = 4) in comparison with the tumor-free mice (n = 4) and with the mice injected with 64Cu-trast-DOTA (n = 3) in the femurs, tibias, humeri, and scapola, known sites of MM cell invasion (Figure 1F). The same biodistribution trend observed in the control mice was observed in the organs of the mice injected with U266 cells or with cold unlabeled Dara (1:50) (Figure 1F). Higher signals were also observed in all organs made of bone tissue such as sternum, skull, and spinal column and in the lung (supplemental Figure 5A-B). 64Cu-DOTA-Dara was also significantly detected in the spleen of MM.1S GFP+/Luc+–engrafted mice (Figure 1F; supplemental Figure 5A), which was also confirmed by flow cytometry (supplemental Figure 5B). In contrast, the majority of 64Cu-DOTA-Dara remained in the blood of the control mice (P < .0001) and was not significantly different in the other organs (supplemental Figure 5A). To assess the sensitivity of the 64Cu-Dara signal in comparison with the standard 18F-FDG PET-CT scan, we analyzed an independent group of animals bearing CD38+ tumors. At 21 days after cell injection, mice were first assessed by BLI and then injected with 18F-FDG (Figure 2A-B). The same group of mice were then injected with 64Cu-DOTA-Dara the next day, and PET imaging was performed (Figure 2C; supplemental video A). BLI and 64Cu-DOTA-Dara PET/CT revealed the presence of MM cells, whereas tumors were almost undetectable with the 18F-FDG PET/CT (Figure 2C-D). The 18F-FDG PET signals were mainly associated with the nonspecific uptake of glucose in leg muscles and the brain. The 18F-FDG bone signal was almost equal to the background signal of 18F-FDG in the heart, a contrast to the highly specific bone signal detected by 64Cu-DOTA-Dara (Figure 2D; see also supplemental videos A and B).
64Cu-DOTA-Dara detects MM cell dissemination in vivo. (A) The radiolabeled antibody 64Cu-DOTA-Dara (3.7 MBq, 10 µg) was administered to 3 mice that had been transplanted by IV injection with a human MM cell line (MM.1S GFP+/Luc+) before (7 days post-IV) and after (21 days post-IV) signs of MM bone metastasis as detected by BLI. The mice were also imaged with 64Cu-DOTA-Dara radiolabeled antibody on the following days, and PET imaging identified MM cell dissemination in the bone (yellow boxes), which overlap with the BLI signals in the same mouse. (B) 64Cu-DOTA-Dara (3.7 MBq, 10 µg) was also administered to 2 mice that had been transplanted by IV injection with U266 Luc+ MM cells, when clear signs (5 weeks post-IV) of MM bone engraftment were detected by BLI. The mice were imaged with 64Cu-DOTA-Dara radiolabeled antibody after 1 day from the radiolabeled Dara injection (see also supplemental Figure 4B). Of note, no sign of engraftment was observed by PET/CT scan. (C) 64Cu-DOTA-trastuzumab (64Cu-DOTA-trast) (3.7 MBq, 10 µg) was administrated to 3 MM.1S GFP+/Luc+–engrafted mice, as assessed by BLI imaging, and a PET/CT scan was performed 1 day after 64Cu-DOTA-trast injection. No bone signals were observed, but only high background associated with the circulating radiolabeled antibody. (D-E) Mice bearing CD38+ MM cells (MM.1S GFP+/Luc+) with a high level of engraftment (see also supplemental Figure 4C), as assessed by BLI images, that were injected with 64Cu-DOTA-Dara (3.7 MBq, 10 µg) without (D) and with (E) unlabeled Dara (500 µg) and imaged at day 1 by PET/CT. Almost no signs of engraftments were detected in the presence of cold Dara, independent of the BLI signals. (F) 64Cu-DOTA-Dara PET/CT-positive mice (MM.1S GFP+/Luc+–engrafted mice: 4) and negative mice (tumor free mice: 4); 64Cu-DOTA-trast–treated mice (3); unlabeled Dara (Block) treated mice (2); and U266-engrafted mice (2) were sacrificed, and organs were harvested at 24 hours and used for γ counting to determine their radioactive content, as is presented in the bar graphs as a percentage of the injected dose per gram (%ID/g). See also supplemental Figure 5A.
64Cu-DOTA-Dara detects MM cell dissemination in vivo. (A) The radiolabeled antibody 64Cu-DOTA-Dara (3.7 MBq, 10 µg) was administered to 3 mice that had been transplanted by IV injection with a human MM cell line (MM.1S GFP+/Luc+) before (7 days post-IV) and after (21 days post-IV) signs of MM bone metastasis as detected by BLI. The mice were also imaged with 64Cu-DOTA-Dara radiolabeled antibody on the following days, and PET imaging identified MM cell dissemination in the bone (yellow boxes), which overlap with the BLI signals in the same mouse. (B) 64Cu-DOTA-Dara (3.7 MBq, 10 µg) was also administered to 2 mice that had been transplanted by IV injection with U266 Luc+ MM cells, when clear signs (5 weeks post-IV) of MM bone engraftment were detected by BLI. The mice were imaged with 64Cu-DOTA-Dara radiolabeled antibody after 1 day from the radiolabeled Dara injection (see also supplemental Figure 4B). Of note, no sign of engraftment was observed by PET/CT scan. (C) 64Cu-DOTA-trastuzumab (64Cu-DOTA-trast) (3.7 MBq, 10 µg) was administrated to 3 MM.1S GFP+/Luc+–engrafted mice, as assessed by BLI imaging, and a PET/CT scan was performed 1 day after 64Cu-DOTA-trast injection. No bone signals were observed, but only high background associated with the circulating radiolabeled antibody. (D-E) Mice bearing CD38+ MM cells (MM.1S GFP+/Luc+) with a high level of engraftment (see also supplemental Figure 4C), as assessed by BLI images, that were injected with 64Cu-DOTA-Dara (3.7 MBq, 10 µg) without (D) and with (E) unlabeled Dara (500 µg) and imaged at day 1 by PET/CT. Almost no signs of engraftments were detected in the presence of cold Dara, independent of the BLI signals. (F) 64Cu-DOTA-Dara PET/CT-positive mice (MM.1S GFP+/Luc+–engrafted mice: 4) and negative mice (tumor free mice: 4); 64Cu-DOTA-trast–treated mice (3); unlabeled Dara (Block) treated mice (2); and U266-engrafted mice (2) were sacrificed, and organs were harvested at 24 hours and used for γ counting to determine their radioactive content, as is presented in the bar graphs as a percentage of the injected dose per gram (%ID/g). See also supplemental Figure 5A.
64Cu-DOTA-Dara PET/CT shows higher resolution than does18F-FDG-PET/CT. (A-C) Representative images of 2 NSG mice (mouse 1 and mouse 2), which were injected with 5 × 106 MM.1S GFP+/Luc+ cells. After 2 weeks from the injection, the mice were imaged using BLI (A), and after 24 hours, the same mice were imaged by 18F-FDG-PET (B). The day after, 64Cu-DOTA-Dara (3.7 MBq, 10 µg) was administered to the same mice imaged with 18F-FDG-PET and BLI (C). (D) Graphical representation of the intensity of the signal of 64Cu-DOTA-Dara PET/CT in comparison with 18F-FDG-PET/CT, expressed as a percentage of the increase in percentage of the injected dose per gram (%ID/gr) of bone organs in comparison with the nonspecific signal of each radio nucleotide (%ID/gr) detected in the heart. A radiolabeled antibody identified MM cancer cells, which were undetectable by 18F-FDG-PET scan. (E) Mice with minimum BLI signals were IV-injected with 64Cu-DOTA-Dara (3.7 MBq, 10 µg) and then imaged by PET/CT, which identified MM cell dissemination in the bone (yellow boxes). (F-G) Representative images at different magnifications of tissue sections stained by hematoxylin and eosin stain (F) and CD138 immunohistochemical stain (G) showing MM cell accumulation (black dashed ovals) in the left and right femurs and in the vertebral body (lower lumbar), but not in the left humerus, which did not show PET/CT signals. H&E, hematoxylin and eosin; IHC, immunohistochemical.
64Cu-DOTA-Dara PET/CT shows higher resolution than does18F-FDG-PET/CT. (A-C) Representative images of 2 NSG mice (mouse 1 and mouse 2), which were injected with 5 × 106 MM.1S GFP+/Luc+ cells. After 2 weeks from the injection, the mice were imaged using BLI (A), and after 24 hours, the same mice were imaged by 18F-FDG-PET (B). The day after, 64Cu-DOTA-Dara (3.7 MBq, 10 µg) was administered to the same mice imaged with 18F-FDG-PET and BLI (C). (D) Graphical representation of the intensity of the signal of 64Cu-DOTA-Dara PET/CT in comparison with 18F-FDG-PET/CT, expressed as a percentage of the increase in percentage of the injected dose per gram (%ID/gr) of bone organs in comparison with the nonspecific signal of each radio nucleotide (%ID/gr) detected in the heart. A radiolabeled antibody identified MM cancer cells, which were undetectable by 18F-FDG-PET scan. (E) Mice with minimum BLI signals were IV-injected with 64Cu-DOTA-Dara (3.7 MBq, 10 µg) and then imaged by PET/CT, which identified MM cell dissemination in the bone (yellow boxes). (F-G) Representative images at different magnifications of tissue sections stained by hematoxylin and eosin stain (F) and CD138 immunohistochemical stain (G) showing MM cell accumulation (black dashed ovals) in the left and right femurs and in the vertebral body (lower lumbar), but not in the left humerus, which did not show PET/CT signals. H&E, hematoxylin and eosin; IHC, immunohistochemical.
To assess the ability of 64Cu-DOTA-Dara to recognize MM cells in the bones when the BLI signal was minimal or completely absent, we used an independent group of mice for histology analysis and for immunohistochemical staining for the MM cell marker CD138. Step section of the bones show foci of MM cells, as is also assessed by CD138 staining in the right and left femurs, confirming the presence of MM cells in the location where both BLI and 64Cu-DOTA-Dara signals were present (Figure 2E-G). We also detected MM cells in the lower vertebral body, which had no BLI signal (Figure 2E-G) and no cells in the humerus, which was also found negative by PET/CT scan (Figure 2E-G).
The relatively short half-life of 64Cu (12.7 hours) may be ideal for patient safety and is sufficiently long for PET/CT imaging up to 48 hours after administration. We have used 64Cu-labeled trastuzumab to safely detect human epidermal growth factor receptor 2 (HER2)–positive tissue in metastatic breast cancer (MBC) patients.18 64Cu was safe, and by day 1 after injection, 64Cu-trastuzumab-DOTA was able to detect MBC, its binding correlating with patient HER2 status. Although Dara has been conjugated with the radionuclide 89zirconium (89Zr),19 the tendency of 89Zr to accumulate in the bones,19-21 associated with a longer time requested for imaging in contrast to 64Cu, may be a limitation in specifically detecting MM cell dissemination in the bones. Our data clearly show higher sensitivity of 64Cu-Dara-DOTA not only in comparison with 18F-FDG signal but also in comparison with BLI imaging, an effect that was not seen when Dara was conjugated with 89Zr.19 Because our studies show a high sensitivity of 64Cu-Dara-DOTA in detecting MM cells, we believe that this imaging approach for MM may also be clinically relevant for detecting minimal residual disease (MRD). MRD, as detected by high throughput bone marrow DNA sequencing, is being considered as a surrogate endpoint in clinical trials,22-24 because effective therapies necessitate 5 to 10 years of follow-up to detect differences in survival.25,26 However, irrespective of its sensitivity, this technique is marrow based and cannot account for a nonhomogenous bone marrow infiltration and extramedullary involvement. In support of Dara as a unique tool for imaging in MM, our group developed a 64Cu antibody (elotuzumab [Elo]) against the highly specific MM receptor CS1 (64Cu-DOTA-Elo). Although 64Cu-DOTA-Elo bound the CS1 antigen in vitro, it did not identify bone engraftment of CS1-positive MM cells in in vivo studies (data not shown). We have begun a 64Cu-DOTA-Dara PET/CT imaging trial that has the potential to affect MM clinical cell detection.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge Dori Triplett, Evelyn Flores, and Debbie Flood for administrative support. Research reported in this publication included work performed in the Center for Biomedicine and Genetics facility.
This work was supported by a Steven Gordon and Briskin Family Innovation Grant (F.P., D.C., J.S.). The Small Animal Imaging Center City of Hope (COH) core and Pathology core used for immunohistochemical staining were supported by the National Institutes of Health National Cancer Institute (P30CA033572) and by the COH Veterinary Pathology Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: E.C., S.H., J.K., S.R., J.S., N.C., D.C., A.K., and F.P. designed the research; E.C., J.C., M.M., E.K.P., D.V., S.V., J.K., P.Y., Y.K., and R.B. performed the research; E.C., J.F.S., J.M.P., G.M., J.S., D.C., and F.P. analyzed the data; J.F.S. and F.P. wrote the manuscript; and all authors reviewed the manuscript.
Conflict-of-interest disclosure: A.K. is a consultant for and on the speakers’ bureau of Janssen Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Flavia Pichiorri, Department of Hematology and Hematopoietic Cell Transplantation, City of Hope, 1500 E Duarte Rd, Duarte, CA 91010; e-mail: fpichiorri@coh.org; and David Colcher, Department of Molecular Immunology, City of Hope, 1500 E Duarte Rd, Duarte, CA 91010; e-mail: dcolcher@coh.org.
References
Author notes
E.C. and J.C. contributed equally to this work.