To the editor:
Acute myeloid leukemia (AML) is the most common acute leukemia in adults with an annual, age-adjusted incidence of 3.5/100 000 men and women rising to 15 to 20 above the age of 60 years.1 Extensive work has now led to the elucidation of its genomic backbone, a refined classification scheme, and updated therapeutic recommendations.2-4 Nevertheless, in patients treated with curative intention outside clinical trials, the cornerstones of AML therapy remain chemotherapy and allogeneic hematopoietic stem cell transplantation.5 For this purpose, cytarabine (1-β-d-arabinofuranosylcytosine) has been used as an essential drug in conventional AML protocols at single doses ranging between 100 mg/m2 and 3000 mg/m2 body surface area. Although high-dose (HD) cytarabine has been applied as postremission treatment for decades, recent recommendations favor lower doses, which are accompanied by reduced toxicities.6 On that basis, we hypothesized that HD cytarabine given as consolidation treatment of patients with AML has no significant impact on survival parameters in comparison with intermediate-/low-dose (ID/LD) cytarabine and performed a systematic review and meta-analysis on that topic.
The systematic review and meta-analysis were performed in accordance with the “PRISMA (preferred reporting items for systematic reviews and meta-analyses) Statement.”7 Eligibility criteria were original articles and research letters published in English reporting prospective, randomized controlled trials of conventional consolidation chemotherapy enrolling at least 100 patients with various forms of AML. Of those studies including both types of consolidation treatment, chemotherapy and autologous or allogeneic hematopoietic stem cell transplantation, only the results of the nontransplant arms were used. We searched the databases PubMed/MedLine, Embase, and Cochrane Library from 1990 to 2015 combining the terms acut*/myel*/nonlymph*/leukem*/leukaem*/leucem*/leucaem*/aml/consolidation/postremission/post-remission/treatment or therapy. In addition, a hand search was performed to screen the proceedings of the annual meetings of the American Society of Hematology and European Hematology Association from 2005 onward and the reference lists of the publications selected (supplemental Figure 1, available on the Blood Web site). Abstract evaluation and data extraction were performed by 2 reviewers (K.N.M. and H.S.). Data were extracted in a standardized format and included name of the study, year of publication and enrollment period, number and age of subjects randomized to different treatment arms, and exact dose and timing of all cytotoxic drugs administered. Risk of bias was assessed using the Cochrane Collaboration tool (available at http://www.handbook.cochrane.org) (supplemental Figure 2). Hazard ratios (HRs) and 95% confidence intervals (CIs) for the study end points relapse-free survival (RFS) and overall survival (OS) were extracted if available and estimated otherwise.8 Data synthesis was performed by random-effects meta-analysis using R version 3.3.3 (available at https://www.R-project.org/). Because the included studies differed in single cytarabine doses applied and number of consolidation cycles, we focused on the cumulative cytarabine dose administered for AML consolidation. For that purpose, a threshold of 20 000 mg/m2 was chosen as it discriminated best between HD and ID/LD cytarabine (supplemental Figure 3).
The initial search yielded a total of 1385 records (databases: 1310; proceedings: 75). Following exclusion of nonrelevant titles or duplicates, a total of 633 were screened. Of those, 27 original articles were selected whose full text was assessed, and finally, 10 studies were included into the systematic review and meta-analysis (PRISMA Flow Diagram; supplemental Figure 1).9-18 These studies initially underwent a quality assessment focusing on random sequence generation, allocation concealment, blinding of patients, personnel and outcome, incomplete outcome data, and selective reporting. Overall, they were considered of good quality and constitute multicenter trials performed in the United States, Europe, Australia, and Japan with their results published in peer-reviewed hematology/oncology journals between 1994 and 2013 (supplemental Figure 2). Individual study characteristics are depicted in supplemental Table 1. A total of 8877 patients aged 1 to 73 years were enrolled between May 1985 and November 2009; 87 of those (<1%) were children included in 1 study,17 all others were 13 years of age or older. Complete remission was achieved by 6257 subjects (70%) following induction chemotherapy, 4224 of them (68%) were further randomized for conventional consolidation treatment. Cytarabine was administered as monotherapy in 1 study and in combination with various other drugs (daunorubicin, idarubicin, aclarubicin, azathioprine, etoposide, vincristine, vindesine, amsacrine, cyclophosphamide, diaziquone, and mitoxantrone) in 6 studies. The remaining studies exhibited treatment arms with both cytarabine monotherapy and combination therapy. However, an initial assessment of cytarabine monotherapy vs combination treatment of conventional AML consolidation did not show a significant difference with respect to RFS and OS (supplemental Figure 4) justifying a focus on the cumulative cytarabine dose alone.
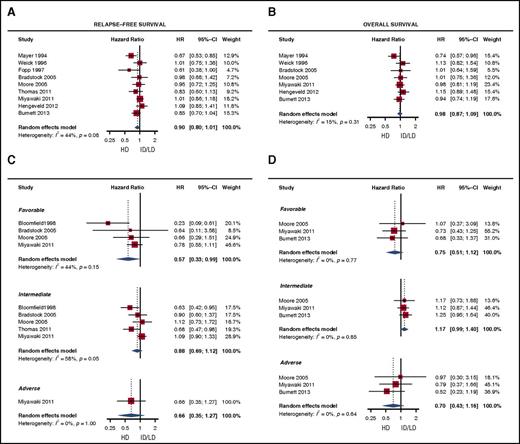
The 9 studies, excluding the study by Bloomfield et al18 reporting outcome data according to cytogenetic subgroups of the cohort initially described by Mayer et al9 , exhibited 20 treatment arms with cumulative HD cytarabine doses between 20 000 mg/m2 and 72 000 mg/m2 and cumulative ID/LD cytarabine doses between 700 mg/m2 and 18 000 mg/m2 (supplemental Figure 3). RFS and OS rates at 3, 4, or 5 years are presented for each study arm in supplemental Table 2. There was no statistically significant difference between both groups with respect to RFS (HR, 0.90; 95% CI, 0.80-1.01) and OS (HR, 0.98; 95% CI, 0.87-1.09) (Figure 1). In a sensitivity analysis, using data from the 8 studies that fulfilled both definitions for ID/LD cytarabine, a cumulative dose of <20 000 mg/m2 and a single dose of <2 g/m2, no statistically significant difference for RFS and OS was observed either (supplemental Figure 5). Similar results were obtained when focusing on studies presenting data on “younger” patients (ie, those of age ≤64 years) (supplemental Figure 6) and on patients having received LD cytarabine induction treatment (supplemental Figure 7). We further performed a stratified analysis on cytogenetic risk groups using data extracted from 4 of the 9 publications and the report by Bloomfield et al.12-15,18 With respect to RFS, a significant risk reduction with HD cytarabine was observed in the favorable cytogenetic risk group (HR, 0.57; 95% CI, 0.33-0.99) but not the intermediate (HR, 0.88; 95% CI, 0.69-1.12) and adverse (HR, 0.66; 95% CI, 0.35-1.27) risk groups. However, concerning OS, no statistically significant difference was demonstrated in any cytogenetic risk group (Figure 1).
Effect of HD vs ID/LD cytarabine as consolidation treatment of patients with AML. The analysis was performed for the total study cohort (A-B) and according to cytogenetic risk groups (C-D). An HR <1 indicates a benefit for HD cytarabine.
Effect of HD vs ID/LD cytarabine as consolidation treatment of patients with AML. The analysis was performed for the total study cohort (A-B) and according to cytogenetic risk groups (C-D). An HR <1 indicates a benefit for HD cytarabine.
Although cytarabine constitutes an indispensable drug in AML therapy, its use is associated with a wide range of adverse reactions including severe neurological and gastrointestinal toxicities that are dose dependent. Therefore, a major goal of numerous studies was to investigate the possibility of reducing cytarabine doses without losing efficacy. Here, we report results of a meta-analysis investigating the issue of ID/LD cytarabine as compared with high doses. However, we were confronted with a number of limitations; among those a considerable variation of the single cytarabine dose, its repetition within a particular consolidation cycle, and the total number of cycles were the most striking ones. In addition, the studies selected encompassed a period of almost 25 years during which supportive care strategies have substantially improved, also affecting treatment outcomes. We, therefore, applied rigid inclusion criteria and focused on a cumulative cytarabine dose given during consolidation treatment. In this way, we were able to show that HD cytarabine provides a statistically significant RFS advantage for the favorable cytogenetic risk group, which is in line with preliminary data also demonstrating an OS benefit for HD cytarabine in this group of patients.19 However, applying an evidence-based approach, we were not able to demonstrate an OS benefit for HD cytarabine consolidation in any of the groups analyzed.
Authorship
Contribution: H.S. and A.B. designed and supervised the study; K.N.M., G.P., and H.S. acquired data; G.P. and A.B. performed statistical analyses; K.N.M., G.P., A.Z., A.W., P.N., H.T.G., A.B., and H.S. interpreted data; H.S. wrote the manuscript; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Heinz Sill, Division of Hematology, Medical University of Graz, Auenbruggerplatz 38, A-8036 Graz, Austria; e-mail: heinz.sill@medunigraz.at.
The online version of this article contains a data supplement.
References
Author notes
K.N.M., G.P., A.B., and H.S. contributed equally to this study.