Key Points
Rd continuous significantly extended OS compared with MPT and resulted in comparable OS to that with Rd18 in patients with multiple myeloma.
Patients achieving complete or very good partial response with Rd benefited greatly from continuous vs fixed treatment in terms of PFS.
Abstract
This FIRST trial final analysis examined survival outcomes in patients with transplant-ineligible newly diagnosed multiple myeloma (NDMM) treated with lenalidomide and low-dose dexamethasone until disease progression (Rd continuous), Rd for 72 weeks (18 cycles; Rd18), or melphalan, prednisone, and thalidomide (MPT; 72 weeks). The primary endpoint was progression-free survival (PFS; primary comparison: Rd continuous vs MPT). Overall survival (OS) was a key secondary endpoint (final analysis prespecified ≥60 months’ follow-up). Patients were randomized to Rd continuous (n = 535), Rd18 (n = 541), or MPT (n = 547). At a median follow-up of 67 months, PFS was significantly longer with Rd continuous vs MPT (hazard ratio [HR], 0.69; 95% confidence interval [CI], 0.59-0.79; P < .00001) and was similarly extended vs Rd18. Median OS was 10 months longer with Rd continuous vs MPT (59.1 vs 49.1 months; HR, 0.78; 95% CI, 0.67-0.92; P = .0023), and similar with Rd18 (62.3 months). In patients achieving complete or very good partial responses, Rd continuous had an ≈30-month longer median time to next treatment vs Rd18 (69.5 vs 39.9 months). Over half of all patients who received second-line treatment were given a bortezomib-based therapy. Second-line outcomes were improved in patients receiving bortezomib after Rd continuous and Rd18 vs after MPT. No new safety concerns, including risk for secondary malignancies, were observed. Treatment with Rd continuous significantly improved survival outcomes vs MPT, supporting Rd continuous as a standard of care for patients with transplant-ineligible NDMM. This trial was registered at www.clinicaltrials.gov as #NCT00689936 and EudraCT as 2007-004823-39.
Introduction
Lenalidomide is an immunomodulatory agent that has been used to treat patients with multiple myeloma. The direct tumoricidal effects of lenalidomide are mediated in myeloma cells by targeting cereblon, a component of the E3 ubiquitin ligase complex.1-4 This interaction triggers proteasome degradation of transcription factors Ikaros and Aiolos, resulting in downregulation of myeloma survival signals IRF4 and MYC and upregulation of the immunoregulatory molecule interleukin 2.2,4,5 Synergistic effects are observed when lenalidomide is administered in combination with dexamethasone, making this regimen one of the standards of care in patients with newly diagnosed and relapsed multiple myeloma.6-11
Lenalidomide and low-dose dexamethasone (Rd) and melphalan plus prednisone and thalidomide (MPT) are 2 treatment options for patients with newly diagnosed multiple myeloma (NDMM) ineligible for autologous stem cell transplant.8-13 These regimens were investigated in the pivotal phase 3 FIRST (Frontline Investigation of Revlimid Plus Dexamethasone Versus Standard Thalidomide) trial.8 This trial compared the efficacy and safety of Rd until disease progression (Rd continuous), Rd for 72 weeks (18 cycles; Rd18), and MPT for 72 weeks (12 cycles) in patients with transplant-ineligible NDMM.8 The study was powered for the primary comparison of progression-free survival (PFS) between Rd continuous and MPT. Nominal power for the comparison of overall survival (OS) was also calculated.
At the original data cutoff of 24 May 2013 (median follow-up of 37 months), the prespecified analysis for the primary endpoint of PFS revealed that treatment with Rd continuous reduced the risk of progression or death by 28% compared with MPT.8 Risk of progression or death was also reduced with Rd continuous compared with Rd18. In addition, although the criteria for the final analysis of OS had not been met, the concurrent, preplanned, interim analysis demonstrated that Rd continuous prolonged OS compared with MPT and was similar to Rd18. At the request of regulatory authorities, an updated analysis was conducted at a median follow-up of 45.5 months, which demonstrated continued improvements in PFS and OS with Rd continuous vs MPT, and in PFS with Rd continuous vs Rd18.14
As of the final data cutoff, January 21, 2016, the median duration of follow-up in the study was 5.6 years and the criterion to proceed with the final OS analysis was met. Here, we present the prespecified final analysis of OS from the FIRST trial and provide an update on the other efficacy and safety outcomes, including those by cytogenetics.
Methods
Study design and patients
The randomized, global, phase 3, multicenter FIRST trial compared Rd continuous with MPT and with Rd18 in patients with NDMM who were ineligible for stem cell transplant. Details of the trial have been described previously.8 Briefly, patients were ≥18 years of age with previously untreated, symptomatic, and measurable multiple myeloma. Key eligibility criteria included an Eastern Cooperative Oncology Group performance status ≤2 and either age ≥65 years or age <65 years and ineligible for autologous stem cell transplant. Patients with severe renal impairment (creatinine clearance <30 mL/min) were eligible, except for those who required dialysis. Fluorescence in situ hybridization analyses were performed on bone marrow cells, and patients with t(4;14), t(14;16), and/or del(17p) were categorized as high risk. All patients provided written informed consent, and the study was approved by institutional review boards or ethics committees at all sites before initiation and conducted according to the Declaration of Helsinki and the Harmonization E6 Guidelines for Good Clinical Practice.
Treatment
Patients were randomized 1:1:1 to receive open-label treatment with Rd continuous, Rd18, or MPT and were stratified by age (≤75 vs >75 years), International Staging System disease stage (I/II vs III), and country. Rd18 was added as a third arm; this enabled investigation into whether treatment with Rd beyond 18 months (72 weeks) in the Rd continuous arm would improve clinical outcomes vs treatment with a fixed duration for 18 months. In the Rd continuous and Rd18 arms, patients received oral lenalidomide on days 1-21 plus oral dexamethasone on days 1, 8, 15, and 22 of 28-day treatment cycles continuously until disease progression (Rd continuous) or for 18 cycles (72 weeks; Rd18), respectively (see supplemental Table 1, available on the Blood Web site, for starting doses). Patients in the MPT arm received oral melphalan on days 1-4, oral prednisone on days 1-4, and oral thalidomide daily in twelve 42-day cycles (72 weeks). Starting doses were adjusted for patients >75 years of age.
Outcomes
With a primary endpoint of PFS, the primary comparison was between the Rd continuous and MPT treatment arms. Comparison of PFS between Rd continuous vs Rd18 arms and MPT vs Rd18 arms was a secondary objective. The key secondary endpoint was OS; additional secondary endpoints included overall response rate (ORR; ≥ partial response [PR]), time to next antimyeloma treatment (TTNT; which censors deaths), and safety, including second primary malignancies. Exploratory endpoints included time from randomization to second progression or death (PFS2) and response to second antimyeloma treatment. Response to treatment was evaluated using the International Myeloma Working Group criteria for multiple myeloma15 and was assessed after each treatment cycle and every 28 days during follow-up. Adverse events were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0).
Statistical analysis
Efficacy analyses were performed on all randomized patients (intention-to-treat [ITT] population), and safety analyses were performed on all patients who received ≥1 dose of study drug (safety population). The prespecified OS analysis was to be performed after all patients were followed for ≥60 months from randomization or died or were lost to follow-up before 60 months. An estimated 597 deaths would be expected in the Rd continuous and MPT arms (896 deaths across the 3 arms), which would have a power of 78% to detect a 25% improvement in median OS (from 45 to 56 months) using a 2-sided log-rank test at the 0.05 significance level. The Kaplan-Meier product limit method was used to estimate time-to-event endpoints (eg, PFS and OS); differences between treatment arms were compared using an unstratified log-rank test.
Results
A total of 1623 patients were randomized to receive Rd continuous (n = 535), Rd18 (n = 541), or MPT (n = 547; supplemental Figure 1). As previously described, baseline characteristics were well balanced among the treatment arms.8 Validated fluorescence in situ hybridization results were available in less than half of the patients (762/1623; supplemental Table 2). Patients with high-risk cytogenetics were evenly distributed across the Rd continuous (17%), Rd18 (20%), and MPT (19%) arms. A large portion of patients in the Rd continuous arm received long-term treatment, with 51%, 39%, 26%, and 18% still on treatment after 18 months, 2, 3, and 4 years, respectively. Additional treatment duration data are provided in the supplemental data. At the time of final data cutoff (21 January 2016), the median duration of follow-up for surviving patients was 67 months (range, 0-86.8 months). Fifty-two (10%) patients were still receiving treatment in the Rd continuous arm at the time of analysis (supplemental Table 3), whereas all patients in the Rd18 and MPT arms had discontinued treatment per the protocol-defined schedule. Disease progression was the most common reason for study discontinuation, with a higher incidence in the Rd18 (67%) and MPT (62%) arms than in the Rd continuous arm (51%).
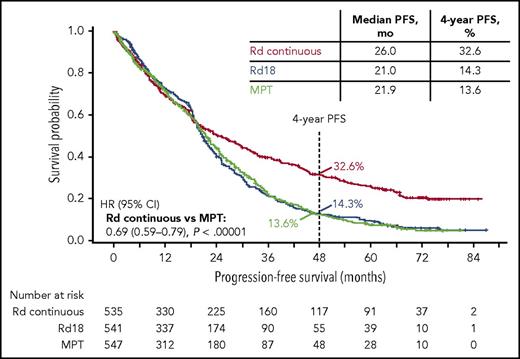
Nearly 3 years after the original analysis of the primary endpoint, PFS, the results remain consistent: Rd continuous significantly improved PFS compared with MPT (hazard ratio [HR], 0.69; 95% confidence interval [CI], 0.59-0.79; P < .00001; Figure 1). Rd continuous also reduced the risk of progression or death compared with Rd18 (HR, 0.70; 95% CI, 0.60-0.81). The median PFS was 26.0 months with Rd continuous, 21.0 months with Rd18, and 21.9 months with MPT. In addition, the 4-year PFS rate more than doubled with Rd continuous (32.6%) compared with Rd18 (14.3%) and MPT (13.6%). In the analysis of PFS by patient subgroups, Rd continuous was favored over MPT in the majority of subgroups analyzed (Figure 2). Rd continuous prolonged PFS compared with MPT and Rd18 in patients with non–high-risk cytogenetics (hereafter referred to as standard risk); however, there was no statistical difference in the group of patients with high-risk cytogenetics (supplemental Table 4). An analysis of PFS by age is provided in the supplemental data.
Effect of patient subgroup on PFS. *Number of events per number of patients. †Complete cytogenetics profile for 501 patients (248 in Rd continuous and 253 in MPT); high-risk cytogenetics included t(4;14), t(14;16), and del(17p). cont, continuous; CrCl, creatinine clearance; ECOG PS, Eastern Cooperative Oncology Group performance status; ISS, International Staging System.
Effect of patient subgroup on PFS. *Number of events per number of patients. †Complete cytogenetics profile for 501 patients (248 in Rd continuous and 253 in MPT); high-risk cytogenetics included t(4;14), t(14;16), and del(17p). cont, continuous; CrCl, creatinine clearance; ECOG PS, Eastern Cooperative Oncology Group performance status; ISS, International Staging System.
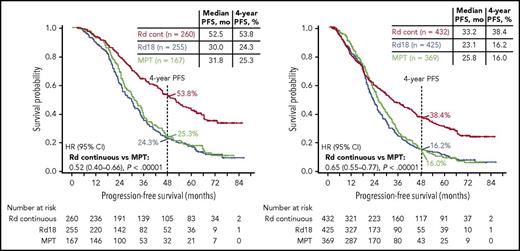
PFS analyzed by best response (Figure 3) revealed that patients who achieved complete response (CR) or very good partial response (VGPR) had greater PFS benefit with Rd continuous than with MPT (HR, 0.52; 95% CI, 0.40-0.66) or Rd18 (HR, 0.47; 95% CI, 0.38-0.59). The median PFS in patients achieving CR with Rd continuous was not reached and was 41.0 and 37.6 months in the Rd18 and MPT arms, respectively. Taken together, good responders (VGPR or CR) still benefited from Rd continuous therapy. A higher proportion of patients who were progression free at 18 months achieved ≥VGPR with Rd continuous (218/276 [79%]) and Rd18 (194/276 [70%]) than with MPT (131/249 [53%]).
PFS by response subgroup. PFS in patients achieving ≥VGPR (left) and ≥PR (right).
PFS by response subgroup. PFS in patients achieving ≥VGPR (left) and ≥PR (right).
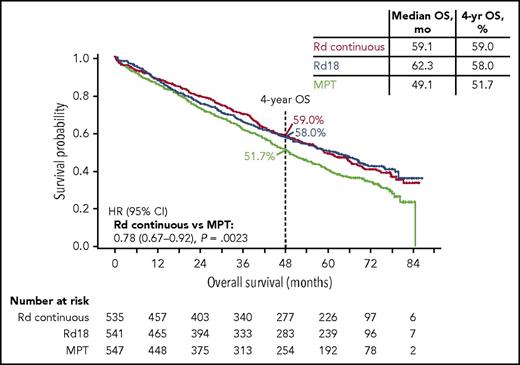
At the median follow-up of 5.6 years, 56% of all patients in the study had died, with 286, 283, and 337 deaths in the Rd continuous, Rd18, and MPT arms, respectively (906 total deaths). The prespecified final OS analysis for the primary comparison revealed that Rd continuous significantly extended OS compared with MPT (HR, 0.78; 95% CI, 0.67-0.92; P = .0023; Figure 4) and resulted in comparable OS to that with Rd18 (HR 1.02; 95% CI, 0.86-1.20). The median OS was similar in the Rd continuous (59.1 months) and Rd18 arms (62.3 months), and both were longer than that in the MPT arm (49.1 months). Similar results were observed with Rd continuous vs MPT and Rd18 in patients with standard-risk cytogenetics, yet the subgroup of patients with high-risk cytogenetics did not experience OS benefit with Rd continuous vs MPT (Figure 5; supplemental Table 4). An analysis of OS by age is provided in the supplemental data.
Effect of patient subgroup on OS. *Number of events per number of patients. †Complete cytogenetics profile for 501 patients (248 in Rd continuous and 253 in MPT); high-risk cytogenetics included t(4;14), t(14;16), and del(17p).
Effect of patient subgroup on OS. *Number of events per number of patients. †Complete cytogenetics profile for 501 patients (248 in Rd continuous and 253 in MPT); high-risk cytogenetics included t(4;14), t(14;16), and del(17p).
In patients achieving ≥VGPR, the median OS was extended by 23.8 months with Rd continuous (79.5 months) compared with MPT (55.7 months; HR, 0.63; 95% CI, 0.48-0.83). The median OS in patients achieving ≥VGPR was similar between Rd continuous and Rd18 (80.1 months).
Higher ORRs (≥PR) were observed with Rd continuous (81%) than with MPT (67%) in the ITT population as well as in patients with standard-risk cytogenetics (81% vs 71%, respectively; Table 1). Rd continuous also resulted in higher-quality responses than MPT in both the ITT population and the patients with standard-risk cytogenetics. ORR and quality of responses with Rd18 were similar to those with Rd continuous in both the ITT population and patients with standard-risk cytogenetics.
Response to treatment as assessed by IMWG criteria
| . | ITT population . | High-risk cytogenetics . | Standard-risk cytogenetics . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Response . | Rd continuous (n = 535) . | Rd18 (n = 541) . | MPT (n = 547) . | Rd continuous (n = 43) . | Rd18 (n = 52) . | MPT (n = 47) . | Rd continuous (n = 205) . | Rd18 (n = 209) . | MPT (n = 206) . |
| ORR, n (%) | 432 (81) | 425 (79) | 369 (67) | 33 (77) | 35 (67) | 32 (68) | 166 (81) | 167 (80) | 146 (71) |
| CR | 119 (22) | 110 (20) | 68 (12) | 5 (12) | 7 (13) | 0 | 43 (21) | 45 (22) | 35 (17) |
| VGPR | 141 (26) | 145 (27) | 99 (18) | 8 (19) | 11 (21) | 5 (11) | 58 (28) | 54 (26) | 45 (22) |
| PR | 172 (32) | 170 (31) | 202 (37) | 20 (47) | 17 (33) | 27 (57) | 65 (32) | 68 (33) | 66 (32) |
| SD, n (%) | 66 (12) | 83 (15) | 116 (21) | 6 (14) | 12 (23) | 11 (23) | 26 (13) | 29 (14) | 37 (18) |
| PD, n (%) | 10 (2) | 6 (1) | 17 (3) | 1 (2) | 0 | 2 (4) | 4 (2) | 4 (2) | 5 (2) |
| NE, n (%) | 27 (5) | 27 (5) | 45 (8) | 3 (7) | 5 (10) | 2 (4) | 9 (4) | 9 (4) | 18 (9) |
| Odds ratio, Rd continuous vs MPT | 2.02 | 1.55 | 1.75 | ||||||
| 95% CI | 1.53-2.68 | 0.61-3.95 | 1.10-2.77 | ||||||
| . | ITT population . | High-risk cytogenetics . | Standard-risk cytogenetics . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Response . | Rd continuous (n = 535) . | Rd18 (n = 541) . | MPT (n = 547) . | Rd continuous (n = 43) . | Rd18 (n = 52) . | MPT (n = 47) . | Rd continuous (n = 205) . | Rd18 (n = 209) . | MPT (n = 206) . |
| ORR, n (%) | 432 (81) | 425 (79) | 369 (67) | 33 (77) | 35 (67) | 32 (68) | 166 (81) | 167 (80) | 146 (71) |
| CR | 119 (22) | 110 (20) | 68 (12) | 5 (12) | 7 (13) | 0 | 43 (21) | 45 (22) | 35 (17) |
| VGPR | 141 (26) | 145 (27) | 99 (18) | 8 (19) | 11 (21) | 5 (11) | 58 (28) | 54 (26) | 45 (22) |
| PR | 172 (32) | 170 (31) | 202 (37) | 20 (47) | 17 (33) | 27 (57) | 65 (32) | 68 (33) | 66 (32) |
| SD, n (%) | 66 (12) | 83 (15) | 116 (21) | 6 (14) | 12 (23) | 11 (23) | 26 (13) | 29 (14) | 37 (18) |
| PD, n (%) | 10 (2) | 6 (1) | 17 (3) | 1 (2) | 0 | 2 (4) | 4 (2) | 4 (2) | 5 (2) |
| NE, n (%) | 27 (5) | 27 (5) | 45 (8) | 3 (7) | 5 (10) | 2 (4) | 9 (4) | 9 (4) | 18 (9) |
| Odds ratio, Rd continuous vs MPT | 2.02 | 1.55 | 1.75 | ||||||
| 95% CI | 1.53-2.68 | 0.61-3.95 | 1.10-2.77 | ||||||
IMWG, International Myeloma Working Group; NE, not evaluable; PD, progressive disease; SD, stable disease.
Median TTNT was longer with Rd continuous (36.7 months) than with MPT (26.7 months) or Rd18 (28.5 months; Figure 6). In patients who achieved ≥VGPR, median TTNT was substantially longer (69.5 months with Rd continuous vs 37.7 months with MPT and 39.9 months with Rd18; Figure 6). In the Rd continuous arm, 299 patients (56%) started second-line antimyeloma treatment, whereas 381 and 377 patients in the MPT and Rd18 arms, respectively (70% in both arms), started second-line treatment (Table 2). Nineteen (4%) patients treated with Rd continuous, 29 (5%) with Rd18, and 23 (4%) with MPT had a documented progression and died without receiving any second-line therapy. A similar proportion of patients who were >75 and ≤75 years of age and treated with Rd continuous (7/186 [4%] vs 12/349 [3%]), Rd18 (10/193 [5%] vs 19/348 [5%]), and MPT (11/188 [6%] vs 12/359 [3%]) progressed and died without receiving any second-line therapy. However, more patients >75 than ≤75 years of age who were treated with Rd continuous (60/186 [32%] vs 48/349 [14%]), Rd18 (37/193 [19%] vs 40/348 [11%]), and MPT (48/188 [26%] vs 42/359 [12%]) died (with or without a documented progression) without receiving any second-line therapy.
Subsequent treatment and outcomes
| . | Rd continuous . | Rd18 . | MPT . |
|---|---|---|---|
| All second-line treatment, n/n (%) | 299/535 (56) | 377/541 (70) | 381/547 (70) |
| CR, n (%) | 17 (6) | 23 (6) | 19 (5) |
| VGPR, n (%) | 41 (14) | 51 (14) | 50 (13) |
| PR or better, n (%) | 134 (45) | 196 (52) | 173 (45) |
| Median time from 2nd to 3rd line, mo | 16.6 | 18.9 | 14.1 |
| Bortezomib-based second-line treatment, n/n (%)* | 179/299 (60) | 208/377 (55) | 170/381 (45) |
| CR, n (%) | 11 (6) | 14 (7) | 7 (4) |
| VGPR, n (%) | 33 (18) | 33 (16) | 24 (14) |
| PR or better, n (%) | 96 (54) | 110 (53) | 74 (44) |
| Median time from 2nd to 3rd line, mo | 16.4 | 15.9 | 10.6 |
| Median OS, mo | 51.8 | 51.9 | 42.2 |
| Lenalidomide-based second-line treatment, n/n (%)† | 41/299 (14) | 82/377 (22) | 150/381 (39) |
| CR, n (%) | 4 (10) | 3 (4) | 10 (7) |
| VGPR, n (%) | 3 (7) | 14 (17) | 20 (13) |
| PR or better, n (%) | 15 (37) | 51 (62) | 77 (51) |
| Median time from 2nd to 3rd line, mo | 15.5 | 23.2 | 17.7 |
| Median OS, mo | NR | 80.1 | 62.6 |
| Thalidomide-based second-line treatment, n/n (%)‡ | 36/299 (12) | 30/377 (8) | 25/381 (7) |
| CR, n (%) | 2 (6) | 1 (3) | 0 |
| VGPR, n (%) | 3 (8) | 3 (10) | 3 (12) |
| PR or better, n (%) | 16 (44) | 12 (40) | 10 (40) |
| Median time from 2nd to 3rd line, mo | 19.6 | 16.5 | 14.9 |
| Median OS, mo | 52.9 | 62.3 | 46.2 |
| Other second-line treatment, n/n (%)§ | 41/299 (14) | 49/377 (13) | 33/381 (9) |
| CR, n (%) | 0 | 4 (8) | 1 (3) |
| VGPR, n (%) | 2 (5) | 1 (2) | 3 (9) |
| PR or better, n (%) | 6 (15) | 19 (39) | 11 (33) |
| Median time from 2nd to 3rd line, mo | 14.2 | 34.8 | 19.4 |
| Median OS, mo | 41.1 | 79.1 | 62.1 |
| . | Rd continuous . | Rd18 . | MPT . |
|---|---|---|---|
| All second-line treatment, n/n (%) | 299/535 (56) | 377/541 (70) | 381/547 (70) |
| CR, n (%) | 17 (6) | 23 (6) | 19 (5) |
| VGPR, n (%) | 41 (14) | 51 (14) | 50 (13) |
| PR or better, n (%) | 134 (45) | 196 (52) | 173 (45) |
| Median time from 2nd to 3rd line, mo | 16.6 | 18.9 | 14.1 |
| Bortezomib-based second-line treatment, n/n (%)* | 179/299 (60) | 208/377 (55) | 170/381 (45) |
| CR, n (%) | 11 (6) | 14 (7) | 7 (4) |
| VGPR, n (%) | 33 (18) | 33 (16) | 24 (14) |
| PR or better, n (%) | 96 (54) | 110 (53) | 74 (44) |
| Median time from 2nd to 3rd line, mo | 16.4 | 15.9 | 10.6 |
| Median OS, mo | 51.8 | 51.9 | 42.2 |
| Lenalidomide-based second-line treatment, n/n (%)† | 41/299 (14) | 82/377 (22) | 150/381 (39) |
| CR, n (%) | 4 (10) | 3 (4) | 10 (7) |
| VGPR, n (%) | 3 (7) | 14 (17) | 20 (13) |
| PR or better, n (%) | 15 (37) | 51 (62) | 77 (51) |
| Median time from 2nd to 3rd line, mo | 15.5 | 23.2 | 17.7 |
| Median OS, mo | NR | 80.1 | 62.6 |
| Thalidomide-based second-line treatment, n/n (%)‡ | 36/299 (12) | 30/377 (8) | 25/381 (7) |
| CR, n (%) | 2 (6) | 1 (3) | 0 |
| VGPR, n (%) | 3 (8) | 3 (10) | 3 (12) |
| PR or better, n (%) | 16 (44) | 12 (40) | 10 (40) |
| Median time from 2nd to 3rd line, mo | 19.6 | 16.5 | 14.9 |
| Median OS, mo | 52.9 | 62.3 | 46.2 |
| Other second-line treatment, n/n (%)§ | 41/299 (14) | 49/377 (13) | 33/381 (9) |
| CR, n (%) | 0 | 4 (8) | 1 (3) |
| VGPR, n (%) | 2 (5) | 1 (2) | 3 (9) |
| PR or better, n (%) | 6 (15) | 19 (39) | 11 (33) |
| Median time from 2nd to 3rd line, mo | 14.2 | 34.8 | 19.4 |
| Median OS, mo | 41.1 | 79.1 | 62.1 |
The most common second-line bortezomib-based regimens included bortezomib + dexamethasone (213 patients; 38%), bortezomib + melphalan + prednisone (110 patients; 20%), bortezomib + cyclophosphamide + dexamethasone (63 patients; 11%), bortezomib monotherapy (32 patients; 6%), and bendamustine + bortezomib + dexamethasone (26 patients; 5%).
The most common lenalidomide-based second-line regimens in the Rd continuous, Rd18, and MPT arms were Rd (24 [59%], 60 [73%], and 110 [73%] patients), lenalidomide monotherapy (13 [32%], 7 [9%], and 12 [8%]), Rd + investigational drug (1 [2%], 6 [7%], and 3 [2%]), Rd + monoclonal antibodies (0, 0, and 7 [5%]), and Rd + carfilzomib (0, 3 [4%], and 2 [1%]).
The most common thalidomide-based regimens included MPT (52 [57%]) and thalidomide monotherapy (14 [15%]).
The most common regimens in this category included melphalan + prednisone (41 [33%]) and dexamethasone monotherapy (18 [15%]); carfilzomib (as either carfilzomib + dexamethasone or carfilzomib + cyclophosphamide + dexamethasone) was given to 1 (2%), 6 (12%), and 2 (6%) patients in Rd continuous, Rd18, and MPT, respectively.
NR, not reached.
Among 1057 patients who received second-line therapy, 557 (53%) were given bortezomib-based regimens: 179 (60%) patients in the Rd continuous arm, 208 (55%) patients in the Rd18 arm, and 170 (45%) patients in the MPT arm (Table 2). Higher-quality responses (ie, ≥VGPR) were more frequent with bortezomib as second-line antimyeloma treatment following Rd continuous and Rd18 than following MPT (Table 2). In addition, median time from second-line bortezomib to third-line treatment was 16.4, 15.9, and 10.6 months. Other second-line regimens, either based on lenalidomide or thalidomide or not incorporating bortezomib, lenalidomide, or thalidomide (referred to as other regimens), were given less frequently and are described in Table 2 with times from second to third line and OS outcomes. Carfilzomib-based second-line therapy was infrequent (14 patients), but more common after Rd18 (9 patients) or MPT (4 patients) than Rd continuous (1 patient). PFS2 was improved in patients who received Rd continuous vs MPT (HR, 0.74; 95% CI, 0.64-0.85). Median PFS2 was 42.9 months with Rd continuous, 40.9 months with Rd18, and 35.0 months with MPT. The number of patients receiving third-line antimyeloma treatment was similar across arms (Rd continuous, 180/299 [60%]; Rd18, 206/377 [55%]; MPT, 231/381 [61%]).
Patients receiving long-term treatment with Rd continuous had similar baseline demographics as those in the ITT population, with a few notable exceptions. Compared with the ITT population, fewer patients with International Staging System stage 3 disease, high-risk cytogenetics, or age >75 years received long-term treatment with Rd continuous (supplemental Table 5). Of note, only 10% of the 52 patients still receiving Rd treatment at the time of this analysis had high lactate dehydrogenase at time of diagnosis, and no patients had high-risk cytogenetics. The majority of patients continued to receive the initial dose of lenalidomide with long-term administration of Rd continuous, whereas the number of patients receiving dexamethasone was reduced over time (supplemental Table 6). High-quality responses were observed in the majority of patients receiving long-term treatment with Rd continuous, with 77%, 86%, and 92% of the patients who were treated for >18 months, ≥3 years, and at data cutoff, respectively, achieving ≥VGPR (supplemental Table 7).
Grade 3/4 adverse events are presented in Table 3 and show no new safety concerns compared with earlier analyses.8,14 More patients experienced grade 3 or 4 neutropenia with MPT (45%) than with Rd continuous (30%) or Rd18 (26%). Grade 3 or 4 infections were observed in a greater proportion of patients treated with Rd continuous (32%) than with Rd18 (22%) or MPT (17%). Similar to results of previous analyses, hematologic secondary primary malignancy (SPM) was more frequent with MPT (3%) than with Rd continuous (1%) or Rd18 (<1%; Table 4). The incidence of solid tumor SPM was similar across treatment arms.
Selected grade 3/4 adverse events
| Patients with selected grade 3/4 adverse events . | Rd continuous (n = 532) . | Rd18 (n = 540) . | MPT (n = 541) . |
|---|---|---|---|
| Hematologic, % | |||
| Neutropenia | 30 | 26 | 45 |
| Anemia | 19 | 16 | 19 |
| Thrombocytopenia | 9 | 8 | 11 |
| Febrile neutropenia | 1 | 3 | 3 |
| Nonhematologic, % | |||
| Infections | 32 | 22 | 17 |
| Pneumonia | 9 | 8 | 6 |
| Cataract | 7 | 3 | 1 |
| Deep vein thrombosis | 5 | 4 | 3 |
| Diarrhea | 5 | 3 | 1 |
| Pulmonary embolism | 4 | 3 | 4 |
| Constipation | 2 | 2 | 5 |
| Peripheral sensory neuropathy | 1 | <1 | 9 |
| Patients with selected grade 3/4 adverse events . | Rd continuous (n = 532) . | Rd18 (n = 540) . | MPT (n = 541) . |
|---|---|---|---|
| Hematologic, % | |||
| Neutropenia | 30 | 26 | 45 |
| Anemia | 19 | 16 | 19 |
| Thrombocytopenia | 9 | 8 | 11 |
| Febrile neutropenia | 1 | 3 | 3 |
| Nonhematologic, % | |||
| Infections | 32 | 22 | 17 |
| Pneumonia | 9 | 8 | 6 |
| Cataract | 7 | 3 | 1 |
| Deep vein thrombosis | 5 | 4 | 3 |
| Diarrhea | 5 | 3 | 1 |
| Pulmonary embolism | 4 | 3 | 4 |
| Constipation | 2 | 2 | 5 |
| Peripheral sensory neuropathy | 1 | <1 | 9 |
Second primary malignancies
| SPM . | Rd continuous (n = 532) . | Rd18 (n = 540) . | MPT (n = 541) . |
|---|---|---|---|
| Invasive, n (%) | 36 (7) | 38 (7) | 46 (9) |
| Hematologic | 4 (1) | 2 (<1) | 14 (3) |
| AML | 1 (<1) | 1 (<1) | 5 (1) |
| MDS | 2 (<1) | 1 (<1) | 5 (1) |
| MDS to AML | 0 | 0 | 4 (1) |
| B-cell leukemia | 1 (<1) | 0 | 0 |
| Solid tumor | 32 (6) | 37 (7) | 32 (6) |
| SPM . | Rd continuous (n = 532) . | Rd18 (n = 540) . | MPT (n = 541) . |
|---|---|---|---|
| Invasive, n (%) | 36 (7) | 38 (7) | 46 (9) |
| Hematologic | 4 (1) | 2 (<1) | 14 (3) |
| AML | 1 (<1) | 1 (<1) | 5 (1) |
| MDS | 2 (<1) | 1 (<1) | 5 (1) |
| MDS to AML | 0 | 0 | 4 (1) |
| B-cell leukemia | 1 (<1) | 0 | 0 |
| Solid tumor | 32 (6) | 37 (7) | 32 (6) |
AML, acute myeloid leukemia; MDS, myelodysplastic syndromes.
Discussion
In this prespecified final OS analysis of the FIRST trial, the primary comparison revealed that Rd continuous significantly prolonged PFS and OS compared with MPT in transplant-ineligible patients with NDMM. Rd continuous also prolonged PFS but not OS compared with Rd18. Of note, both Rd arms extended OS by ∼1 year compared with MPT, with a median OS of ∼5 years in these elderly patients with NDMM.
The benefit of OS observed with Rd continuous and Rd18 may be driven in part by optimized rescue therapy possibilities in patients treated with Rd vs those treated with MPT. Patients who received bortezomib-based therapy (the most common second-line therapy) after Rd continuous or Rd18 had better-quality responses to second-line treatment and a longer median time from second- to third-line treatment than those who received bortezomib-based therapy after MPT. One potential explanation is that patients previously exposed to both melphalan and thalidomide, which are often associated with more hematologic and neurological toxicity, may have had inferior outcomes with bortezomib due to lower bone marrow reserves and residual symptoms of peripheral neuropathy.16,17 Regardless of the second or subsequent lines of therapy they received, however, patients in the Rd continuous and Rd18 arms had higher median OS and better outcomes after second-line treatment compared with patients in the MPT arm. Moreover, the benefit of Rd continuous vs MPT is further evidenced by the increasing differences in outcomes between the 2 arms over time, with Rd continuous extending PFS by 4 months, PFS2 by 8 months, and OS by 10 months. Taken together, these findings suggest that Rd affords a clinical advantage in subsequent lines of therapy and highlight the importance of using Rd continuous and not MPT as first-line treatment of transplant-ineligible patients with NDMM.
OS was similar in the Rd continuous and Rd18 arms, which may have been due to a combination of factors, including the impact of subsequent lines of treatment, the choices of which were up to the treating physician, and the older age of the patient population. The study was conducted in 18 countries in Europe, North America, and Asia Pacific, and second-line regimens were greatly variable. Similar OS results were achieved between Rd continuous and Rd18 patients who received either a bortezomib- or lenalidomide-based second-line therapy. In contrast, patients treated with Rd continuous who received either a thalidomide-based second-line therapy or other regimens had a less favorable OS outcome compared with patients treated with Rd18. Rd18 patients were offered innovative regimens for the second line, such as Rd plus either carfilzomib or an investigational agent, slightly more frequently. Although OS was similar in the Rd-containing arms, continuous treatment with Rd greatly extended PFS and TTNT in patients who responded to Rd compared with responders who discontinued after a fixed treatment duration of 18 months. TTNT was nearly 6 years in patients who achieved deeper responses (≥VGPR) with Rd continuous vs 3 years in patients who achieved ≥VGPR but discontinued after 18 months. One should note, however, the challenge of making direct associations between response and PFS outcomes with longer-term outcomes such as OS. This is particularly limiting in a post hoc analysis of small patient subgroups using data from a clinical trial designed before the availability of agents approved within the last decade.
All 52 patients who were still on treatment after nearly 6 years in the Rd continuous arm achieved PR or better, and 92% achieved ≥VGPR. Among these patients, none had high-risk cytogenetics and only 10% had high lactate dehydrogenase at diagnosis. About 40% of the 52 patients had discontinued dexamethasone and 21% were over 75 years of age (26% of patients treated beyond 3 years and 35% of patients at enrollment were >75 years of age), confirming that Rd is manageable in very elderly patients.14 Overall, these results support the use of Rd continuous in patients without high-risk cytogenetics who respond well (especially those who achieve ≥VGPR) and have good tolerance to treatment with the potential option to discontinue dexamethasone in the long term.
Rd continuous and Rd18 were not superior to MPT for the subgroup of patients with high-risk cytogenetics for both PFS and OS. Of note, treatment approaches have changed since the initiation of the FIRST study, and novel therapies including triplets are becoming increasingly available to high-risk patients. Rd has been explored as a backbone for combination studies, and when combined with bortezomib (RVd) has shown some benefit in high-risk patients with NDMM.18 In addition, RVd was investigated in the large, phase 3 Southwest Oncology Group S0777 study in patients with NDMM without the intention to transplant immediately.19 Southwest Oncology Group S0777 showed both a PFS and an OS benefit in favor of RVd vs Rd (median PFS, 43 vs 30 months; median OS, 75 vs 64 months). However, the study population was very different from that of the FIRST trial, with only 43% of patients aged ≥65 years (vs 94% FIRST trial8 ), 69% of patients intending to transplant (although not immediately), and very few patients with renal failure. RVd was also associated with an increase in neurological toxicity. Overall, these results are difficult to extrapolate to an elderly population, especially in a real-world setting, but RVd might be preferred to Rd for some fit elderly patients. Furthermore, Rd combined with carfilzomib, ixazomib, elotuzumab, or daratumumab has demonstrated improved clinical outcomes in patients with relapsed/refractory multiple myeloma.20-23 These findings support the use of triplet regimens containing the Rd backbone in high-risk patients.
The safety profiles of Rd continuous, Rd18, and MPT reported in this analysis are consistent with those observed in previous analyses, with no new safety concerns observed.8,14 As reported previously in the FIRST trial, hematologic SPM were more frequent with MPT than with Rd continuous or Rd18, but the incidence of solid tumor SPM was similar across treatment arms.8,14 These results are also consistent with those of a meta-analysis that found no increased risk of SPM when lenalidomide was used in combination with dexamethasone.24
In conclusion, Rd continuous prolongs OS compared with MPT and is one of the standards of care for transplant-ineligible patient with NDMM.
Presented in abstract form at the 58th annual meeting of the American Society of Hematology, San Diego, CA, 3 December 2016.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Stephanie Morgan and Peter Simon for medical writing assistance, and Thea Gray for medical editing assistance, which was sponsored by Celgene Corporation.
This study was funded by Celgene Corporation. The study was designed by the Intergroupe Francophone du Myélome (study number 2007-01/Celgene MM-020) in collaboration with Celgene, which sponsored the study and was involved in the data collection, data analysis, and writing of the manuscript in collaboration with the senior academic authors.
Authorship
Contribution: T.F., M.A.D., A.D., J.V.C., A. Belch, M.C., A. Pinto, K.W., H.L., N.J.B., A. Banos, M.T., M.D., J.D.C., C.G., J.-J.L., C.C., A.O., J.D.L.R., D.W., D.B., J.L., K.C.A., P.M., M.A., A. Perrot, B.A., L.Q., M.R., E.B., S.M., M.M., H. A.-L., X.L., A.E.-H., G.C., V.H., L.B., and C.H. contributed to the acquisition, analysis, or interpretation of data for this article, revised the manuscript critically for important intellectual content, approved the final version to be published, and agreed to be accountable for all aspects of the article. The first and revised drafts of the manuscript were developed by T.F. All authors had full access to all data and had sole discretion in the data interpretation, writing of the manuscript, review and approval prior to submission, and decision to submit for publication. T.F. had full access to all data in the study and had final responsibility for the decision to submit for publication.
Conflict-of-interest disclosure: T.F. reports advisory board fees from Amgen, Celgene, Janssen, Karyopharm, Pharmamar, and Takeda and speakers bureau fees from Amgen, Celgene, Janssen, and Takeda; M.A.D. reports honoraria and consulting/advisory fees from Amgen, Celgene, Janssen, and Takeda; A.D. reports grants from Alnylam, Celgene, Pfizer, Prothena, and Takeda; M.C. reports honoraria from Amgen, Bristol-Myers Squibb, Celgene, Janssen, and Takeda; A. Pinto reports honoraria from Celgene, Spectrum, and Takeda and research funding from Takeda; K.W. reports research funding from Celgene and Janssen and honoraria from Amgen, Bristol-Myers Squibb, Celgene, Janssen, Novartis, Onyx, and Takeda and advisory board membership for Amgen, Bristol-Myers Squibb, Celgene, Janssen, Novartis, Onyx, and Takeda; H.L. reports consulting or advisory fees and speakers bureau fees from Amgen, Bristol-Myers Squibb, Celgene, and Janssen and research funding from Takeda; N.J.B. reports honoraria, consulting, or advisory fees and travel expenses from Amgen, Celgene, Janssen, and Takeda and research funding and fees for expert testimony from Celgene and Janssen; M.D. reports research grants, consultancy fees, and speaker’s honoraria from Celgene and Janssen, consultancy fees and speaker’s honoraria from Amgen, and consultancy fees from Bristol-Myers Squibb and Takeda; C.G. reports honoraria from and advisory board membership for Amgen, Bristol-Myers Squibb, Celgene, and Janssen; C.C. reports consulting for and research funding and honoraria from Celgene; A.O. reports advisory board fees from Amgen and Janssen; D.W. reports grants from Celgene and grants and personal fees from Amgen, Bristol-Myers Squibb, Celgene, Janssen, Novartis, and Takeda; K.C.A. reports advisory board fees from Bristol-Myers Squibb, Celgene, Gilead, and Millennium; P.M. reports honoraria from Bristol-Myers Squibb, Celgene, Janssen, and Takeda; A. Perrot reports honoraria and consulting fees from Amgen, Celgene, Janssen, Novartis, and Takeda; M.R. reports grants, personal fees, and nonfinancial support from Amgen, Celgene, and Janssen; E.B. reports advisory board fees from Celgene; M.M. reports personal fees from Celgene; A.E.-H. reports employment and stock options from Celgene; G.C. reports employment, stock options, and travel expenses from Celgene; V.H. reports employment from Celgene; L.B. reports grants, personal fees, and nonfinancial support from Celgene and Janssen, personal fees and nonfinancial support from Amgen, and personal fees from Takeda; C.H. reports honoraria from Celgene; the remaining authors declare no competing financial interests.
Correspondence: Thierry Facon, Centre Hospitalier Régional Universitaire de Lille, Rue Michel Polonovski, 59037 Lille, France; e-mail: thierry.facon@chru-lille.fr.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal