To the editor:
Chronic lymphocytic leukemia (CLL) exhibits an indolent precursor phase prior to development of a more aggressive phenotype which requires treatment. Understanding the early pathogenesis of CLL offers the opportunity to better implement more effective intervention for this disease. The Eµ-TCL1 murine model mimics many features of human CLL and is widely used to interrogate CLL biology.1 Our group has previously reported that during disease progression in this model, genes become silenced progressively over time.2 This is initiated through transcriptional silencing followed by epigenetic regulation of select genes, which recapitulates the pathogenesis of human CLL. The mechanism of gene silencing involves the p50 (Nfkb1) subunit of NF-κB, a family of transcription factors which is known to play an important role in the progression of CLL.2 In fact, a mutagenesis screen in the Eµ-TCL1 mouse found that mutations leading to the activation of p50 exhibit more aggressive disease.3 Therefore, in our present study, we generated a new mouse model by crossing the Eµ-TCL1 mouse with the previously described p50 knockout mouse4 to study the role of p50 in CLL pathogenesis. Novel treatment strategies are necessary in CLL (particularly resistant disease), and our findings provide support for therapeutic targeting of p50 in CLL and related B-cell malignancies.
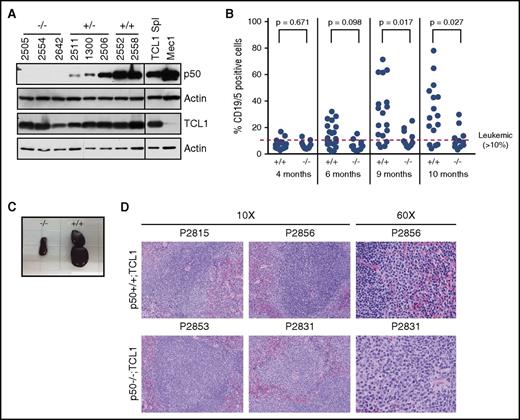
We crossed Eμ-TCL1 mice with p50−/− mice and examined p50+/+, p50+/−, and p50−/− animals (all TCL1+) for disease onset and survival. Immunoblots for TCL1 and p50 were performed on a subset of study animals as previously described5 to confirm that the expression of TCL1 is not compromised due to the loss of p50 (Figure 1A). Starting at 4 months, the animals were monitored by monthly flow cytometry analysis for CD19 and CD5+ B cells in the PB. To assess disease burden over time, mixed-effects models were used to allow for correlations among observations from the same mouse, and data were log-transformed to stabilize variances. At an early stage (4 months of age), there was no statistical difference between the p50+/+;TCL1 and p50−/−;TCL1 mice (Figure 1B; P = .671). However, we see that p50+/+;TCL1 appears to have greater disease burden than p50−/−;TCL1 at later time points (P < .03 at months 9 and 10). The difference between genotypes at specific time points is shown in supplemental Table 1 (available on the Blood Web site). We also see increased white blood cell counts by modified Giemsa stain at 9 and 10 months in the p50+/+;TCL1 animals compared with the p50−/−;TCL1 (supplemental Figure 1).
Peripheral blood (PB) and spleen leukemic burden is decreased in the p50−/−;TCL1 mice. (A) Immunoblots were performed in mouse spleen cell lysate from p50+/+, p50+/−, and p50−/− mice (all TCL1+). Spleen lysate from a TCL1-transgenic animal and lysate from the Mec1 cell line were used as a positive controls for TCL1 and p50, respectively. Actin is shown as a loading control. The line on the blots indicates where additional control lanes have been removed. (B) PB was monitored for the presence of CD19+/CD5+ leukemic cells starting at 4 months. Leukemia is defined as >10% CD19+/CD5+ cells. (C) Spleens were isolated from mice at time of sacrifice based on meeting early removal criteria. A representative image of spleens from littermate p50+/+;TCL1 and p50−/−;TCL1 animals is shown. (D) Hematoxylin-and-eosin (H&E) histology was performed on sections of spleens isolated from 4- to 7-month-old p50+/+;TCL1 and p50−/−;TCL1 animals (N = 6 per genotype). Representative images from each genotype at original magnification ×10 and ×60 are shown.
Peripheral blood (PB) and spleen leukemic burden is decreased in the p50−/−;TCL1 mice. (A) Immunoblots were performed in mouse spleen cell lysate from p50+/+, p50+/−, and p50−/− mice (all TCL1+). Spleen lysate from a TCL1-transgenic animal and lysate from the Mec1 cell line were used as a positive controls for TCL1 and p50, respectively. Actin is shown as a loading control. The line on the blots indicates where additional control lanes have been removed. (B) PB was monitored for the presence of CD19+/CD5+ leukemic cells starting at 4 months. Leukemia is defined as >10% CD19+/CD5+ cells. (C) Spleens were isolated from mice at time of sacrifice based on meeting early removal criteria. A representative image of spleens from littermate p50+/+;TCL1 and p50−/−;TCL1 animals is shown. (D) Hematoxylin-and-eosin (H&E) histology was performed on sections of spleens isolated from 4- to 7-month-old p50+/+;TCL1 and p50−/−;TCL1 animals (N = 6 per genotype). Representative images from each genotype at original magnification ×10 and ×60 are shown.
We next examined disease in the spleen to rule out the possibility that loss of p50 impairs the localization of the leukemic cells to the PB without affecting total leukemic burden in the animals. We found that when the animals met removal criteria, spleens from p50−/−;TCL1 mice were consistently smaller than the p50+/+;TCL1 littermates (Figure 1C). We euthanized p50+/+;TCL1 and p50−/−;TCL1 mice (aged 4-7 months) to look at PB and spleen leukemia burden prior to terminal disease and examined splenic structure using H&E histology. In p50+/+;TCL1, small well-differentiated lymphocytes sometimes forming germinal centers are in the white pulp, whereas large atypical lymphocytes are limited to the marginal zone and red pulp, which is consistent with increased PB disease in these mice. In contrast, neoplastic cells in the p50−/−;TCL1 efface the white pulp (Figure 1D). We verified that CD19/CD5+ cells were decreased in p50−/−;TCL1 mice in the PB and spleen (supplemental Figure 2). Finally, we examined gene expression and found that both IL-6 and CXCL9, which have been previously described as genes repressed by p50,6 were upregulated in the p50−/−;TCL1 B cells although this was not significant (supplemental Figure 3).
We next analyzed disease development and survival in the different genotype groups. For time to leukemia, estimates of the cumulative incidence function and competing risks regression using the Fine and Gray model7 were used to account for the mice who either died young without disease or developed T-cell instead of B-cell leukemia. Kaplan-Meier plots and the log-rank test were used to assess differences in overall survival (all analyses were performed using SAS/STAT software). The p50−/−;TCL1 mice (N = 11) had a significantly lower incidence of leukemia compared with p50+/+;TCL1 (N = 20) (Figure 2A; subdistribution hazard ratio [SHR] for p50+/+;TCL1 vs p50−/−;TCL1 = 3.53; 95% confidence interval [CI], 1.28, 9.72; P = .015). The p50+/−;TCL1 mice (N = 25) exhibit a phenotype indistinguishable from the p50−/−;TCL1 (SHR = 0.81; 95% CI, 0.28, 2.34; P = .699), while still showing significantly reduced leukemia compared with the p50+/+;TCL1 (p50+/+;TCL1 vs p50+/−;TCL1, SHR = 4.35; 95% CI, 1.99, 9.54; P < .001), suggesting that even a reduction in the total amount of p50 (compared with a total loss) can significantly impact disease development. Despite the significant difference in the development of leukemia, overall survival was not significantly improved in the p50−/−;TCL1 animals compared with p50+/+;TCL1 (Figure 2B). Due to the described immune dysfunction in the TCL1 mouse,8 we examined the B and T cells to determine whether this could account for the impaired survival in p50−/−;TCL1 mice. We found no difference in the relative percentage of CD3, CD4, or CD8 T cells, nor was there a difference in the activation of B or T cells between p50+/+;TCL1 vs p50−/−;TCL1 (supplemental Table 2). Although the original paper describing the p50−/− animals did not report a difference in survival relative to wild type, we have identified recent reports that p50−/− mice do have inferior overall survival due to premature aging, and the lifespan of the p50−/− in these studies was ∼15 months, similar to the survival of the p50−/−;TCL1 in our study.9
Cumulative incidence of leukemia is delayed in the p50−/−;TCL1 mice. All experiments were carried out under protocols approved by The Ohio State University Institutional Animal Care and Use Committee. (A) A competing risks model was used to assess the occurrence of B-cell leukemia. The p50−/−;TCL1 mice had a significantly lower incidence of leukemia compared with p50+/+;TCL1 mice (subdistribution hazard ratio [SHR] for p50+/+ vs p50−/− = 3.53; 95% CI, 1.28, 9.72; P = .015). (B) The overall log-rank P value among the 3 genotypes was calculated and Kaplan-Meier estimates of the survival function are presented.
Cumulative incidence of leukemia is delayed in the p50−/−;TCL1 mice. All experiments were carried out under protocols approved by The Ohio State University Institutional Animal Care and Use Committee. (A) A competing risks model was used to assess the occurrence of B-cell leukemia. The p50−/−;TCL1 mice had a significantly lower incidence of leukemia compared with p50+/+;TCL1 mice (subdistribution hazard ratio [SHR] for p50+/+ vs p50−/− = 3.53; 95% CI, 1.28, 9.72; P = .015). (B) The overall log-rank P value among the 3 genotypes was calculated and Kaplan-Meier estimates of the survival function are presented.
Overall, this study highlights the importance NF-κB-p50 in CLL development. Agents such as ibrutinib that target B-cell receptor signaling have proven very effective in treating B-cell malignancies, in part through targeting downstream NF-κB signaling.10,11 In addition, numerous studies have proposed that NF-κB inhibitors effectively target survival signaling in CLL cells.12-14 However, loss of key NF-κB subunits such as p65, IκB kinase α or β exhibit a lethal phenotype,15 and therefore therapies targeting broad NF-κB signaling have not advanced in a clinical setting. On the other hand, loss of p50 in a murine system produces predominately a B-cell defect.4 The more recent reports of premature aging in the p50 knockout animals9 is likely due to prolonged lack of activity from an early age, and would not be a complication with transient inhibition that would be clinically pursued. Therefore, therapies targeting p50 may provide an antileukemia effect without the toxicities associated with broad NF-κB inhibitors.
The online version of this article contains a data supplement.
Authorship
Acknowledgments: The authors thank Carlo Croce for the TCL1 mouse.
This work was supported by the National Institutes of Health, National Cancer Institute: T32 CA009338 and K12 CA133250 (E.H.), P01 CA81534 (A.J.J.), and P30 CA016058, P01 CA95426, and R35 CA197734 (J.C.B.); the Leukemia & Lymphoma Society (special scholar grant [E.H.]); the Sullivan Foundation; Michael and Judy Thomas; and the D. Warren Brown Foundation.
Contribution: T.L.C. performed experiments, analyzed data, and wrote the manuscript; A.L. and N.G. performed experiments and analyzed data; M.T., B.K.H., and V.M.G. performed animal experiments; A.M.L. performed statistical analysis; S.T., D.M.L., and A.J.J. analyzed data; J.C.B. analyzed data and reviewed the manuscript; E.H. designed and performed experiments, analyzed data, supervised the study, and wrote the manuscript; and all authors read and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Erin Hertlein, Division of Hematology, Department of Internal Medicine, Comprehensive Cancer Center, The Ohio State University, 462 Wiseman, 410 West 12th Ave, Columbus, OH 43210; e-mail: erin.hertlein@osumc.edu.

![Figure 2. Cumulative incidence of leukemia is delayed in the p50−/−;TCL1 mice. All experiments were carried out under protocols approved by The Ohio State University Institutional Animal Care and Use Committee. (A) A competing risks model was used to assess the occurrence of B-cell leukemia. The p50−/−;TCL1 mice had a significantly lower incidence of leukemia compared with p50+/+;TCL1 mice (subdistribution hazard ratio [SHR] for p50+/+ vs p50−/− = 3.53; 95% CI, 1.28, 9.72; P = .015). (B) The overall log-rank P value among the 3 genotypes was calculated and Kaplan-Meier estimates of the survival function are presented.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/130/3/10.1182_blood-2017-01-761130/4/m_blood761130f2.jpeg?Expires=1769088645&Signature=hiQ3YKznwQSBGJoGtnybW5VMkE7rNXhFx-7Kcuwf~pVSJRzhI9x0SVCJGaocytiv7FC3VoFegukdDPxbSgru4U9wklzsnAwTftP5dcEUlfIpGmExbDGuFhPNYJh-MejWM-KngU88bIjYUB3GhLvf~svIK6zGSJEdJnLL8pqwBTo11ZU50PXR919S4IsnX2LQvy~p-wbJrz4A1bFc1V5iv-kzAVV7s0MGMXJBq1EvSGk~SCa~vuXXmWjnu2vqIrknuOmhdnYkB4lGNS~GRjeS18SjZwUEq91tAxEoITpnythfPam2jrkz-4EgJoc8Ewa3K9Xp-XQHfxwCEP2rlglnJg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal