Key Points
Tcl1 cooperates with the NF-kB pathway in the pathogenesis of the aggressive form of CLL.
Abstract
TCL1 oncogene is overexpressed in aggressive form of human chronic lymphocytic leukemia (CLL) and its dysregulation in mouse B cells causes a CD5-positive leukemia similar to the aggressive form of human CLLs. To identify oncogenes that cooperate with Tcl1, we performed genetic screen in Eμ−TCL1 mice using Sleeping Beauty transposon-mediated mutagenesis. Analysis of transposon common insertion sites identified 7 genes activated by transposon insertions. Overexpression of these genes in mouse CLL was confirmed by real time reverse transcription-polymerase chain reaction. Interestingly, the main known function of 4 of 7 genes (Nfkb1, Tab2, Map3K14, and Nfkbid) is participation in or activation of the nuclear factor-kB (NF-kB) pathway. In addition, activation of the NF-kB is 1 of main functions of Akt2, also identified in the screen. These findings demonstrate cooperation of Tcl1 and the NF-kB pathway in the pathogenesis of aggressive CLL. Identification cooperating cancer genes will result in the development of combinatorial therapies to treat CLL.
Introduction
B-cell chronic lymphocytic leukemia (CLL) is the most common adult leukemia in the Western world.1,2 We have previously shown that transgenic mice overexpressing TCL1 gene in B cells (Eμ-TCL1) develop the aggressive form of CLL3 and that aggressive human CLLs overexpress Tcl1.4 These results indicate that dysregulation of TCL1 is critically important in the pathogenesis of the aggressive form of CLL.5 Because Eµ-TCL1 mice developed CLL in relatively old age, it is likely that deregulation of Tcl1 is an initiating event in CLL pathogenesis, but additional genetic changes are necessary for CLL development. To identify these changes, we performed genetic screen in Eμ−TCL1 mice using Sleeping Beauty (SB) transposon-mediated mutagenesis. The Sleeping Beauty approach is based on the ability of SB transposons containing murine stem-cell virus long terminal repeat and splice donor site to activate the expression of nearby oncogenes.6,7 This system was successfully used in the past to identify genes contributing to development of several tumor types.8,9
Study design
Mouse strains
Homozygous Eµ-TCL1 transgenic mice as well as genotyping protocol were described previously.3 CD19–Cre homozygous transgenic mice were purchased from The Jackson Laboratory and genotyped according to the manufacturer recommendations. The SB strains homozygous for TgTn(sb-T2/Onc2)6113Njen (SB6113) or TgTn(sb-T2/Onc2)6070Njen (SB6070) SB mutagenic transgenes and a Cre-inducible SBase knock-in allele at ROSA26 were previously described.6,7 The 2 SB strains are different by chromosomal locations of the transgenes. B-cell populations were isolated from spleens and lymph nodes of sick Tcl1/SB and control animals using B-Cell Isolation Kit (Miltenyi Biotec) as previously described.10 Isolation and flow cytometry analysis of spleen lymphocytes were carried out as previously described.11 The animal studies (The Ohio State University protocol 2010A00000146) were approved by The Ohio State University Institutional Animal Care and Use Committee and were conducted under National Institutes of Health guidelines.
Identification and analysis of genes affected by SB mutagenesis
DNA was extracted from B cells of mouse CLL tumors with the DNeasy Blood & Tissue Kit (Qiagen). To identify insertion sites, DNA was shared, ligated to adaptors, and analyzed using Illumina-based ligation-mediate polymerase chain reaction (PCR) as previously described.12 Sequencing reads were analyzed using gene-centric common insertion site method as previously described.12 Real time reverse transcription (RT)-PCR was carried out using indicated Applied Biosystems assays according to the manufacturer’s protocol.
Results and discussion
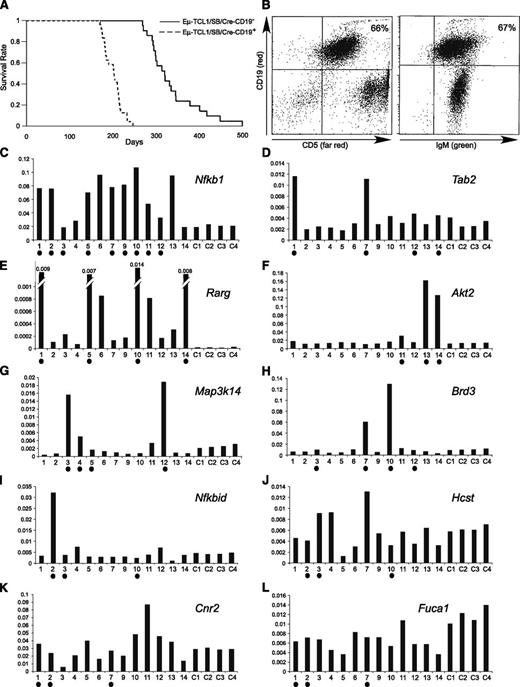
To generate Eµ-TCL1/SB animals undergoing B cell–specific transposon mutagenesis, we first crossed Eµ-TCL1 and CD19–Cre animals. The resulting progeny (hemizygous for both, Eµ-TCL1 and CD19–Cre; F1) were crossed with SB6113 or SB6070 strains. Two genotypes from this transgenic progeny (F2), Eµ-TCL1/SB/CD19–Cre+ and Eµ-TCL1/SB/CD19–Cre−, were studied. Twenty-four mice of Eµ-TCL1/SB/CD19–Cre+ genotype and 21 mice of Eµ-TCL1/SB/CD19–Cre− genotype were produced. Figure 1A shows Kaplan-Meier survival plot comparing Eµ-TCL1/SB/CD19–Cre+ (dashed line) and Eµ-TCL1/SB/CD19–Cre− (solid line) genotypes. Strikingly, all Eµ-TCL1/SB/CD19–Cre+ mice died within 250 days before the first casualty occurred in the second group (at least 20 days later, Figure 1A). Sick mice presented with enlarged abdomen resulting from an oversized spleen and lymph nodes. Fluorescence-activated cell sorter analysis revealed expansion of the CD19+IgM+CD5+ lymphocyte population in spleens and lymph nodes (up 100% of all B cells were IgM+CD5+, Figure 1B). All animals showed a phenotype very similar to that of Eµ-TCL1 mice.3
Analysis of Eμ−TCL1/SB mice. (A) Kaplan-Meier survival plot of Eμ−TCL1/SB and Eμ−TCL1 control mice. (B) Fluorescence-activated cell sorter analysis of a representative Eμ−TCL1/SB CLL case (the T cells are green because in this system cells that do not undergo Cre recombination are green fluorescent protein–positive12 ). (C-L) Real-time RT-PCR analysis of genes affected by CISs. Shown values are relative to actin expression. Samples containing insertions near corresponding genes are marked with solid dots. The following Applied Biosystems assays were used: Hcst (Mm01172975_m1), Nfkbid (Mm00549082_m1), Map3k14 (Mm00444166_m1), Rarg (Mm00441091_m1), Brd3 (Mm00469733_m1), Tab2 (Mm00663112_m1), Fuca1 (Mm00502778_m1), Nfkb1 (Mm00476361_m1), Akt2 (Mm02026778_g1) and Cnr2 (Mm02620087_s1), and Actin (Mm00607939_s1).
Analysis of Eμ−TCL1/SB mice. (A) Kaplan-Meier survival plot of Eμ−TCL1/SB and Eμ−TCL1 control mice. (B) Fluorescence-activated cell sorter analysis of a representative Eμ−TCL1/SB CLL case (the T cells are green because in this system cells that do not undergo Cre recombination are green fluorescent protein–positive12 ). (C-L) Real-time RT-PCR analysis of genes affected by CISs. Shown values are relative to actin expression. Samples containing insertions near corresponding genes are marked with solid dots. The following Applied Biosystems assays were used: Hcst (Mm01172975_m1), Nfkbid (Mm00549082_m1), Map3k14 (Mm00444166_m1), Rarg (Mm00441091_m1), Brd3 (Mm00469733_m1), Tab2 (Mm00663112_m1), Fuca1 (Mm00502778_m1), Nfkb1 (Mm00476361_m1), Akt2 (Mm02026778_g1) and Cnr2 (Mm02620087_s1), and Actin (Mm00607939_s1).
To determine common transposon insertion sites (CISs) tumor DNA was analyzed using Illumina-based ligand-mediated PCR followed by gene-centric common insertion site analysis.12 Fifteen animals of Eµ-TCL1/SB/CD19–Cre+ genotype (8 from SB6113 strain and 7 from SB6070 strain) were analyzed and CISs and affected genes were identified in at least 3 tumors (Table 1). In most of the cases, CISs occurred in the proximity of a single gene, except in 2 pairs, Cnr2 and Fuca1 and Hcst and Nfkbid, which appeared as significant because of the same 3 CISs (Table 1 and supplemental Figure 1 and supplemental Table 1). To determine if CISs resulted in activation of affected genes, we carried out real-time RT-PCR for all affected genes (samples 1-7 from SB6113 strain, samples 9-14 from SB6070 strain; C1-C4 are normal CD19+ B-cell controls, RNA samples from samples 8 and 15 were not available) (Figure 1C-L). Interestingly, Nfkb1 encoding p105 and p50 components of nuclear factor (NF)-kB signaling pathway13 was by far the most affected gene in this screen. Ten of 15 samples showed a total of 14 transposon insertions within intron 1 of the gene, and its expression was significantly increased in 8 of 9 affected samples. Nfkb1 was an average of approximately threefold overexpressed in CLL samples vs normal controls (Figure 1C and supplemental Figure 1 and supplemental Table 1). Two other activators of the NF-kB signaling pathway—Tab214 and Map3K1415 —were also activated because of insertions in 4 tumors each (Tab2 was activated in all 4 tumors on average ∼2.5-fold compared with controls, Map3K14 in 3 of 4 tumors on average approximately fourfold) (Figure 1D,G). The Akt2 oncogene was also among genes identified in the screen, and its overexpression (eightfold on average) was confirmed in 3 of 3 insertion cases (Figure 1F). Although Akt2 exerts its oncogenic activity through multiple targets, it can also activate NF-kB pathway through phosphorylation of IKKα.16 In addition, Nfkbid,17 another participant of the NF-kB pathway, was activated in 1 case, whereas it was targeted by insertions in 3 tumors (Figure 1I). The transposon integrations in Nfkbid are all at the 3′ end of the gene (supplemental Figure 1); it is possible that transposon integration in this case generates a truncated transcript, as was previously reported for endothelial growth factor receptor.9 Thus, it is not necessarily unexpected to find a lack of Nfkbid overexpression in 2 of 3 tumors with insertions.
Genes affected by CISs in Eμ−TCL1/SB mice
| Gene symbol . | P value . | Number of tumors . | Tumors with insertions . | Real-time RT-PCR conformation . |
|---|---|---|---|---|
| Nfkb1 | <.0001 | 10 | 1,2,3,5,7,9,10,11,12,15 | 8 of 9 higher than control |
| Tab2 | <.0001 | 4 | 1,7,12,14 | 4 of 4 higher than control* |
| Map3K14 | <.0001 | 4 | 3,4,5,12 | 3 of 4 higher than control* |
| Rarg | <.0001 | 4 | 1,5,10,14 | 4 of 4 higher than control* |
| Akt2 | <.0001 | 3 | 11,13,14 | 3 of 3 higher than control* |
| Brd3 | <.0001 | 4 | 3,7,10,12 | 2 of 4 higher than control* |
| Hcst/Nfkbid | <.0001 | 3 | 2,3,10 | 1 of 3 higher than control* |
| Cnr2/Fuca1 | <.0001 | 3 | 1,2,7 | Not confirmed |
| Gene symbol . | P value . | Number of tumors . | Tumors with insertions . | Real-time RT-PCR conformation . |
|---|---|---|---|---|
| Nfkb1 | <.0001 | 10 | 1,2,3,5,7,9,10,11,12,15 | 8 of 9 higher than control |
| Tab2 | <.0001 | 4 | 1,7,12,14 | 4 of 4 higher than control* |
| Map3K14 | <.0001 | 4 | 3,4,5,12 | 3 of 4 higher than control* |
| Rarg | <.0001 | 4 | 1,5,10,14 | 4 of 4 higher than control* |
| Akt2 | <.0001 | 3 | 11,13,14 | 3 of 3 higher than control* |
| Brd3 | <.0001 | 4 | 3,7,10,12 | 2 of 4 higher than control* |
| Hcst/Nfkbid | <.0001 | 3 | 2,3,10 | 1 of 3 higher than control* |
| Cnr2/Fuca1 | <.0001 | 3 | 1,2,7 | Not confirmed |
P values are calculated by χ-squared test.
*Specifies that expression levels of all indicated samples were highest among all samples tested.
The most dramatic expression differences were observed for retinoic acid receptor γ (Rarg). Nine tumors without insertions showed on average ∼20-fold induction of Rarg vs controls; 4 tumors with insertions showed additional ∼30-fold induction (∼600-fold induction vs controls) (Figure 1E). Interestingly, Rarg is a member of the retinoic acid receptor family, which also includes Rarα.18 In humans, RARα is involved in recurrent 17q21 translocations in myeloid malignancies, and generally resulting fusion proteins considered as a gain of function for RARα19 In summary, SB screen identified 8 CISs, affecting 10 genes. Seven of 8 CISs resulted in the activation of 7 corresponding genes. For 5 of these genes (Nfkb1, Tab2, Map3k14, Akt2, and Rarg), we observed a good correlation between insertions and expression activation, for 2 others, Nfkbid and Brd3, there was only partial correlation (Figure 1H-I). Interestingly, Brd3 was highly activated (approximately sevenfold and ∼12-fold) in 2 of 4 insertion cases (Figure 1H). A CIS between Cnr2 and Fuca1 did not affect their expression (Figure 1K-L). Because multiple insertions were found in each tumor (supplemental Table 1), the shorter mean survival of mice that had insertions near genes in which no difference in expression was noted was likely due to insertions near other genes, particularly Nfkb1. Specifically, all tumors that had insertions near genes that had low or no correlation with increased gene expression had Nfkb1 insertions as well (Figure 1).
To identify oncogenes that cooperate with Tcl1 in the aggressive form of CLL, we carried out an SB screen in Eμ−TCL1 mice. Our findings demonstrate cooperation of Tcl1 and the NF-kB pathway because we found that 5 of 7 genes activated by CISs can either activate or participate in the NF-kB pathway. We and others previously showed the importance of NF-kB in CLL.20-23 Because TCL1 is dysregulated in most of aggressive CLLs, it seems likely that the same genes are dysregulated in human CLLs overexpressing Tcl1.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dorothee Wernicke for valuable suggestions and critical discussion of the manuscript and Joshua Trenkamp for technical assistance.
This work was supported by the American Cancer Society Research Scholar Grant, Swan family funds, and CLL global research foundation grant (Y.P.), and National Institutes of Health (grant P01-CA81534) of the CLL Research Consortium (C.M.C.).
Authorship
Contribution: Y.P., C.M.C., and A.J.D. designed research; A.J.D. provided SB mice; N.Z., V.B., J.R., A.B., Y.P., A.P., L.R., L.C., and A.L. performed research and analyzed data; Y.P. and C.M.C. wrote the paper; and all authors critically reviewed and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yuri Pekarsky, The Ohio State University, Comprehensive Cancer Center, Biomedical Research Tower, Room 1090, 460 West 12th Ave, Columbus, OH 43210; e-mail: pekarsky.yuri@osumc.edu.