Abstract
Patients who present with severe manifestations of acute venous thromboembolism (VTE) are at higher risk for premature death and long-term disability. In recent years, catheter-based interventional procedures have shown strong potential to improve clinical outcomes in selected VTE patients. However, physicians continue to be routinely faced with challenging decisions that pertain to the utilization of these risky and costly treatment strategies, and there is a relative paucity of published clinical trials with sufficient rigor and directness to inform clinical practice. In this article, using 3 distinct clinical scenario presentations, we draw from the available published literature describing the natural history, pathophysiology, treatments, and outcomes of VTE to illustrate the key factors that should influence clinical decision making for patients with severe manifestations of deep vein thrombosis and pulmonary embolism. The results of a recently completed pivotal multicenter randomized trial are also discussed.
Introduction
Hematologists play a crucial role in managing patients with severe manifestations of acute venous thromboembolism (VTE). Although they are often asked to advise physicians on when to use catheter-based therapies, many hematologists may feel less familiar with their nuances and pathophysiological underpinnings. Moreover, many important clinical questions are not yet directly addressed by high-quality clinical trials or evidence-based practice guidelines. In this article, we delineate key considerations relating to the natural history, pathophysiology, and outcomes of VTE, and the potential impact of interventional VTE therapies. Our goal is to strengthen the clinician’s ability to individualize care for patients with severe manifestations of deep vein thrombosis (DVT) and pulmonary embolism (PE).
In addition to the biology of VTE, the anatomy and physiology also matter
Most recognized VTE risk factors are clinical factors that affect the physiological balance between clotting and bleeding, including both inherited conditions (eg, genetic mutations of clotting factors) and acquired conditions (eg, cancer, postoperative state, trauma, hormonal exposure). Although Virchow’s Triad also includes stasis and endothelial injury, the search for therapeutic targets has been primarily focused on the biology of coagulation, and major advances in care have been largely limited to new anticoagulant drugs that prevent recurrent VTE at least as effectively and safely as do predecessor drugs.1 Anticoagulant therapy is unquestionably tremendously effective in reducing fatal PE and recurrent VTE, both important outcomes in patients with DVT and PE. However, it is also true that anticoagulant therapy is often insufficient to prevent the full range of VTE consequences that are important to patients’ ability to function normally.
In patients with symptomatic PE and signs of right heart strain (“submassive PE”), anticoagulant therapy is unable to prevent short-term deterioration, death, or both in a small but significant minority of patients.2 In addition, because anticoagulant therapy does not actively dissolve thrombus, residual thrombus remains in the pulmonary arteries and increases pulmonary vascular resistance and right ventricular (RV) afterload; although studies have varied in the proportion of patients who are affected, some studies suggest that this may include up to 50% of PE survivors.3-5 RV damage from acute PE can reduce the heart’s ability to adapt to exercise.6-9 In a prospective cohort study of 100 patients with acute PE and a low rate of baseline comorbidities, 46.5% had an abnormally low maximum rate of oxygen consumption 1 year after the PE.10 On average, these subjects had poorer health-related quality of life, a shorter 6-minute walk distance, and higher dyspnea scores. In some studies, incomplete recovery of lung perfusion has been associated with chronic respiratory symptoms,11 hypoxemia,12-14 gas exchange deficits,13,15,16 exercise intolerance,11-18 and increased pulmonary artery pressure.3,18-25 Full-blown chronic thromboembolic pulmonary hypertension occurs in about 3% of patients with a first-time PE,24 but chronic cardiopulmonary disability of lesser severity occurs to some degree in 25% to 50% of PE survivors.26-28
The postthrombotic syndrome (PTS) is a chronic condition that develops in about 40% of patients with a first episode of lower-extremity DVT despite the use of anticoagulant therapy.29 PTS causes chronic limb heaviness, fatigue, swelling, and pain, with a minority of patients developing severe venous claudication, skin changes, and leg ulcers. As a result, fully one third of patients do not return to their baseline quality of life (QOL) after DVT.30 A multicenter study that examined determinants of QOL found the development of PTS to be the strongest predictor of 2-year QOL after DVT; in contrast, the occurrence of recurrent VTE did not exhibit a strong correlation with patient-reported QOL.31 Hence, it is clear that preventing recurrent VTE is only one element of ensuring good long-term health and QOL for VTE patients.
A large body of venous physiological studies over a 50-year period has shown that the final macroscopic pathway that leads to PTS is the onset of ambulatory venous hypertension, which leads to chronic edema, tissue hypoxia, progressive calf pump dysfunction, subcutaneous fibrosis, and ultimately skin ulceration.32-37 Two key physiological contributors to post-DVT ambulatory venous hypertension are valvular reflux and late venous obstruction.
Valvular reflux
In a patient with normal venous function, the venous valves close rapidly when the patient stands upright. In contrast, patients with valvular incompetence (reflux) exhibit delayed valve closure, allowing reversed flow for part of the cardiac cycle, resulting in elevation of venous pressures. DVT triggers inflammation-induced valvular damage in involved segments and also leads to distal reflux by causing venous dilatation and valve leaflet separation.38,39 Deep (femoropopliteal) venous reflux develops in 60% of DVT patients over a 1-year follow-up despite anticoagulant therapy; reflux also develops in the superficial (saphenous) venous system in some patients.40 Of note, veins that exhibit rapid endogenous clot lysis have been found to develop valvular reflux much less frequently, a finding that supports the potential effectiveness of treatment strategies aimed at achieving early clot removal.41,42
Venous obstruction
The importance of early and complete thrombus resolution is strongly supported by clinical studies.43,44 In a subgroup analysis from a randomized trial evaluating compression therapy for proximal DVT, patients with residual thrombus, in 6-month follow-up ultrasound, were significantly more likely to develop PTS.45 In studies including a meta-analysis of 11 anticoagulation randomized controlled trials (RCTs), the residual thrombus burden after initial DVT therapy demonstrated a moderate correlation with the risk of recurrent VTE.46-48 Studies of surgical venous thrombectomy and various forms of thrombolytic therapy have also found early clot lysis and restoration of venous patency to predict better functional outcomes.49-52
Importantly, different venous segments exhibit different tendencies toward endogenous venous recanalization. With the femoral and popliteal veins, recanalization is the rule and occurs in 50% of patients by 3 months and in over 90% of patients by 1 year.41 In contrast, in patients with iliofemoral DVT (DVT involving the common femoral vein or iliac vein, with or without involvement of other veins), complete venous recanalization rarely occurs with anticoagulant therapy alone, and PTS tends to be more frequent and more severe.41,53
Patient 1
Proximal DVT and PTS
A 37-year-old man presented to the hematology clinic. He developed a left lower-extremity DVT involving the lower femoral vein and popliteal vein 3 weeks after knee surgery. He first presented to a doctor 6 days after the onset of the left calf pain and swelling. The patient was anticoagulated with rivaroxaban, but over the subsequent 5 days, the swelling, aching, and discoloration worsened, extending to involve the entire left thigh. A repeat ultrasound found extension of the thrombus into the left common femoral vein. The patient was switched to enoxaparin for 6 weeks, resulting in resolution of the extreme tenderness to touch but with only a slight reduction in daily pain and swelling. He was subsequently transitioned to oral warfarin therapy, on which he now remains 1 year later. Currently, the patient complains of severe left lower-extremity swelling and pain that have occurred daily since his DVT diagnosis and that render him unable to walk for more than 1 block without needing to stop and elevate his leg. He worked as a manual laborer but has not been able to work and is applying for disability support.
Discussion
Catheter-directed thrombolysis (CDT) refers to the direct intrathrombus administration of a fibrinolytic drug via a catheter or device embedded within the thrombus using imaging guidance.54,55 The intrathrombus infusion affords improved drug penetration into the thrombus, enhanced clot removal efficacy, and the ability to use far lower doses of fibrinolytic drug than with systemic administration. The addition of mechanical thrombus disruption or aspiration using catheter-based devices (pharmacomechanical CDT, or PCDT) further enhances clot removal and enables faster treatment with even lower drug doses. Catheter access into the venous system for CDT may also enable endovascular treatment of underlying venous anatomic abnormalities (eg, iliac vein compression syndrome, webs, stenosis).55,56
CDT and PCDT can achieve important goals in selected patients: (1) save life, limb, or organ when used urgently in the rare patient with acute limb-threatening circulatory compromise from DVT or progressive IVC thrombosis with visceral organ compromise; (2) enable faster relief of presenting symptoms (see below); and (3) reduce the severity of PTS, presumably by restoring venous patency and preserving the venous valves (see below). However, because CDT/PCDT is associated with an increased risk of major bleeding (1% to 8%) and intracranial bleeding (0.5%), its appropriate frequency of use is being explored in rigorous studies.1,2,54
In a multicenter RCT (the CAVENT study) of patients with DVT involving the iliac, upper femoral venous system, or both, additional CDT was associated with a 26% relative reduction in the risk of PTS over 2 years (41.1% vs 55.6%; P = .04).57 In this study, 3.2% of patients receiving CDT had a major bleed, but there were no intracranial bleeds, treatment-related deaths, or PE. The difference in PTS occurrence became even greater between 2 and 5 years follow-up (43% vs 71%; P < .001); however, QOL was no different between treatment groups at any time point beyond 6 months.58,59 Limitations of this study include its modest sample size (n = 209), geographical limitation (4 centers in Norway), and use of older CDT methods without thrombectomy devices.
The National Institutes of Health–sponsored ATTRACT Trial is a multicenter, assessor-blinded, US-based RCT that evaluated whether the use of PCDT reduces the occurrence of PTS within 2 years in 692 patients with acute proximal DVT.60 This trial found that (a) PCDT did not reduce the occurrence of PTS over 2 years (47% PCDT vs 48% control; P = .56); (b) PCDT caused additional major bleeding (absolute risk difference 1.4%, P = .049; no fatal or intracranial bleeds were observed); (c) PCDT provided greater reduction of leg pain and swelling within the first 30 days, in comparison with standard therapy alone (.024 < P < .051); (d) PCDT reduced the severity of PTS at all time points between 6 and 24 months (P < .01 for all Villalta score comparisons); and (e) there was no significant difference in recurrent VTE or health-related QOL between the 2 treatment groups.61 The authors concluded that PCDT should not be routinely used as first-line therapy for most DVT patients. However, the findings that PCDT improved leg symptom status to some degree at all time points studied argue for further data exploration to determine whether specific identifiable subgroups may experience clinically meaningful benefits from first-line use. Such studies are underway, as are more detailed assessments of QOL, costs, and imaging findings.
In the meantime, careful patient selection for CDT/PCDT is important and should consider the following factors: (1) risk of bleeding: a very low threshold should be applied to exclude patients with factors that may increase the risk of bleeding, including ongoing or recent active bleeding; recent major surgery, trauma, pregnancy, or other invasive procedure; advanced age; and the presence of lesions in critical areas such as the central nervous system; (2) clinical severity and time of DVT: in the absence of acute limb threat, the use of CDT after failure of initial anticoagulant therapy to alleviate pain and swelling to a degree that permits the patient to ambulate without severe limitation is better justified than is up-front use of CDT as an element of initial DVT care. However, CDT will only achieve substantial thrombus removal if provided within the first 2 to 3 weeks after symptom onset54,56,57 ; (3) anatomic extent of DVT: patients with acute iliofemoral DVT are at much-increased risk for PTS and recurrent VTE and are likely to prove the best candidates for CDT.2,29,54,62,63 In contrast, patients with asymptomatic DVT or isolated calf vein or popliteal DVT should not undergo CDT because benefits do not outweigh risks. It is also important to consider the patient’s baseline ambulatory functional capacity, comorbidities, and personal preferences for the type of treatment.
Patient 1 did not have acute limb threat at the time of the acute DVT, so CDT cannot be considered a required element of care. When the patient initially presented, the DVT did not extend into the iliac or common femoral vein. Because most patients with femoropopliteal DVT ultimately recanalize their veins with anticoagulation alone and only infrequently develop advanced PTS, the risks of CDT outweighed the likely benefits at the time of the patient’s initial presentation. However, the DVT subsequently extended to the iliofemoral venous segment 11 days after initial symptom onset, putting the patient at higher long-term risk of recurrent DVT and PTS. The preponderance of the available data supports the ability of thrombolytic therapy to improve symptoms and reduce the severity of PTS (but not to prevent it overall) in this situation.57,58,61 Therefore, after a careful assessment to exclude factors that might promote bleeding, it would have been reasonable to consult an interventional specialist, have a balanced risk-benefit discussion with the patient (including the choice of simply switching to another anticoagulant regimen), and to proceed with intervention if he or she so desired.
Before and after CDT/PCDT, patients should receive therapeutic anticoagulation with similar dosing, monitoring, and duration as do nonintervened DVT patients. During CDT infusions, the use of unfractionated heparin (UFH) targeted to a subtherapeutic partial thromboplastin time (PTT) (eg, 1.2 to 1.7 times control) is supported by clinical studies and consensus guidelines; low molecular weight heparin can also be used during CDT/PCDT, but direct-acting oral anticoagulants that do not have a reversal agent approved by the US Food and Drug Administration (FDA) should be stopped prior to CDT/PCDT because bleeding can occur during thrombolysis.54,57 During the first weeks after CDT/PCDT, the patient is particularly vulnerable to re-thrombosis. Based on clinical experience, the authors’ preference is to use enoxaparin during this period, but use of a twice-daily oral anticoagulant may also be acceptable.
Unfortunately, patient 1 developed severe PTS, causing major life interference and QOL impairment. Management should include a trial of elastic compression stockings first and then, if needed, a venoactive drug (eg, diosmin, a nutritional supplement that is approved by the FDA for management of chronic venous disease symptoms), a structured exercise program (if available locally and if the patient can tolerate it), or an intermittent compression device (either a home edema pump for massive edema or a portable venous return assist-return device).63
If these modalities fail to provide the patient with relief of symptoms and disability, then referral to an interventional specialist is suggested. A careful clinical examination, supplemented by Duplex ultrasound, is performed to identify 2 potentially reversible contributors to venous hypertension: (1) chronic obstruction of the iliac, common femoral vein, or both; and (2) major saphenous vein reflux. In patient 1, both factors were present. He underwent endovascular stent placement to eliminate the iliac vein obstruction; this step eliminated his pain and venous claudication and partially improved his limb swelling. One month later, he underwent endovenous ablation of the left great saphenous vein, resulting in complete symptom resolution. When seen for follow-up 12 months later, his overall level of function had improved further.
In a recent meta-analysis (n = 1118 patients) of nonrandomized studies and case series, stent placement in PTS patients with chronically occluded iliac veins was associated with resolution of pain, swelling, and ulcer in two thirds of stented patients with infrequent major bleeds (0.9%), pulmonary embolisms (0.6%), and deaths (0.3%).64 However, the vast majority of the included studies provided only low-quality evidence, prospective studies have been limited, and there is only 1 published RCT.65,66 Of note, this small (n = 50) study found that patients randomized to stent placement with standard PTS care experienced significantly greater improvement in pain, PTS severity, ulcer healing, and QOL at 4 to 6 months follow-up in comparison with patients who receive standard PTS care alone.66 Hence, at present, the justification for selectively using an endovascular strategy is based upon the disabling nature of advanced PTS, the apparent benefits of endovascular therapy to at least some patients, and the low observed risk of causing harm.67,68 If stenting is planned, the patient should be informed of the potential risks (eg, late stenosis or occlusion), uncertainties, and off-label nature of currently available stents. A pivotal multicenter RCT is being planned to evaluate endovascular therapy for the management of patients with severe established PTS.69
Patient 2
Submassive PE
A 40-year-old man, 28 days removed from sleeve gastrectomy for morbid obesity, presented with chief complaints of chest pain and shortness of breath. The patient was feeling well until 4 days before arrival. At that time, he developed abdominal pain and nausea, resulting in hospital admission, and an upper gastrointestinal endoscopy showed a small hiatal hernia and superficial gastric erosions without bleeding. In the hospital, he developed sudden chest pain, shortness of breath, and tachycardia. At this time, he had a heart rate of 112 beats/min, respiratory rate of 24 breaths/min, blood pressure of 140/82 mmHg, and oxygen saturation of 97% on 2 L of supplemental oxygen. He was mildly tachypneic but in no acute distress at rest. Upon standing he developed considerable chest pain, dizziness, and tachycardia (up to 138 beats/min) and was unable to walk from his bed.
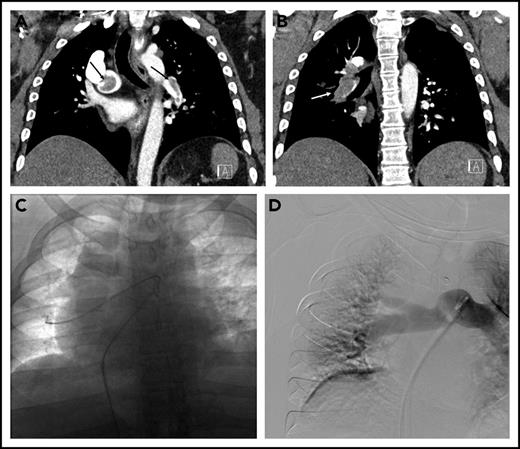
The patient was started on a therapeutic dose of UFH, and diagnostic testing for PE was initiated. Laboratory data included troponin-I of 0.35 ng/mL (normal < 0.04 ng/mL), an N-terminal-pro-brain natriuretic peptide level of 3590 pg/mL (normal < 300 pg/mL), and lactate 2.4 mmol/L (normal < 1.6 mmol/L). Chest computed tomography (CT) angiogram showed a saddle PE in the main pulmonary artery as well as obstructive lobar and segmental thrombus (Figure 1A-B). The RV to left ventricular (LV) ratio was 1.3 (normal < 0.9). Transthoracic echocardiography showed a severely dilated and hypokinetic right ventricle and an estimated pulmonary artery systolic pressure of 61 mmHg.
Submassive pulmonary embolism in patient 2. (A) Coronal CT demonstrating thrombus (arrows) in the right main and left lower-lobe pulmonary arteries. (B) Bulky thrombus is identified in the right lower-lobe pulmonary artery (arrow). (C) Wires and catheters traversing the thrombus in the right main and lower-lobe pulmonary artery. (D) Completion pulmonary angiography demonstrating good pulmonary perfusion.
Submassive pulmonary embolism in patient 2. (A) Coronal CT demonstrating thrombus (arrows) in the right main and left lower-lobe pulmonary arteries. (B) Bulky thrombus is identified in the right lower-lobe pulmonary artery (arrow). (C) Wires and catheters traversing the thrombus in the right main and lower-lobe pulmonary artery. (D) Completion pulmonary angiography demonstrating good pulmonary perfusion.
The hospital’s Pulmonary Embolism Response Team (PERT), consisting of faculty from cardiothoracic surgery, pulmonary/critical care medicine, and interventional radiology (IR), was activated. After a discussion of treatment options, the PERT’s consensus was to proceed with CDT. In the IR suite, the pulmonary arterial system was catheterized (Figure 1C). The mean plasminogen activator (PA) pressure was 37 mmHg. Bilateral multisidehole infusion catheters were positioned within the thrombus. Recombinant tissue PA (rt-PA) was administered through each catheter at a rate of 0.67 mg/h (total dose 1.3 mg/h). UFH was infused through one of the sheaths to maintain a PTT less than 2 times the institutional norm during the infusion. After 18 hours (24 mg rt-PA total), the patient was brought back to the IR suite, where repeat measurements showed a mean PA pressure of 20 mmHg. Repeat angiography showed that the main right and left pulmonary arteries and lobar branches were largely thrombus free (Figure 1D). The sheaths and catheters were removed, and therapeutic-level UFH was resumed. An echocardiogram the next day showed improved RV function. He was discharged on warfarin 4 days postoperative. One month after discharge he reported feeling well without residual dyspnea or other symptoms.
Discussion
Because patients with acute submassive PE (who have normal blood pressure but have RV dysfunction) are at intermediate risk of early clinical deterioration (5%) and death (2% to 3%), escalation of therapy beyond anticoagulation alone is often considered.70,71 More than 1700 patients with submassive PE have been randomized to studies comparing systemic thrombolysis (ST) or anticoagulation alone. The largest of these, the PEITHO trial, randomized 1005 patients to receive bolus tenecteplase plus anticoagulation or anticoagulation alone.71 The primary endpoint (a composite of death or acute clinical deterioration) was met less frequently in the tenecteplase group, but mortality was not significantly different. Furthermore, the tenecteplase group had a fivefold higher risk of major bleeding and a 10-fold higher risk of intracranial bleeding. A meta-analysis that included the PEITHO results concluded that although ST reduces short-term clinical deterioration and may slightly reduce deaths, it increases major bleeding threefold and intracranial/fatal bleeding fivefold.72,73
With ST, a systemically delivered drug gets directed away from obstructed pulmonary arteries, reducing its efficacy and necessitating large doses (eg, 100 mg rt-PA) to remove the clot effectively.74 In contrast, CDT delivers a lower dose of thrombolytic drug (eg, <30 mg rt-PA) directly into the clot. Three prospective studies (of which one, the ULTIMA Study, was a small RCT) have demonstrated that CDT with rt-PA lyses PE, unloads the right ventricle and improves pulmonary arterial blood flow at 48 hours.75-77 In these studies, there was no intracranial or fatal bleeding; however, in the SEATTLE II single-arm cohort study, major bleeds occurred in 11% of CDT recipients.77
Patient 2 had submassive PE; he was hemodynamically stable but had RV dysfunction according to CT, echocardiography, and biomarker assessment. His elevated lactate, postural symptoms, and inability to ambulate raised the concern that he was progressing toward clinical deterioration, so the decision was made to escalate therapy. Given his relatively recent surgery and the presence of recently symptomatic superficial gastric erosions, CDT was chosen over ST to potentially reduce the risk of bleeding. The individual experience with this patient was consistent with the short-term results seen in the limited CDT studies thus far. However, it should be emphasized that the optimal therapeutic approach to prevent short- and long-term deleterious outcomes in submassive PE patients is not known. A rigorous trial of CDT is needed to assess whether this method of thrombolytic drug delivery is safe and improves short- and long-term outcomes.78
Patient 3
Massive PE
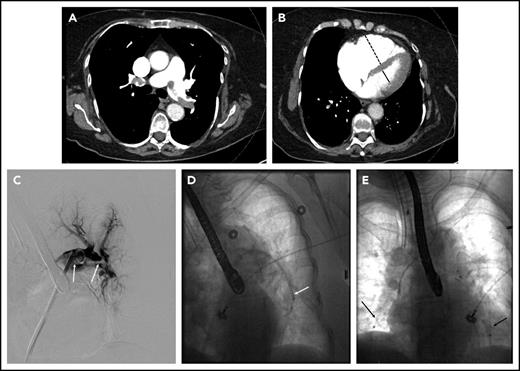
A 78-year-old woman with hypertension, hyperlipidemia, and chronic obstructive pulmonary disease presented to the emergency room with 4 hours of chest pain associated with lightheadedness and diaphoresis. She had an oxygen saturation of 86% on room air and a systolic blood pressure of 80 mmHg. Her oxygen saturation improved to 98% on 2 L of oxygen by nasal cannula. Initial labs were notable for a troponin of 0.44 ng/mL (normal <0.04 ng/mL). Electrocardiography was normal. The d-dimer was 8820 ng/mL, prompting CT angiography of the pulmonary arteries, which revealed multiple bilateral PE in the main pulmonary arteries (Figure 2A), with evidence of right heart dilation (Figure 2B) and strain on transthoracic echocardiography. The patient was started on UFH and transferred to the intensive care unit (ICU) for further management. Lower extremity ultrasound revealed residual acute DVT in the right popliteal and tibial veins. The PERT was activated, and the initial consensus was to not escalate therapy beyond anticoagulation because she had stabilized. However, 12 hours after admission to the ICU, she developed hypotension (systolic blood pressure of 78 mmHg) and was started on vasopressors. The PERT was alerted to this new development, and the decision was made to proceed with catheter-directed therapy.
Massive pulmonary embolism in patient 3. (A) Axial CT image demonstrating thrombus in the right and left main pulmonary arteries. (B) Axial CT image through the heart demonstrating an enlarged RV (dashed line) to LV (solid line) ratio. (C) Thrombus on pulmonary angiography (arrows), extending from the left main pulmonary artery into the left lower-lobe pulmonary artery. (D) Indigo aspiration device (arrow) within the left lower-lobe pulmonary artery. (E) Placement of bilateral CDT infusion catheters (arrows).
Massive pulmonary embolism in patient 3. (A) Axial CT image demonstrating thrombus in the right and left main pulmonary arteries. (B) Axial CT image through the heart demonstrating an enlarged RV (dashed line) to LV (solid line) ratio. (C) Thrombus on pulmonary angiography (arrows), extending from the left main pulmonary artery into the left lower-lobe pulmonary artery. (D) Indigo aspiration device (arrow) within the left lower-lobe pulmonary artery. (E) Placement of bilateral CDT infusion catheters (arrows).
Because of the patient’s instability and hypoxia, general anesthesia with endotracheal intubation was performed by a cardiac anesthesiologist. Pulmonary angiography was performed, showing the bulk of the thrombus in the left lower-lobe pulmonary artery (Figure 2C). The Indigo CAT 8 aspiration device (Penumbra, Inc., Alameda, CA) was used in an attempt to rapidly debulk thrombus (Figure 2D). Bilateral multisidehole infusion catheters were then positioned in each pulmonary artery (Figure 2E), and 0.5 mg rt-PA/catheter/h was initiated, with UFH infusion targeted to PTT less than 2 times the institutional norm. Additionally, a retrievable inferior vena cava (IVC) filter was placed at the time of the procedure. After a total dose of 12 mg, the patient was noted to have significant oozing from the catheter site, so thrombolytic infusion was halted. Her blood pressure was normal, and she was no longer requiring vasopressor support. The catheters and sheaths were removed at the bedside. Therapeutic-level UFH was resumed. The patient was discharged 2 days later on anticoagulation. The IVC filter was removed percutaneously 6 weeks later, at which time the patient was doing well with no functional limitations.
Discussion
Massive PE represents a true emergency, because mortality rates are high with anticoagulation alone. In patients who do not have contraindications, ST is the fastest reperfusion method and should be strongly considered. However, some patients may not be good candidates for ST because of advanced age or comorbidities, and in these settings, catheter-directed therapy or surgical embolectomy may play a role.1,2
The goal of catheter-directed therapy in the setting of massive PE is to rescue the patient from hypotension and death. Because speed is paramount, mechanical methods to rapidly remove a clot, unload the RV, and improve left ventricular filling are often used. In a systematic review and meta-analysis of 592 patients treated with catheter-directed therapy, the most commonly used technique was pigtail catheter rotation to create a channel within the clot, although multiple devices have been used for active thrombus removal in the setting of massive PE.79 Of note, the safety and efficacy of these novel devices for PE are currently unknown.
An alternative approach for massive PE is open surgical thrombectomy, which can have several advantages. First, operative mortality with appropriate patient selection in the current literature is lower than historical rates.80 Second, en bloc removal of thrombus is highly effective in improving pulmonary blood flow. Third, heroic measures such as extracorporeal membrane oxygenation can be quickly instituted. Thus, in centers with the requisite expertise, it may be reasonable to treat massive PE patients in the operating room, if ST is ineffective or contraindicated. In our center, catheter-directed therapy is often preferred because of the availability of local expertise, and it was effective in this patient. However, had it failed, surgical therapy would likely have been attempted.
The PREPIC-2 trial demonstrated that for most patients with DVT who can receive anticoagulation, the addition of an inferior vena cava (IVC) filter does not improve the clinical outcome.81 For massive PE, analyses of registry datasets suggest the possibility of a mortality benefit when IVC filters are used to prevent additional acute emboli in patients.82,83 However, caution is urged in interpreting these nonrandomized studies because of the strong potential for patient selection bias and the fact that unstable patients who died quickly upon arrival to the hospital were counted in the anticoagulation-only group (immortal time bias).
In patient 3, the need for pressors and presence of hypotension increased the risk of death approximately eightfold in comparison with submassive PE. Thus, emergent reperfusion with catheter-directed therapy was performed. The patient’s advanced age was considered to be a relative contraindication to ST, because a disproportionate number of major and intracranial bleeds occur in patients over 65 years of age.72 Thrombus aspiration was used along with rt-PA infusion to achieve more rapid clot removal and RV unloading and to potentially reduce the amount of thrombolytic drug needed. The effectiveness of this strategy has not been validated in prospective trials but is felt by many physicians to be justified on the basis of the possibility that rapid catheter-based therapy can rescue patients known to be at imminent risk of death.
Conclusion
Catheter-based therapies offer the potential for improved clinical outcomes in patients with severe manifestations of DVT and PE. Even as clinical trials continue to establish the appropriate criteria for use of these treatment methods, physicians should continue to individualize care with careful consideration of risks and benefits; close collaboration with colleagues who possess diverse expertise; monitoring of local outcomes including mortality, bleeding, hemodynamic improvement, and long-term function; and implementation of quality improvement interventions to ensure that therapy is targeted to patients most likely to benefit.
Acknowledgments
This work was supported in part by a grant from the National Institutes of Health, National Heart Lung and Blood Institute (U01-HL088476) (S.V.). Washington University School of Medicine receives research grant support from Cook Medical, BSN Medical, and Therakos. New York University Langone School of Medicine receives research grant support from Penumbra, Inc.
Authorship
Contribution: S.V. and A.K.S. wrote the article and take full responsibility for its contents.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Suresh Vedantham, Mallinckrodt Institute of Radiology, Washington University School of Medicine, 510 S Kingshighway, Box 8131, St. Louis, MO 63110; e-mail: vedanthams@mir.wustl.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal