To the editor:
Infants younger than 1 year of age with acute myeloid leukemia (AML) often exhibit high-risk clinical and cytogenetic features.1-4 They are also at increased risk of pulmonary and infectious toxicities, early death (ED), and treatment-related mortality (TRM).3,5,6 Despite these challenges, infants are typically treated with the same AML protocols used for older children and adolescents.2-4,6-8 Outcomes for infants with AML remain unsatisfactory, with overall survival (OS) and event-free survival (EFS) rates of 56% to 76% and 44% to 72%, respectively.2-4,6,9,10 Therefore, there is an urgent need to develop new treatment strategies for infants with AML.
Gemtuzumab ozogamicin (GO), an anti-CD33 humanized antibody conjugated with calicheamicin, a cytotoxic antibiotic, is a promising agent with limited reports of use in infants.8,11-19 GO was safe in combination with intensive chemotherapy in pediatric patients with AML enrolled in the Children’s Oncology Group (COG) pilot trial, AAML03P1,17 and it was associated with reduced relapse risk (RR) and improved EFS in the phase 3 COG trial, AAML0531.8 To determine the safety and efficacy of GO in infants with AML, we combined the data from AAML03P1 and AAML0531 and analyzed survival and toxicity in infants younger than 1 year of age.
Eligibility criteria were identical for infants enrolled in AAML03P1 or AAML0531. Infants 1 month or older with de novo AML were eligible. Infants <1 month of age were also eligible if the leukemia was progressive, because spontaneous remission in newborns is known to occur.20 Infants with acute promyelocytic leukemia, juvenile myelomonocytic leukemia, known bone marrow failure syndromes, or Down syndrome, and infants who had received prior anti–leukemic therapy, with the exception of intrathecal cytarabine, were ineligible. All infants enrolled in AAML03P1 and infants randomized to the experimental arm of AAML0531 received standard therapy, with the addition of 0.1 mg/kg GO, administered IV on day 6 of Induction (Ind) I and day 7 of Intensification (Int) II. The dose of GO was empirical and was converted to milligram per kilogram by dividing 3 mg/m2 by 30 for patients with body surface area <0.6 m2.21 Infants in the control arm of AAML0531 received standard therapy alone (noGO).
Complete remission (CR) was defined as <5% blasts by bone marrow morphology. ED was defined as death during Ind I. EFS and OS were measured from study entry, and disease-free survival (DFS), TRM,22 and RR were measured from the end of Ind I, for patients in CR. Patients lost to follow-up were censored at their last known contact. Differences in percentages of variables among subgroups were tested by the χ2 or Fisher’s exact tests. The Mann-Whitney test was used to determine differences among continuous variables. The Kaplan-Meier method23 was used to estimate OS, EFS, and DFS. Estimates of TRM and RR were calculated by cumulative incidence, considering competing events.24 Cox proportional hazard models25 were used to determine the effect of clinical features on EFS and OS.
Data from 39 infants enrolled in AAML03P1 (2003-2005) and 103 infants enrolled in AAML0531 (2006-2010) were combined and analyzed (supplemental Table 1, available on the Blood Web site). Median follow-up was 5.02 years. Demographics and disease characteristics for infants enrolled in either trial were similar. Abnormalities of 11q23 were identified in 70 (49%) infants. No infants had t(8;21), and only 4 (3%) had inv(16). Of infants who underwent FLT3 testing, none had internal tandem duplication. Six infants had 12p/ETV6 abnormalities and 7 had del(7q). Risk group assignments were as follows: 136 (96%) intermediate risk, 4 (3%) low risk, and 2 (1%) high risk. GO was given to 78 (55%) infants.
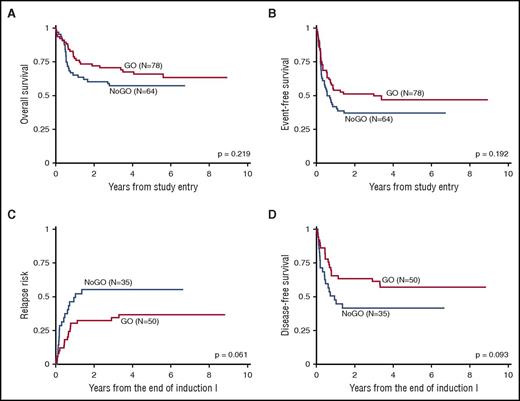
The addition of GO did not result in statistically significant improvement in outcomes of infants (Table 1; Figure 1). The overall outcomes from study entry were as follows: 5-year EFS 42% ± 8%, 5-year OS 62% ± 8%, and RR 44% ± 11%. Eight patients received both GO and stem cell transplantation (SCT), of whom 6 survived without relapse, 1 died following relapse, and 1 died without relapse. Of the 6 infants who underwent SCT without GO, 3 survived without relapse, 1 survived despite relapse, 1 died following relapse, and 1 died without relapse. Younger (0-179 days) and older (180-364 days) age groups, by age at diagnosis, did not show significant differences in 5-year EFS (45% ± 13% vs 40% ± 11%, respectively; P = .767) or 5-year OS (66% ± 12% vs 59% ± 11%; P = .366). Of the 6 infants with 12p/ETV6 abnormalities, 3 experienced relapse, which resulted in an unfavorable EFS for the group (5-year EFS 22% ± 39%). Notably, outcomes of infants with del(7q) were very poor (5-year EFS 0%, 5-year OS 36% ± 40%). Univariable and multivariable Cox regression analyses found no significant differences in EFS or OS from study entry by age group, GO treatment, presence of 11q23 abnormalities, high white blood cell count (>100 000/µL) at diagnosis, 12p/ETV6 abnormalities, or central nervous system involvement (supplemental Tables 2 and 3).
Outcomes of infants, based on the use of GO
| From study entry . | |||
|---|---|---|---|
| noGO (N = 64) | GO (N = 78) | P | |
| ED | 3 (5%) | 5 (6%) | .730 |
| 0-179 d | 3 (14%) | 4 (10%) | .684 |
| 180-364 d | 0% | 1 (3%) | .462 |
| CR after Ind I | 56% | 68% | .182 |
| CR after Ind II | 70% | 75% | .560 |
| 5-y OS | 57% ± 12% | 66% ± 11% | .219 |
| 0-179 d | 59% ± 21% | 70% ± 15% | .261 |
| 180-364 d | 56% ± 16% | 62% ± 17% | .667 |
| 5-y EFS | 37% ± 12% | 47% ± 12% | .192 |
| 0-179 d | 32% ± 20% | 53% ± 16% | .085 |
| 180-364 d | 40% ± 15% | 40% ± 17% | .812 |
| From the end of Ind I | |||
| noGO (N = 35) | GO (N = 50) | P | |
| 5-y RR | 55% ± 18% | 37% ± 14% | .061 |
| 0-179 d | 50% ± 30% | 36% ± 20% | .363 |
| 180-364 d | 58% ± 22% | 37% ± 20% | .109 |
| 5-y TRM | 11% ± 23% | 2% ± 4% | .754 |
| 0-179 d | 38% ± 76% | 0% ± 0% | .085 |
| 180-364 d | 0% ± 0% | 4% ± 8% | .339 |
| 5-y DFS | 42% ± 17% | 57% ± 14% | .093 |
| 0-179 d | 42% ± 0% | 64% ± 20% | .145 |
| 180-364 d | 42% ± 20% | 50% ± 20% | .383 |
| 5-y OS | 65% ± 16% | 69% ± 13% | .547 |
| 0-179 d | 67% ± 0% | 72% ± 18% | .528 |
| 180-364 d | 64% ± 20% | 66% ± 20% | .928 |
| From study entry . | |||
|---|---|---|---|
| noGO (N = 64) | GO (N = 78) | P | |
| ED | 3 (5%) | 5 (6%) | .730 |
| 0-179 d | 3 (14%) | 4 (10%) | .684 |
| 180-364 d | 0% | 1 (3%) | .462 |
| CR after Ind I | 56% | 68% | .182 |
| CR after Ind II | 70% | 75% | .560 |
| 5-y OS | 57% ± 12% | 66% ± 11% | .219 |
| 0-179 d | 59% ± 21% | 70% ± 15% | .261 |
| 180-364 d | 56% ± 16% | 62% ± 17% | .667 |
| 5-y EFS | 37% ± 12% | 47% ± 12% | .192 |
| 0-179 d | 32% ± 20% | 53% ± 16% | .085 |
| 180-364 d | 40% ± 15% | 40% ± 17% | .812 |
| From the end of Ind I | |||
| noGO (N = 35) | GO (N = 50) | P | |
| 5-y RR | 55% ± 18% | 37% ± 14% | .061 |
| 0-179 d | 50% ± 30% | 36% ± 20% | .363 |
| 180-364 d | 58% ± 22% | 37% ± 20% | .109 |
| 5-y TRM | 11% ± 23% | 2% ± 4% | .754 |
| 0-179 d | 38% ± 76% | 0% ± 0% | .085 |
| 180-364 d | 0% ± 0% | 4% ± 8% | .339 |
| 5-y DFS | 42% ± 17% | 57% ± 14% | .093 |
| 0-179 d | 42% ± 0% | 64% ± 20% | .145 |
| 180-364 d | 42% ± 20% | 50% ± 20% | .383 |
| 5-y OS | 65% ± 16% | 69% ± 13% | .547 |
| 0-179 d | 67% ± 0% | 72% ± 18% | .528 |
| 180-364 d | 64% ± 20% | 66% ± 20% | .928 |
Kaplan-Meier curves comparing outcomes with and without GO for infants <1 year of age with AML. Results are combined from AAML03P1 and AAML0531. (A) OS from study entry. (B) EFS from study entry. (C) RR from the end of Ind I. (D) DFS from the end of Ind I.
Kaplan-Meier curves comparing outcomes with and without GO for infants <1 year of age with AML. Results are combined from AAML03P1 and AAML0531. (A) OS from study entry. (B) EFS from study entry. (C) RR from the end of Ind I. (D) DFS from the end of Ind I.
There were no increases in sterile-site bacterial or fungal infections (grades 3-5), left ventricular systolic dysfunction, liver venoocclusive disease (VOD), or median days to absolute neutrophil count or platelet recovery in any single course or overall for infants who received GO (supplemental Table 4). Only 2 cases of VOD were reported during courses containing GO, and both occurred in Ind I. Seven additional cases of VOD were reported: 1 in Int III and 6 during the SCT course. The overall rate of VOD was 6% (5% GO vs 6% noGO; P = .730). Left ventricular systolic dysfunction occurred in 11 infants (13% GO vs 4% noGO; P = .055). The median number of days to recovery for absolute neutrophil count and platelets was similar and consistent across Ind I and Int II, for younger and older infants, and for infants who received or did not receive GO.
There were more EDs in younger than in older infants (7 EDs vs 1 ED, respectively; P = .013). GO was not associated with increased rate of ED in infants. Of the 8 infants who died during Ind I, 3 died of toxicities possibly related to treatment. Of those, 2 were in the younger age group and died of acute respiratory distress syndrome (ARDS) associated with respiratory syncytial virus infection. The third infant was in the older age group, did not receive GO because of disease-related liver dysfunction, and died of ARDS. The remaining 5 EDs were due to disease-related causes: 2 from hemorrhage, 1 from tumor lysis syndrome, 1 from respiratory failure, and 1 from multiorgan fibrosis.
TRM occurred in 2 infants who were in CR. One death occurred during Int I and was due to adenovirus infection, which led to right-sided heart failure, coagulopathy, and small bowel necrosis. The other death occurred during Int III and was caused by ARDS related to respiratory syncytial virus infection.
Our analysis shows that GO was well tolerated in infants and was associated with favorable disease outcomes. Although this study was not powered to detect a statistically significant improvement in survival, the trend toward improved 5-year RR and the favorable hazard ratios for EFS and OS support the clinical benefit of GO in infants with AML. Toxic death in infants in remission was very low overall and did not increase with GO. We conclude that GO can be safely combined with intensive chemotherapy in infants with AML. Future studies may wish to focus on the pharmacokinetics of GO in infants, to optimize dosing.
Presented in abstract form at the 56th annual meeting of the American Society of Hematology, San Francisco, CA, 7 December 2014.
The online version of this article contains a data supplement.
Authorship
Acknowledgments: This study was supported by research funding from the National Institutes of Health, National Cancer Institute under award numbers U10CA180899 (T.A.A., R.B.G., and Y.-C.J.W.), U10CA180886 (R.A., L.S., S.C.R., B.A.H., S.B.K., A.H.-M., S.M., and A.S.G.), and U10CA098543 (R.A., L.S., S.C.R., B.A.H., S.B.K., A.H.-M., S.M., and A.S.G.), and the Andrew McDonough B+ Foundation (Y.-C.J.W.).
Contribution: E.M.G. designed the analysis, analyzed the data, and wrote the manuscript; A.S.G., T.A.A., R.B.G., and Y.-C.J.W. designed the study, analyzed the data, and edited the manuscript; and R.A., L.S., S.C.R., B.A.H., S.B.K, A.H.-M., and S.M. analyzed the data and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Erin M. Guest, Children’s Mercy Hospital, 2401 Gillham Rd, Kansas City, MO 64108; e-mail: eguest@cmh.edu.