Abstract
There are no data on the role of postconsolidation therapy with gemtuzumab ozogamicin (GO; Mylotarg) in children with acute myeloid leukemia (AML). The NOPHO-AML 2004 protocol studied postconsolidation randomization to GO or no further therapy. GO was administered at 5 mg/m2 and repeated after 3 weeks. We randomized 120 patients; 59 to receive GO. Survival was analyzed on an intention-to-treat basis. The median follow-up for patients who were alive was 4.2 years. Children who received GO showed modest elevation of transaminase and bilirubin without signs of veno-occlusive disease. Severe neutropenia followed 95% and febrile neutropenia 40% of the GO courses. Only a moderate decline in platelet count and a minor decrease in hemoglobin occurred. Relapse occurred in 24 and 25 of those randomized to GO or no further therapy. The median time to relapse was 16 months versus 10 months (nonsignificant). The 5-year event-free survival and overall survival was 55% versus 51% and 74% versus 80% in those randomized to receive GO or no further therapy, respectively. Results were similar in all subgroups. In conclusion, GO therapy postconsolidation as given in this trial was well tolerated, showed a nonsignificant delay in time to relapse, but did not change the rate of relapse or survival (clinicaltrials.gov identifier NCT00476541).
Introduction
Improvements in induction and consolidation therapy as well as supportive care in pediatric acute myeloid leukemia (AML) have resulted in remission rates above 90% and overall survival (OS) above 65%.1-4 Despite the progress, approximately one-third of the patients eventually relapse.
Gemtuzumab ozogamicin (GO; Mylotarg) is a humanized anti-CD33–calicheamicin conjugate developed for targeted treatment of AML and was approved for use by the US Food and Drug Administration in 2000 but withdrawn in October 2010 because of concerns about both toxicity and efficacy in adults. The major side effects are myelosuppression and liver dysfunction.5 Despite the withdrawal of GO, studies on the safety and efficacy of GO are ongoing and continuously published6,7 and the final role of GO in AML therapy is unsettled.8
There is only limited experience of using GO in children. A phase 1 trial performed by the Children's Oncology Group (COG) started at a dose of 9 mg/m2 but was reduced to 6 mg/m2 because of liver toxicity.9 A phase 2 study with GO administered to relapsed AML as 2 doses of 7.5 mg/m2 with a 14-day interval was well tolerated with response rates of 30%-40%.10,11 GO has been applied during induction in a few protocols for de novo pediatric AML,2,7,12 demonstrating that the combination of GO with intensive chemotherapy is safe and feasible but the benefit of the approach is still unclear.
Postconsolidation GO therapy has been used in elderly AML patients with variable results. A small study indicated a benefit from 3 low-dose 3 mg/m2 monthly doses,13 whereas a phase 3 study using 3 cycles of monthly GO at 6 mg/m2 did not result in any benefit for the GO-treated group.14 There are no experiences of postconsolidation GO therapy in children. We report the results of the Nordic Society of Paediatric Hematology and Oncology (NOPHO) 2004 protocol including postconsolidation randomization to GO or no further therapy.
Methods
The NOPHO-AML 2004 protocol (clinicaltrials.gov identifier NCT00476541) opened in January 2004 and included all children diagnosed with AML in the 5 Nordic countries (Denmark, Finland, Iceland, Norway, and Sweden); from November 2007, Hong Kong was also included. NOPHO-AML 2004 included induction with AIET (cytarabine, idarubicin, etoposide, and 6-thioguanine).3 The second induction course and the 4 consolidation courses were similar to the NOPHO-AML 93 protocol.15 The flowchart of the protocol is shown in Figure 1. The cumulated doses were 6-thioguanin 800 mg/m2, cytarabine 49 300 mg/m2, etoposide 1200 mg/m2, idarubicin 36 mg/m2, and mitoxantrone 60 mg/m2.
Flowchart of the NOPHO-AML 2004 protocol. High-risk patients were eligible for HSCT after completing 3 courses but before the last consolidation (HA2E). AIET indicates cytarabine 200 mg/m2 continuous infusion days 1-4, 6-thioguanine 100 mg/m2 bis in die (bid) days 1-4, etoposide 100 mg/m2 days 1-4, idarubicin 12 mg/m2 days 2, 4, and 6. AM indicates cytarabine 100 mg/m2 continuous infusion days 1-5, mitoxantrone 10 mg/m2 days 1-3. HA1M indicates cytarabine 1 g/m2 bid days 1-3, mitoxantrone 10 mg/m2 days 3-5. HA2E indicates cytarabine 2 g/m2 bid days 1-3, etoposide 100 mg/m2 days 2-5. HA3 indicates cytarabine 3 g/m2 bid days 1-3. GO indicates gemtuzumab ozogamicin 5 mg/m2 days 1 and 21.
Flowchart of the NOPHO-AML 2004 protocol. High-risk patients were eligible for HSCT after completing 3 courses but before the last consolidation (HA2E). AIET indicates cytarabine 200 mg/m2 continuous infusion days 1-4, 6-thioguanine 100 mg/m2 bis in die (bid) days 1-4, etoposide 100 mg/m2 days 1-4, idarubicin 12 mg/m2 days 2, 4, and 6. AM indicates cytarabine 100 mg/m2 continuous infusion days 1-5, mitoxantrone 10 mg/m2 days 1-3. HA1M indicates cytarabine 1 g/m2 bid days 1-3, mitoxantrone 10 mg/m2 days 3-5. HA2E indicates cytarabine 2 g/m2 bid days 1-3, etoposide 100 mg/m2 days 2-5. HA3 indicates cytarabine 3 g/m2 bid days 1-3. GO indicates gemtuzumab ozogamicin 5 mg/m2 days 1 and 21.
High-risk patients, defined as having poor response to induction (> 15% blasts after AIET or no remission after second induction) or the presence of MLL rearrangements other than t(9;11)(p21;q23), were offered hematopoietic stem cell transplantation (HSCT) with a matched sibling or unrelated donor if a matched donor was identified before the last consolidation. An amendment of the protocol in June 2009 restricted the high-risk criteria to poor response only. Complete remission (CR) was defined as a cellular bone marrow with blasts below 5% and neutrophils above 1 × 109/L and platelets above 100 × 109/L.
Randomization to GO or no further therapy was offered to standard-risk patients and high-risk patients in first complete remission (CR1) who completed consolidation without HSCT because of lack of donor. Randomization was balanced according to risk group. Patients were only eligible for randomization when they had started the last consolidation course (second HA2E). National ethics committees and institutional review boards in each country approved the study, and randomization was only done after obtaining informed consent according to national guidelines following the Declaration of Helsinki.
GO was administered as a 2-hour infusion of 5 mg/m2 at least 4 weeks after the last consolidation course and repeated after an interval of 3 weeks. GO was only given when transaminase levels were less than 5 times the upper normal limit, bilirubin < 20μM, neutrophils > 1.0 × 109/L, and platelets > 80 × 109/L. Antifungal prophylaxis was discontinued during GO therapy. Premedication with acetaminophen, clemastine (meclastin), and methylprednisolone was given before GO. Blood samples and clinical history were obtained at least twice a week for a minimum of 3 weeks after each of the 2 GO infusions. End points were toxicity of the GO therapy, relapse rate, and survival in the 2 randomized arms.
Statistical methods
The study was designed with a power of 82% to detect a difference in relapse rate of 25% by including 120 patients. Interim analyses were performed annually by the data monitoring committee. The randomization was closed in December 2010 when 120 patients were randomized. To compare the distribution of categorical or dichotomized variables, the χ2 test was used and the Fisher exact test was used when the expected count in any cell of the table was < 5.
Event-free survival (EFS) was defined as the time from diagnosis until death in remission, relapse, second malignancy, or last follow-up, whatever occurred first. OS was defined as the time from diagnosis to death from any cause or last follow-up. All patients were followed to death or February 2012, 15 months after the last patient was randomized. No patients were lost to follow-up. The median time of follow-up for patients who were alive was 4.2 years from diagnosis (range 1.7 to 7.8 years). The probabilities of EFS and OS were estimated by the Kaplan-Meier method and differences between survival distributions were compared with the log-rank test.
Results
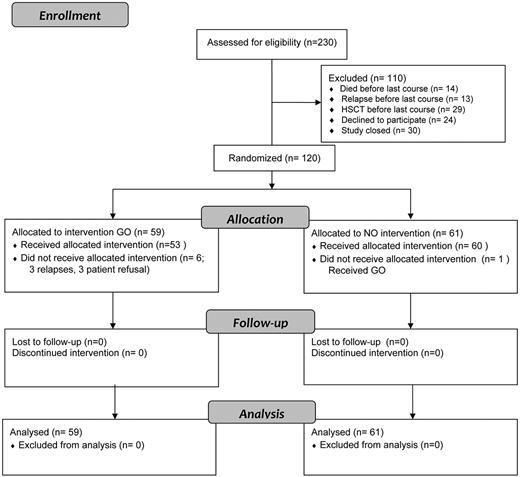
When the targeted 120 patients were randomized by December 2010, a total of 230 patients had been entered on NOPHO-AML 2004. Randomization had not been offered to 86 patients because of death (n = 14), relapse (n = 13), HSCT (n = 29), or closure of the randomization (n = 30) before they reached last consolidation. Participation in the randomization was declined by the parents/children or the treating physicians in 24 (17%) of the 144 eligible patients (Figure 2).
Patient flow and randomization in the NOPHO-AML 2004 protocol. Postconsolidation GO randomization in patients diagnosed from 2004 to 2010.
Patient flow and randomization in the NOPHO-AML 2004 protocol. Postconsolidation GO randomization in patients diagnosed from 2004 to 2010.
The clinical characteristics of the 120 patients randomized are presented in Table 1. There were no statistically significant differences between the 2 groups. There was a nonsignificant excess of patients with white blood cell count (WBC) more than 100 × 109/L (P = .053) and FLT3-ITD aberration (P = .06) in the group randomized to no further therapy.
Characteristics of the 120 patients randomized
| Characteristics . | Randomized to GO, N = 59, n (%) . | Randomized to no further therapy, N = 61, n (%) . | P . |
|---|---|---|---|
| Sex | .30 | ||
| Male | 30 (51) | 37 (61) | |
| Female | 29 (49) | 24 (39) | |
| Age, y | .62 | ||
| 0-1 | 16 (27) | 15 (25) | |
| 2-9 | 22 (37) | 28 (46) | |
| 10+ | 21 (36) | 18 (30) | |
| White blood count, × 109/L | .12 | ||
| 0-9.9 | 22 (37) | 24 (39) | |
| 10-99 | 35 (59) | 29 (48) | |
| > 100 | 2 (3) | 8 (13) | |
| FAB classification | .86 | ||
| M0 | 3 (5) | 4 (7) | |
| M1 | 7 (12) | 7 (12) | |
| M2 | 16 (27) | 22 (36) | |
| M4 | 10 (17) | 7 (12) | |
| M5 | 14 (24) | 13 (21) | |
| M6 | 1 (2) | 1 (2) | |
| M7 | 6 (10) | 4 (7) | |
| Other and missing | 2 (3) | 3 (5) | |
| CD33 expression | .07 | ||
| Positive | 50 (85) | 59 (97) | |
| Negative | 7 (12) | 2 (3) | |
| No data | 2 (3) | 0 | |
| CNS disease | .55 | ||
| Yes | 7 (12) | 6 (10) | |
| No | 51 (86) | 55 (90) | |
| Data missing | 1 (2) | 0 (0) | |
| Cytogenetics | .93 | ||
| Normal karyotype | 11 (19) | 9 (15) | |
| t(8;21) | 13 (22) | 14 (23) | |
| inv(16) | 4 (7) | 6 (10) | |
| t(9;11) | 10 (17) | 8 (13) | |
| 11q23 non t(9;11) | 6 (10) | 5 (8) | |
| Other aberrations | 15 (25) | 19 (31) | |
| FLT3 aberrations | .23 | ||
| ITD | 0 (0) | 4 (7) | |
| ALM (D835/I836) | 4 (7) | 4 (7) | |
| Wild type | 41 (70) | 37 (61) | |
| Not tested | 14 (24) | 16 (26) | |
| Risk group | .69 | ||
| Standard risk | 54 (92) | 57 (93) | |
| High risk | 5 (8) | 4 (7) | |
| Remission achieved | .22 | ||
| After first induction | 44 (75) | 51 (84) | |
| After second induction | 15 (25) | 10 (16) |
| Characteristics . | Randomized to GO, N = 59, n (%) . | Randomized to no further therapy, N = 61, n (%) . | P . |
|---|---|---|---|
| Sex | .30 | ||
| Male | 30 (51) | 37 (61) | |
| Female | 29 (49) | 24 (39) | |
| Age, y | .62 | ||
| 0-1 | 16 (27) | 15 (25) | |
| 2-9 | 22 (37) | 28 (46) | |
| 10+ | 21 (36) | 18 (30) | |
| White blood count, × 109/L | .12 | ||
| 0-9.9 | 22 (37) | 24 (39) | |
| 10-99 | 35 (59) | 29 (48) | |
| > 100 | 2 (3) | 8 (13) | |
| FAB classification | .86 | ||
| M0 | 3 (5) | 4 (7) | |
| M1 | 7 (12) | 7 (12) | |
| M2 | 16 (27) | 22 (36) | |
| M4 | 10 (17) | 7 (12) | |
| M5 | 14 (24) | 13 (21) | |
| M6 | 1 (2) | 1 (2) | |
| M7 | 6 (10) | 4 (7) | |
| Other and missing | 2 (3) | 3 (5) | |
| CD33 expression | .07 | ||
| Positive | 50 (85) | 59 (97) | |
| Negative | 7 (12) | 2 (3) | |
| No data | 2 (3) | 0 | |
| CNS disease | .55 | ||
| Yes | 7 (12) | 6 (10) | |
| No | 51 (86) | 55 (90) | |
| Data missing | 1 (2) | 0 (0) | |
| Cytogenetics | .93 | ||
| Normal karyotype | 11 (19) | 9 (15) | |
| t(8;21) | 13 (22) | 14 (23) | |
| inv(16) | 4 (7) | 6 (10) | |
| t(9;11) | 10 (17) | 8 (13) | |
| 11q23 non t(9;11) | 6 (10) | 5 (8) | |
| Other aberrations | 15 (25) | 19 (31) | |
| FLT3 aberrations | .23 | ||
| ITD | 0 (0) | 4 (7) | |
| ALM (D835/I836) | 4 (7) | 4 (7) | |
| Wild type | 41 (70) | 37 (61) | |
| Not tested | 14 (24) | 16 (26) | |
| Risk group | .69 | ||
| Standard risk | 54 (92) | 57 (93) | |
| High risk | 5 (8) | 4 (7) | |
| Remission achieved | .22 | ||
| After first induction | 44 (75) | 51 (84) | |
| After second induction | 15 (25) | 10 (16) |
GO indicates gemtuzumab ozogamicin; FAB, French-American-British; ITD, internal tandem duplication; and ALM, activation loop mutation.
Three of the 59 patients randomized to receive GO withdrew the acceptance for participation and 3 relapsed before therapy was completed. One patient randomized to no further therapy received GO. Analyses were performed on an intention-to-treat basis according to the result of the randomization.
Toxicity
Detailed toxicity data were available from all 53 patients who received both GO courses as randomized. The median interval from last consolidation to GO was 31 days (range 27-50) and the median interval between first and second GO was 21 days (range 20-41). The interval between first and second GO was more than 4 weeks in 3 patients because of prolonged neutropenia, elevated transaminase, and shingles. No major events were reported in relation to the infusions.
The hematologic toxicity is shown in Table 2. Only a minor decrease in hemoglobin was observed after both GO infusions. No patients received red cell transfusion. Severe leukopenia, WHO grade 4, WBC < 1.0 × 109/L was seen in 81% after first GO and in 67% after second GO. Severe neutropenia was almost universal with a median neutrophil nadir of 0.0 × 109/L (neutrophils < 0.5 in 94%-96% after first and second GO). Neutropenia < 0.5 × 109/L lasted a median of 15 days (range 0-43) after both GO courses. Neutrophils < 0.5 for more than 20 days was seen in one patient after the first GO and in 4 patients after the second GO. Febrile neutropenia treated with antibiotics followed 42 (40%) of the GO courses (23 episodes [43%] after the first and 19 [36%] after the second GO). Those with febrile neutropenia after the first course had a 74% risk of a febrile episode after the second course compared with only 7% of those without febrile episode after the first course (P < .001). None of the infectious episodes were life-threatening (no cases of hypotension or need of intensive care).
Hematologic and hepatic toxicity in 53 patients who received 2 courses of GO after randomization
| . | GO1 baseline . | GO1 nadir . | GO1 peak . | GO2 baseline . | GO2 nadir . | GO2 peak . |
|---|---|---|---|---|---|---|
| Hematologic | ||||||
| Hb, g/dL | 10.5 | 10.1 | 12.0 | 10.8 | ||
| 8.1-14.3 | 7.8-14.0 | 9.8-15.0 | 8.2-14.8 | |||
| WHO grade 3/4 (%) | 2/0 (4) | 0/0 (0) | ||||
| WBC, ×109/L | 2.7 | 0.5 | 3.7 | 0.7 | ||
| 1.5-9.8 | 0.0-4.1 | 1.6-16.8 | 0.2-4.4 | |||
| WHO grade 3/4 (%) | 7/43 (94) | 13/35 (92) | ||||
| Neutrophils, ×109/L | 1.3 | 0.0 | 1.6 | 0.0 | ||
| 0.7-8.6 | 0.0-3.2 | 0.4-13.3 | 0.0-1.4 | |||
| WHO grade 3/4 (%) | 0/50 (96) | 1/47 (96) | ||||
| Platelets, ×109/L | 199 | 83 | 238 | 62 | ||
| 43-470 | 10-239 | 97-460 | 11-202 | |||
| WHO grade 3/4 (%) | 5/3 (15) | 16/4 (39) | ||||
| Hepatic | ||||||
| ALAT, U/L | 48 | 82 | 61 | 72 | ||
| 14-204 | 20-279 | 12-121 | 21-282 | |||
| WHO grade 3/4 (%) | 2/0 (4) | 1/0 (2) | ||||
| Bilirubin, μmol/L | 5 | 8 | 5 | 7 | ||
| 2-15 | 2-28 | 1-15 | 2-36 | |||
| WHO grade 3/4 (%) | 0/0 (0) | 0/0 (0) |
| . | GO1 baseline . | GO1 nadir . | GO1 peak . | GO2 baseline . | GO2 nadir . | GO2 peak . |
|---|---|---|---|---|---|---|
| Hematologic | ||||||
| Hb, g/dL | 10.5 | 10.1 | 12.0 | 10.8 | ||
| 8.1-14.3 | 7.8-14.0 | 9.8-15.0 | 8.2-14.8 | |||
| WHO grade 3/4 (%) | 2/0 (4) | 0/0 (0) | ||||
| WBC, ×109/L | 2.7 | 0.5 | 3.7 | 0.7 | ||
| 1.5-9.8 | 0.0-4.1 | 1.6-16.8 | 0.2-4.4 | |||
| WHO grade 3/4 (%) | 7/43 (94) | 13/35 (92) | ||||
| Neutrophils, ×109/L | 1.3 | 0.0 | 1.6 | 0.0 | ||
| 0.7-8.6 | 0.0-3.2 | 0.4-13.3 | 0.0-1.4 | |||
| WHO grade 3/4 (%) | 0/50 (96) | 1/47 (96) | ||||
| Platelets, ×109/L | 199 | 83 | 238 | 62 | ||
| 43-470 | 10-239 | 97-460 | 11-202 | |||
| WHO grade 3/4 (%) | 5/3 (15) | 16/4 (39) | ||||
| Hepatic | ||||||
| ALAT, U/L | 48 | 82 | 61 | 72 | ||
| 14-204 | 20-279 | 12-121 | 21-282 | |||
| WHO grade 3/4 (%) | 2/0 (4) | 1/0 (2) | ||||
| Bilirubin, μmol/L | 5 | 8 | 5 | 7 | ||
| 2-15 | 2-28 | 1-15 | 2-36 | |||
| WHO grade 3/4 (%) | 0/0 (0) | 0/0 (0) |
Median values and ranges with WHO toxicity grade 3 and 4 are presented.
GO indicates gemtuzumab ozogamicin; Hb, hemoglobin; and ALAT, alanine aminotransferase.
A moderate decline in platelet count was noted with median nadir of 83 × 109/L after the first GO and 62 × 109/L after the second GO. Platelet nadir was < 50 × 109/L in 15% after the first and in 39% after the second GO (P < .01). Platelet transfusion was given after 11 (10%) of the GO courses to 9 different patients (17%).
Liver toxicity is shown in Table 2. Alanine aminotransferase (ALAT) and bilirubin showed only a slight increase from the baseline values. Moderate elevation of transaminase was common, whereas only 2 episodes of bilirubin level above 25 μmol/L occurred. None of the patients showed signs of hepatic veno-occlusive disease (VOD).
Survival
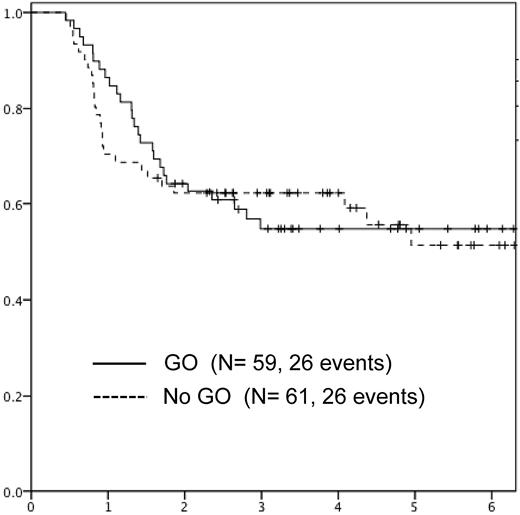
No patients died in CR1 after GO therapy. Therapy-related myelodysplastic syndrome (t-MDS) developed in 2 patients 1.7 and 3.0 years from AML diagnosis, and 12 and 29 months from GO therapy. Relapse occurred in 24 patients among those randomized to GO (including 3 relapses before start of GO) and in 25 of those randomized to no further therapy. The median time to relapse was 16 months in the GO arm versus 11 months in those receiving no GO (nonsignificant; Table 3).
Follow-up of the 120 patients randomized
| Characteristics . | Randomized to GO, N = 59, n (%) . | Randomized to no further therapy, N = 61, n (%) . |
|---|---|---|
| Treated with GO | ||
| No | 5 (8) | 60 (98) |
| 1 course | 1 (2) | 0 |
| 2 courses | 53 (90) | 1 (2) |
| Events | ||
| No event | 33 (56) | 35 (57) |
| Death in CR1 | 0 (0) | 1 (2) |
| MDS | 2 (3) | 0 (0) |
| Relapse | 24 (41) | 25 (41) |
| Median time from diagnosis to relapse, mo | 16 | 11 |
| Median follow-up for patients alive, y | 4.3 | 4.1 |
| HSCT in CR2 | ||
| Yes | 23* | 21 |
| Median days from last consolidation to SCT | 458 | 286 |
| Survival | ||
| 5-year EFS, % | 55 | 51 |
| 95% confidence interval, % | 51-59 | 42-59 |
| 5-year OS, % | 74 | 80 |
| 95% confidence interval, % | 70-78 | 72-86 |
| Characteristics . | Randomized to GO, N = 59, n (%) . | Randomized to no further therapy, N = 61, n (%) . |
|---|---|---|
| Treated with GO | ||
| No | 5 (8) | 60 (98) |
| 1 course | 1 (2) | 0 |
| 2 courses | 53 (90) | 1 (2) |
| Events | ||
| No event | 33 (56) | 35 (57) |
| Death in CR1 | 0 (0) | 1 (2) |
| MDS | 2 (3) | 0 (0) |
| Relapse | 24 (41) | 25 (41) |
| Median time from diagnosis to relapse, mo | 16 | 11 |
| Median follow-up for patients alive, y | 4.3 | 4.1 |
| HSCT in CR2 | ||
| Yes | 23* | 21 |
| Median days from last consolidation to SCT | 458 | 286 |
| Survival | ||
| 5-year EFS, % | 55 | 51 |
| 95% confidence interval, % | 51-59 | 42-59 |
| 5-year OS, % | 74 | 80 |
| 95% confidence interval, % | 70-78 | 72-86 |
GO indicates gemtuzumab ozogamicin; CR1, first complete remission; MDS, myelodysplastic syndrome; HSCT, hematopoietic stem cell transplantation; EFS, event-free survival; and OS, overall survival.
Including 2 patients with therapy-related MDS.
After relapse, second complete remission (CR2) was achieved in 44 (90%), 88% in the GO arm, and 92% in those who were randomized to no further therapy. HSCT was performed in 42 of the 44 patients who achieved CR2 and the 2 patients with t-MDS. The median time from last GO to HSCT was 405 days (range 47-866). No patients had signs of VOD after HSCT.
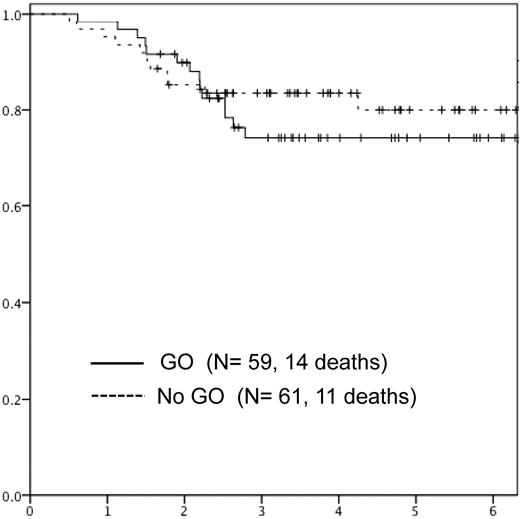
The 5-year EFS and OS was 55% (95% confidence interval [CI]: 51-59) versus 51% (95% CI: 42-59; nonsignificant) and 74% (95% CI: 70-78) versus 80% (95% CI: 72-86; nonsignificant) in those randomized to receive GO versus no further therapy, respectively (Figures 3 and 4). EFS was analyzed according to randomization by sex, age, WBC, French-American-British (FAB), CD33 positivity, cytogenetics, and response to induction (Table 4). There were no significant differences between EFS according to GO randomization in any of the subgroups analyzed.
Overall survival in patients randomized to GO versus no further therapy (no GO).
Overall survival in patients randomized to GO versus no further therapy (no GO).
Five-year EFS in subgroups with 10 or more patients according to the result of randomization
| Characteristics . | Randomized to GO EFS, % . | Randomized to no further therapy EFS, % . | P . |
|---|---|---|---|
| Sex | |||
| Male | 42 | 46 | .5 |
| Female | 68 | 57 | .5 |
| Age, y | |||
| 0-1 | 74 | 80 | .8 |
| 2-9 | 51 | 36 | .3 |
| 10+ | 42 | 50 | .3 |
| White blood count, × 109/L | |||
| 0-9.9 | 44 | 47 | .7 |
| 10+ | 61 | 53 | .7 |
| FAB classification | |||
| M1/M2 | 28 | 32 | .5 |
| M4/M5 | 70 | 71 | .8 |
| Cytogenetics | |||
| Normal karyotype | 53 | 44 | .4 |
| t(8;21) or inv(16) | 38 | 50 | .9 |
| 11q23 | 68 | 62 | .9 |
| Other aberrations | 59 | 49 | .9 |
| Remission achieved | |||
| After first induction | 66 | 59 | .7 |
| After second induction | 11 | 17 | .4 |
| Characteristics . | Randomized to GO EFS, % . | Randomized to no further therapy EFS, % . | P . |
|---|---|---|---|
| Sex | |||
| Male | 42 | 46 | .5 |
| Female | 68 | 57 | .5 |
| Age, y | |||
| 0-1 | 74 | 80 | .8 |
| 2-9 | 51 | 36 | .3 |
| 10+ | 42 | 50 | .3 |
| White blood count, × 109/L | |||
| 0-9.9 | 44 | 47 | .7 |
| 10+ | 61 | 53 | .7 |
| FAB classification | |||
| M1/M2 | 28 | 32 | .5 |
| M4/M5 | 70 | 71 | .8 |
| Cytogenetics | |||
| Normal karyotype | 53 | 44 | .4 |
| t(8;21) or inv(16) | 38 | 50 | .9 |
| 11q23 | 68 | 62 | .9 |
| Other aberrations | 59 | 49 | .9 |
| Remission achieved | |||
| After first induction | 66 | 59 | .7 |
| After second induction | 11 | 17 | .4 |
GO indicates gemtuzumab ozogamicin; EFS, event-free survival; and FAB, French-American-British.
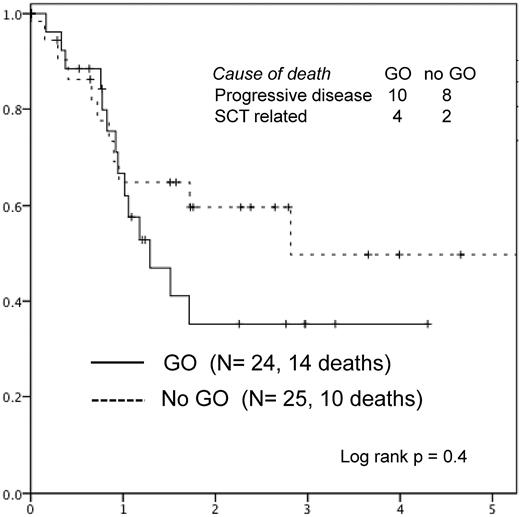
The 3-year survival after relapse was 43% without significant difference between the 2 randomized arms (Figure 5). Deaths occurred in 24: in 18 from progressive disease (in 12 after HSCT in CR2) and in 6 from HSCT-related complications, mostly infections (Figure 5).
Survival after relapse in patients randomized to gemtuzumab ozogamicin (GO) versus no further therapy (no GO).
Survival after relapse in patients randomized to gemtuzumab ozogamicin (GO) versus no further therapy (no GO).
Discussion
The overall results of NOPHO-AML 2004 are favorable with a high remission rate and OS around 70%3 but the high relapse rate remains a problem. GO given after consolidation postponed the relapses a median of 5 months but it did not change the cumulated rate of relapse. The delay of the relapses in the GO arm was not statistically significant and could be a chance finding. The late relapses did not translate into a superior survival as would be expected because time of relapse is the most important prognostic factor in relapsed AML.16,17
The randomized study of 6-mercaptopurine and cytarabine maintenance therapy by the French LAME (Leucemie Aigue Myeloblastique Enfant) group documented that those who relapsed after maintenance were more refractory to reinduction resulting in a poorer outcome after relapse.18 Similar results were observed after thioguanine, vincristine, azacitidine, cytarabine, and cyclophosphamide maintenance reported by the Children's Cancer Groups (CCG).19 In contrast, reinduction in the present study was successful with a CR2 rate of 90% without any increased risk of refractory relapsed AML in patients previously treated with GO and no difference between survival in the 2 groups. A relatively large fraction of the relapsed patients (17 of 49, 35%) had core-binding factor (CBF) AML contribution to the high CR2 rate.
GO during induction seems to be especially beneficial for adult patients with t(8;21)(q22;q22) or inv(16)(p13q22) AML.6 Patients with t(8;21) had a high relapse rate in the NOPHO-AML 2004 study which was not influenced by the addition of GO therapy after consolidation. Patients with poor response also had a high relapse rate that was not influenced by GO therapy. In contrast, young children and those with MLL aberration had a very favorable EFS regardless of GO therapy.
The addition of GO to induction chemotherapy does not change the remission rate but reduces the relapse rate and increases survival in adults.6,20 We did not find any benefit giving GO as a postconsolidation therapy, and similar results are reported in adults,14 suggesting that only early GO therapy may prevent relapse. An additional fifth course of chemotherapy does not improve the relapse-free survival,4 indicating that only the first months of therapy predict the relapse rate.
High expression of CD33 is associated with adverse disease features and is an independent predictor of inferior outcome in pediatric AML.21 However, the correlation between CD33 expression and response to GO is still under debate. We did not find any trend toward different EFS in those who were CD33 positive versus negative but we did not perform quantitative studies of CD33 expression. Antigen change from diagnosis to relapse is common in pediatric AML but the expression of CD33 is stable with changes in only 2% of the patients.22 Although we do not have data on CD33 status at relapse, it seems unlikely that a shift in CD33 expression is the cause of the failure of GO to prevent relapse in this trial.
Therapy-related MDS occurred in 2 patients treated with GO. Second malignant neoplasms are rare in pediatric AML with a 15-year cumulated incidence of less than 2%.23,24 GO is not known to be associated with secondary malignancies and our observation may be a chance finding.
The most common acute side effect of GO was severe neutropenia seen in 96%. Febrile neutropenia occurred in 40%, similar to the frequency in children with relapsed AML.11 There was a strong association between fever after the first course and the risk of febrile neutropenia after the second course. The median time to neutrophil recovery was 15 days which is a little shorter than the 20 days observed in elderly AML patients receiving 3 cycles of postremission therapy with 6 mg/m2 of GO.14
Platelet transfusions were given to 45% of older patients receiving GO14 but only to 17% in our cohort and only 27% of the courses were followed by platelet nadir below 50. Significantly more patients had platelet nadir < 50 after the second course than after the first course (39% vs 15%).
Hemoglobin was barely affected by the GO therapy. The syndrome of toxic symptoms during intravascular hemolysis and impaired hemoglobin scavenging described in children with relapsed AML treated with GO25 was not observed in any of the patients.
No patients had hyperbilirubinemia above 40 μmol/L or VOD in contrast to the 23% with grade 3 or 4 liver toxicity in adults.26,27 A high rate of VOD has primarily been reported when GO was given after HSCT.28 Treatment with GO in heavily pretreated children with relapsed AML has been with manageable toxicity, including VOD in only 1 of 15.29 The risk of VOD seems to be increased with concomitant use of thioguanine,30 and when GO is followed by HSCT within 3 months.31,32 All our patients received thioguanine as part of induction but more than 6 months before GO. None of the patients who received transplants after GO (at a median interval of 405 days from second GO) showed signs of VOD.
The toxicity profile for GO used as monotherapy in this postconsolidation setting in heavily pretreated children with AML is favorable, but the lack of improvements in the final survival indicates little if any role of GO in the postconsolidation phase of AML therapy.
Our patients were all in CR1 with a low CD33-antigen load in peripheral blood which is associated with complete GO saturation of the CD33 cells and an efficient bone marrow cell kill.33 GO might have had some effect as indicated by the nonsignificant postponing of the relapses. Although alternative schedules of GO may have produced different results, postconsolidation monotherapy with GO does not, despite the good tolerability, seem a promising tool for preventing relapse.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The study received funding from the Swedish Childhood Cancer Foundation, the Danish Childhood Cancer Foundation, and the Karen Elise Jensen Foundation. This study was supported in part by research funding from Wyeth (later Pfizer).
Authorship
Contribution: The study was designed by the NOPHO-AML Study Group, of which the authors are members; H.H. wrote the first and subsequent drafts of the manuscript; and all authors provided feedback on the manuscript and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete listing of the participating NOPHO-AML institutions and investigators appears in the supplemental Appendix (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Correspondence: Henrik Hasle, Department of Pediatrics, Aarhus University Hospital, 8200 Skejby, Denmark; e-mail: hasle@dadlnet.dk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal