Key Points
SYK is a suitable molecular target for nanotechnology-enabled therapy against ALL.
Nanoscale liposomal formulation of SYK inhibitor C61 displayed a promising preclinical profile as an antileukemic drug candidate.
Abstract
We report preclinical proof of principle for effective treatment of B-precursor acute lymphoblastic leukemia (ALL) by targeting the spleen tyrosine kinase (SYK)–dependent antiapoptotic blast cell survival machinery with a unique nanoscale pharmaceutical composition. This nanoscale liposomal formulation (NLF) contains the pentapeptide mimic 1,4-Bis (9-O dihydroquinidinyl) phthalazine/hydroquinidine 1,4-phathalazinediyl diether (C61) as the first and only selective inhibitor of the substrate binding P-site of SYK. The C61 NLF exhibited a very favorable pharmacokinetic and safety profile in mice, induced apoptosis in primary B-precursor ALL blast cells taken directly from patients as well as in vivo clonogenic ALL xenograft cells, destroyed the in vivo clonogenic fraction of ALL blast cells, and, at nontoxic dose levels, exhibited potent in vivo antileukemic activity against patient-derived ALL cells in xenograft models of aggressive B-precursor ALL. Our findings establish SYK as an attractive molecular target for therapy of B-precursor ALL. Further development of the C61 NLF may provide the foundation for therapeutic innovation against therapy-refractory B-precursor ALL.
Introduction
B-precursor acute lymphoblastic leukemia (ALL) is the most common form of cancer in children and adolescents.1,2 Currently, the major challenge in the treatment of B-precursor ALL is to cure patients who have relapsed despite intensive frontline chemotherapy.1,3-5 There is an urgent and unmet need to identify new drug candidates capable of destroying therapy-resistant leukemic B-cell precursors. Spleen tyrosine kinase (SYK) is a cytoplasmic protein tyrosine kinase with multiple important regulatory functions in B-lineage lymphoid cells.6,7 Notably, the antiapoptotic nuclear factor B, phosphatidylinositol 3-kinase/protein kinase B, and signal transducer and activator of transcription 3 (STAT3) pathways are regulated by SYK-mediated tyrosine phosphorylation events.8,9 Constitutive activation and antiapoptotic function of SYK have been documented for several B-lineage lymphoid malignancies, including B-precursor ALL.8,10 The identification of SYK as a key regulator of multiple antiapoptotic pathways in B-lineage lymphoid cells prompts the hypothesis that rationally designed inhibitors targeting SYK may overcome the resistance of leukemic cells to apoptosis and thereby provide the foundation for more effective multimodality treatment regimens for patients with a poor prognosis for B-precursor ALL.
We recently identified the pentapeptide mimic 1,4-Bis (9-O-dihydroquinidinyl) phthalazine/hydroquinidine 1,4-phathalazinediyl diether (“compound 61,” or C61) as a highly selective and potent inhibitor targeting the substrate binding P-site of SYK.9 However, the poor water solubility of this cinchona alkaloid and its life-threatening off-target side effects owing to its quininelike chemical structure, especially the development of peracute severe intravascular hemolysis and shock with secondary kidney failure and seizures at moderate-to-high dose levels, have emerged as major obstacles to its further development as an antileukemic drug candidate.10 Liposomal nanoparticle (LNP) therapeutics containing active anticancer agents may provide the foundation for potentially more effective and less toxic anticancer treatment strategies due to their improved pharmacokinetics, reduced systemic toxicity, and increased intratumoral/intracellular delivery.11-13 Therefore, we developed a multifunctional, PEGylated LNP formulation of C61, designated “formulation 25A,” as a unique nanoscale pharmaceutical composition for therapeutic application against B-precursor ALL. The purpose of the present study was to perform a preclinical evaluation of the potential of this nanoscale liposomal formulation (NLF) of C61 as a new pharmaceutical composition against ALL cells.
Methods
Synthesis and chemical characteristics of C61 and its salt formulation
C61 is a C2-symmetrical cinchona alkaloid derivative, which was prepared using previously described synthetic procedures (see Uckun et al9 and Uckun et al10 ). C61 is a potent and selective chemical inhibitor of SYK.9,10 C61-salt [C61-(H2SO4)4] was synthesized by treating C61 with H2SO4 in ethanol followed by recrystallization, as previously described in Uckun et al.10
Preparation and characterization of the liposomal C61 formulation
The C61 LNP formulation, 25A, was prepared using the standard thin film evaporation method. Size measurement by the dynamic light scattering technique, ζ potential measurements, analytical high-performance liquid chromatography, and transmission electron microscopy were used for physicochemical characterization of the LNP. The activity of the C61 LNP formulation was measured using kinase assays and apoptosis assays.9,10 See supplemental Methods for a description of these methodologies (available on the Blood website).
Clinical samples
Leukemia cells isolated from bone marrow (N = 17) or peripheral blood (N = 1) specimens of 18 patients with B-precursor ALL (new diagnosis = 16; relapse = 2) and from 11 spleen specimens of xenografted nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice were used in the described experiments. The xenografts were established using primary cells from 7 newly diagnosed and 4 relapsed pediatric patients with B-precursor ALL. The secondary use of leukemic cells for subsequent molecular studies did not meet the definition of human subject research per 45 CFR 46.102 (d and f) of the Code of Federal Regulations because it does not include identifiable private information, and it was approved by the Institutional Review Board Committee on Clinical Investigations at the Children’s Hospital Los Angeles (Human Subject Assurance Number FWA0001914). The research was also reviewed and approved by the Memorialcare Health System Institutional Review Board at Miller Children’s Hospital, Long Beach, CA. This study was conducted in accordance with the Declaration of Helsinki.
Immunofluorescence staining and multiparameter flow cytometry
A broad panel of commercially available monoclonal antibodies were used for the immunophenotyping of primary leukemic cells from pediatric patients with ALL, as well as leukemic cells from spleen specimens of NOD/SCID mice xenografted with primary human ALL cells by standard immunofluorescent staining and multiparameter flow cytometry, as previously reported in Uckun et al9 and Uckun et al.10 The SYK expression level of leukemic cells was examined by intracytoplasmic flow cytometry using BD Phosflow Fix Buffer 1 (catalog number 557870, BD Biosciences, San Jose, CA) for permeabilization of the cell membrane and SYK–fluorescein isothiocyanate antibody (catalog number 552476, BD Biosciences). The labeled cells were analyzed on a LSR II flow cytometer (Becton, Dickinson, Lakes, NJ). Confocal microscopy was used to study SYK expression in xenograft cells using previously published procedures (see Uckun et al9 ). See supplemental Methods for a description of these methodologies as well as the list of monoclonal antibodies used for immunophenotyping.
Preclinical studies of the liposomal C61 formulation in rodents
The toxicity and pharmacokinetics of the liposomal C61 formulation were studied in rodents using Animal Care and Use Committee–approved standard procedures.10 The antileukemic activity of 25A was studied in a NOD/SCID mouse model of human B-precursor ALL. See supplemental Methods for a description of these methodologies.
Results
Physicochemical characteristics and in vitro activity profile of the NLF of the SYK P-site inhibitor C61
The generated ellipsoid C61 LNP formulation 25A had a diameter of 136.3 ± 1.2 nm and a negative surface charge with a ζ potential of −12.1 ± 0.8 mV in solution, consistent with the use of the negatively charged phospholipid DSPE, and contained 8.7 ± 0.1 mg/mL C61 (supplemental Figure 1). Formulated C61 inhibited the constitutive activity of SYK (but not Bruton's tyrosine kinase [BTK]) in the BCR-ABL+ B-precursor ALL cell line ALL-1 in a concentration-dependent fashion and prevented the CD19-mediated activation of SYK without affecting SYK protein expression levels (supplemental Figure 2). C61-loaded LNP formulation 25A (but not empty LNP formulation 25B) caused apoptosis in the B-lineage ALL cell lines DAUDI and ALL-1 in a concentration-dependent manner at concentrations ≥3 µg/mL with an average 50% effective concentration value of 3.4 ± 1.0 µg/mL. But it did not affect the viability of SYK− nontarget human medulloblastoma and fibrosarcoma cell lines, even at 30 µg/mL (supplemental Figure 3). The ability of 25A to consistently cause apoptosis of SYK+ ALL cell lines was confirmed in 3 additional independent validation experiments using the B-precursor ALL cell line ALL-1 (supplemental Figure 4) and 4 independent validation experiments using the T-lineage ALL cell line LOUCY (supplemental Figure 5). The C61 LNP caused more than 90% apoptosis at ≥10 µg/mL, whereas the drug-free LNP formulation 25B had minimal effect on the viability of either cell line. In a preliminary stability study using extended storage at 4°C, the average radius values of the C61-LNP, measured by dynamic light scattering, did not show significant changes up to 16 weeks to suggest the emergence of aggregates or the alteration of the size distribution profile (supplemental Figure 6). The C-61 content of the 25A LNP, measured by high-performance liquid chromatography, showed no detectable decrease after extended storage to suggest leakage or burst release from the LNP. The ability of the 25A LNP to cause apoptosis of the B-precursor ALL cell line ALL-1 was sustained over a 3-month storage time (supplemental Figure 6).
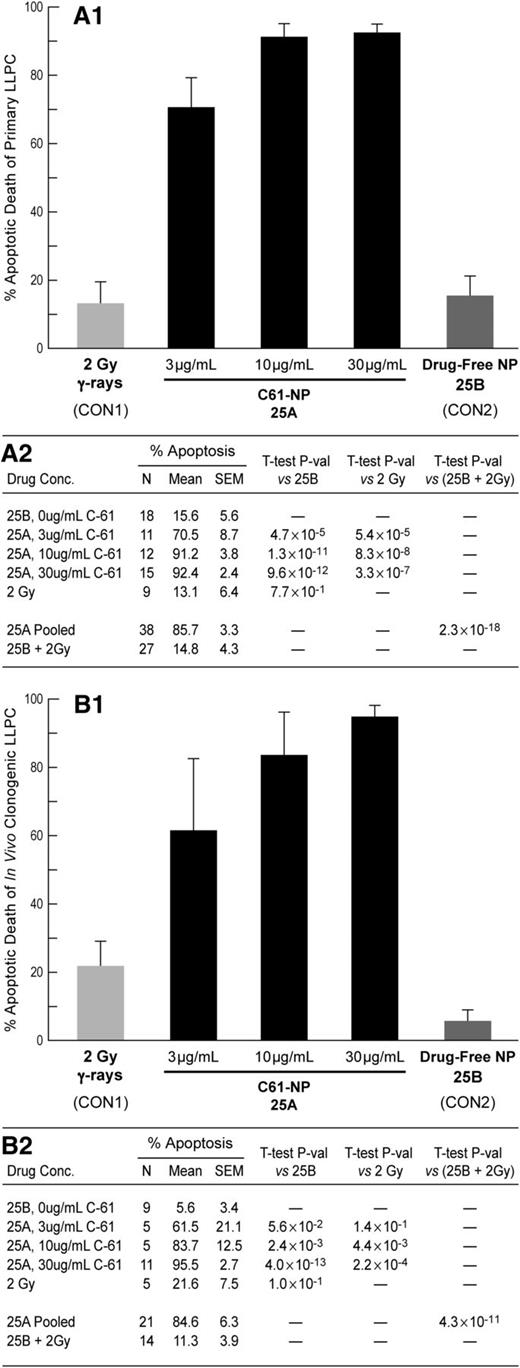
In vitro antileukemic activity of the NLF of the SYK P-site inhibitor C61 against primary and xenografted B-precursor ALL cells
C61-LNP 25A (but not control LNP 25B), when used at 3 to 30 µg/mL concentrations, consistently caused apoptosis in primary leukemic lymphocyte precursor cells (LLPCs) taken directly from 18 children with newly diagnosed or relapsed ALL (Figure 1A and supplemental Figure 7), and its antileukemic potency was markedly superior to that of irradiation with 200 rad γ-rays (P < .00001) (Figure 1A). We also examined the ability of 3 to 30 µg/mL 25A to cause apoptotic destruction of very aggressive in vivo clonogenic human LLPCs isolated from spleens of xenografted NOD/SCID mice that developed overt leukemia after inoculation with primary leukemic cells from 11 patients with B-precursor ALL, including 4 patients with relapsed ALL (Figure 1B, supplemental Figure 8, and supplemental Table 1). We previously reported that primary leukemia cells from relapsed patients with B-precursor ALL express abundant levels of SYK protein.10 Likewise, xenograft cells were SYK+, as determined by western blot analysis and confocal microscopy (supplemental Figure 9 A-B). Notably, 91.4% ± 2.2% of xenograft cells expressed SYK, as determined by quantitative intracellular flow cytometry (supplemental Table 1 and supplemental Figure 9C). Xenograft cells were exquisitely sensitive to 25A. While only 5.6% ± 3.4% of xenograft cells (N = 9) treated with 25B (162 µg/mL lipid) and 21.6% ± 7.5% of xenograft cells irradiated with 200 rad γ-rays showed evidence of apoptosis, 95.5% of xenograft cells treated with 25A (162 µg/mL lipid, 30 µg/mL C61) became apoptotic (Figure 1B, P value 25A vs 25B = 4.0 × 10−13, and supplemental Table 1).
Apoptotic destruction of primary B-precursor ALL cells and their in vivo clonogenic fraction by liposomal C61 nanoparticle (NP) formulation 25A. (A) Concentration-dependent apoptosis induction of primary LLPCs from patients with B-precursor ALL (N = 18) after treatment with 25A is shown. Treatment with 3 µg/mL 25A resulted in 70.5% ± 8.7% apoptosis, whereas 10 µg/mL caused 91.2% ± 3.8% apoptosis (P = .047) and 30 µg/mL caused 92.4% ± 2.4% apoptosis (P = .032). Neither drug-free control NP formulation 25B nor 2 Gy γ-rays caused a significant degree of apoptosis. In each of the 18 cases, there were at least 1 untreated control, 1 25B-treated control, and at least 1 test sample treated with 25A at 3, 10, or 30 µg/mL. Cumulatively, 0 µg/mL was tested in 18, 3 µg/mL in 11, 10 µg/mL in 12, and 30 µg/mL in 15 cases (see supplemental Methods). Ionizing radiation was tested in 9 cases based on the availability of the irradiator and specimen size. Cells were incubated with 25A or 25B for 24 hours in 2 cases, 48 hours in 13 cases, and 72 hours in 3 cases. (B) Concentration-dependent apoptosis induction of in vivo clonogenic LLPCs obtained from spleens of xenografted NOD/SCID mice challenged with primary LLPCs from 11 patients with B-precursor ALL after treatment with 25A is shown. In each of the 11 cases, there were at least 1 untreated control (in 9 cases there also was a 25B-treated control) and at least 1 test sample treated with 25A. Cumulatively, 0 µg/mL was tested in 11, 3 µg/mL in 5, 10 µg/mL in 5, and 30 µg/mL in 11 cases (see supplemental Methods). Ionizing radiation was tested in 5 cases based on the availability of the irradiator and sufficient cell numbers. Xenograft cells were incubated with 25A or 25B for 24 hours in 1 case, 48 hours in 9 cases, and 72 hours in 1 case. As in (A), neither drug-free control NP formulation 25B nor 2 Gy γ-rays caused a significant degree of apoptosis. SEM, standard error of the mean.
Apoptotic destruction of primary B-precursor ALL cells and their in vivo clonogenic fraction by liposomal C61 nanoparticle (NP) formulation 25A. (A) Concentration-dependent apoptosis induction of primary LLPCs from patients with B-precursor ALL (N = 18) after treatment with 25A is shown. Treatment with 3 µg/mL 25A resulted in 70.5% ± 8.7% apoptosis, whereas 10 µg/mL caused 91.2% ± 3.8% apoptosis (P = .047) and 30 µg/mL caused 92.4% ± 2.4% apoptosis (P = .032). Neither drug-free control NP formulation 25B nor 2 Gy γ-rays caused a significant degree of apoptosis. In each of the 18 cases, there were at least 1 untreated control, 1 25B-treated control, and at least 1 test sample treated with 25A at 3, 10, or 30 µg/mL. Cumulatively, 0 µg/mL was tested in 18, 3 µg/mL in 11, 10 µg/mL in 12, and 30 µg/mL in 15 cases (see supplemental Methods). Ionizing radiation was tested in 9 cases based on the availability of the irradiator and specimen size. Cells were incubated with 25A or 25B for 24 hours in 2 cases, 48 hours in 13 cases, and 72 hours in 3 cases. (B) Concentration-dependent apoptosis induction of in vivo clonogenic LLPCs obtained from spleens of xenografted NOD/SCID mice challenged with primary LLPCs from 11 patients with B-precursor ALL after treatment with 25A is shown. In each of the 11 cases, there were at least 1 untreated control (in 9 cases there also was a 25B-treated control) and at least 1 test sample treated with 25A. Cumulatively, 0 µg/mL was tested in 11, 3 µg/mL in 5, 10 µg/mL in 5, and 30 µg/mL in 11 cases (see supplemental Methods). Ionizing radiation was tested in 5 cases based on the availability of the irradiator and sufficient cell numbers. Xenograft cells were incubated with 25A or 25B for 24 hours in 1 case, 48 hours in 9 cases, and 72 hours in 1 case. As in (A), neither drug-free control NP formulation 25B nor 2 Gy γ-rays caused a significant degree of apoptosis. SEM, standard error of the mean.
Potency of the NLF of the SYK P-site inhibitor C61 against leukemia-initiating xenograft cells derived from patients with B-precursor ALL.
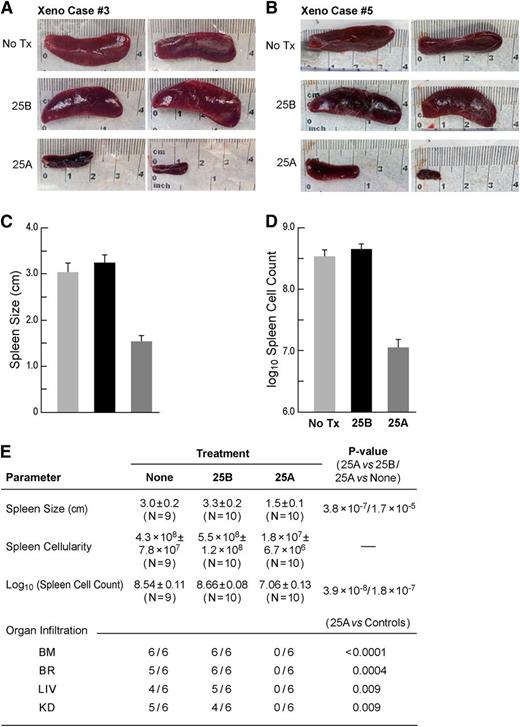
Immunophenotyping of xenograft cells by multiparameter flow cytometry confirmed the coexpression of multiple ALL-associated human lymphoid differentiation antigens, including high levels of CD10, CD19, and CD34 that have been reported as markers of putative leukemic stem cells in B-precursor ALL14-16 (supplemental Figure 10). While the majority of the xenograft ALL cells were CD10+CD19+CD34+, there was case-to-case variation in the composite immunophenotypes consistent with the immunophenotypic heterogeneity of pediatric B-precursor ALL. We next sought to determine if the C61-LNP formulation 25A is capable of destroying the leukemia-initiating in vivo clonogenic fraction in xenograft specimens in the 5 most-aggressive B-precursor ALL cases (xenograft case numbers 3, 4, 5/derived from a relapsed patient, 10/derived from a relapsed patient, and 11; supplemental Figure 10). Xenograft cells isolated from the spleens of leukemic NOD/SCID mice were treated with 25A (lipid concentration: 162 µg/mL; C61 concentration: 30 µg/mL), 25B (lipid concentration: 162 µg/mL; C61 concentration: 0 µg/mL), or left untreated for 48 hours at 37°C and then reinjected into NOD/SCID mice. Control mice challenged with untreated or 25B-treated xenograft cells invariably developed overt leukemia between 20 days and 123 days (median: 91 days) (Figure 2). Necropsy revealed massive splenomegaly at the time of death in each of 19 control mice (Figure 2A-C). The spleens of mice inoculated with untreated xenograft cells (N = 9) measured 3.0 ± 0.2 cm and contained an average of 4.3 × 108 cells. Likewise, the spleens measured 3.3 ± 0.2 cm and contained an average of 5.5 × 108 cells in mice inoculated with 25B-treated xenograft cells (N = 10). In contrast, the spleens measured only 1.5 ± 0.1 cm (P < 1 × 10−6) and contained 1.8 × 107 cells (P < 1 × 10−6) in mice inoculated with 25A-treated xenograft cells (N = 10) (Figure 2A-E). In 3 xenograft cases, multiple tissues of mice from the different treatment groups were examined for evidence of leukemic infiltration. Most important, all of the 12 mice receiving untreated (N = 6) or 25B-treated (N = 6) xenograft cells showed evidence of disseminated leukemia with leukemic infiltrates in multiple organs, including bone marrow, the brain, the liver, and the kidney (Figure 2E and supplemental Figure 11). By comparison, none of the 6 mice (2 mice per case × 3 cases) that were inoculated with 25A-treated xenograft cells had evidence of disseminated leukemia (P = .009-<.0001; Figure 2E). These findings provide direct experimental evidence that C61-LNP 25A severely damages the in vivo clonogenic fraction in xenograft cell populations derived from patients with aggressive B-precursor ALL and abrogates their ability to engraft and initiate leukemia in NOD/SCID mice.
Treatment with 25A destroys in vivo clonogenic B-precursor ALL xenograft cells. Xenograft cells isolated from the spleens of leukemic NOD/SCID mice were treated with 25A (lipid concentration: 162 µg/mL; C61 concentration: 30 µg/mL) or 25B (lipid concentration: 162 µg/mL; C61 concentration: 0 µg/mL), or were left untreated (Tx) for 48 hours at 37°C and then reinjected into NOD/SCID mice (2 mice per group per case, 10 mice per group for all 5 cases combined). The starting cell number was identical for each treatment condition, and all mice in a given xenograft case received the same volume of cells with the same starting cell number in the inoculum. The untreated control inoculation samples in the 5 cases contained 200 000 to 400 000 cells. Experiments were terminated by the euthanasia of all mice in all treatment groups as soon as control mice showed signs of morbidity. The actual times to termination in the 5 independent experiments were 20 days, 25 days, 91 days, 112 days, and 123 days after leukemic cell inoculation. In each of the 5 independent experiments, NOD/SCID mice that were challenged with untreated or 25B-treated xenograft cells rapidly developed overt leukemia with massive splenomegaly and multiorgan infiltration. By contrast, the spleens of mice that were challenged with 25A-treated xenograft cells were much smaller and no multiorgan involvement was observed in any of the 3 cases examined histopathologically. (A-B) depict the spleens of mice from 2 representative experiments. The spleen images were obtained using an iPhone 4S equipped with an 8-megapixel iSight camera (Apple, Cupertino, CA). The cumulative data are shown in (C-D). The statistical comparisons as well as organ infiltration data are shown in (E). BM, bone marrow; BR, brain; KD, kidneys; LIV, liver.
Treatment with 25A destroys in vivo clonogenic B-precursor ALL xenograft cells. Xenograft cells isolated from the spleens of leukemic NOD/SCID mice were treated with 25A (lipid concentration: 162 µg/mL; C61 concentration: 30 µg/mL) or 25B (lipid concentration: 162 µg/mL; C61 concentration: 0 µg/mL), or were left untreated (Tx) for 48 hours at 37°C and then reinjected into NOD/SCID mice (2 mice per group per case, 10 mice per group for all 5 cases combined). The starting cell number was identical for each treatment condition, and all mice in a given xenograft case received the same volume of cells with the same starting cell number in the inoculum. The untreated control inoculation samples in the 5 cases contained 200 000 to 400 000 cells. Experiments were terminated by the euthanasia of all mice in all treatment groups as soon as control mice showed signs of morbidity. The actual times to termination in the 5 independent experiments were 20 days, 25 days, 91 days, 112 days, and 123 days after leukemic cell inoculation. In each of the 5 independent experiments, NOD/SCID mice that were challenged with untreated or 25B-treated xenograft cells rapidly developed overt leukemia with massive splenomegaly and multiorgan infiltration. By contrast, the spleens of mice that were challenged with 25A-treated xenograft cells were much smaller and no multiorgan involvement was observed in any of the 3 cases examined histopathologically. (A-B) depict the spleens of mice from 2 representative experiments. The spleen images were obtained using an iPhone 4S equipped with an 8-megapixel iSight camera (Apple, Cupertino, CA). The cumulative data are shown in (C-D). The statistical comparisons as well as organ infiltration data are shown in (E). BM, bone marrow; BR, brain; KD, kidneys; LIV, liver.
In vivo pharmacodynamic features and antileukemic efficacy of the NLF of the SYK P-site inhibitor C61.
C61-LNP formulation 25A was overall very well tolerated by mice (supplemental Table 2) without any clinical or laboratory evidence of moderate to severe acute toxicity at cumulative dose levels ranging from 5 mg/kg to 500 mg/kg. At the highest dose level, histopathological examination revealed a few small clusters of inflammatory cells in the intestinal mucosa and a mild to moderate segmental hydropic degeneration/intracellular edema in the renal tubular epithelium of 2 of 5 mice (supplemental Figure 12). There were no toxic lesions suggestive of any significant parenchymal organ damage (supplemental Table 2). Likewise, C61-LNP was very well tolerated in rats at dose levels ranging from 10 mg/kg (mouse equivalent dose: 20 mg/kg) and 25 mg/kg (mouse equivalent dose: 50 mg/kg) (supplemental Table 3).
We next set out to study the pharmacokinetics (PK) of 25A in healthy BALB/c mice in an attempt to determine if effective antileukemic concentrations of this unique C61-LNP formulation can be achieved at nontoxic safe dose levels (Figure 3). The composite plasma concentration–time curve of total C61 after the IV injection of a nontoxic, 80-mg/kg (68-µmol/kg) bolus dose of 25A is shown in Figures 3A1 and A2. A single-compartment, first-order pharmacokinetic model was fit to the plasma concentration–time curves, and the calculated pharmacokinetic parameter values are shown in Figures 3 A1 and A2. 25A had a modest volume of distribution at the central compartment (VC) (125 mL/kg), which is only twice the plasma volume (∼50 mL/kg) and less than 20% of the total body water volume in mice (∼725 mL/kg). Consequently, concentrations of C61 in excess of 500 µg/mL, which is >150-fold higher than the concentrations required to cause apoptosis in primary leukemia cells, could be easily achieved at this dose level of 25A. The predicted maximum plasma concentration (Cmax) was 642 µg/mL, the elimination half-life 1.97 hours (elimination rate constant = 0.352 ± 0.036 hour−1), and the systemic exposure (area under the curve [AUC]) at this dose level was 1826 ± 141 µg/mL·h (Figure 3 A2). This PK profile of the unique C61-LNP formulation was substantially superior to that of C61-salt, which yielded >3-logs lower plasma concentrations of C61 (Figure 3A3). This is because of its very large VC of 75 758 mL/kg, which is 1515-fold larger than the plasma volume and 104-fold larger than the total body water volume in mice consistent with an extensive distribution of C61-salt into extravascular compartments. The C61-salt dose was half the dose for 25A, as 80 mg/kg C61-salt administered as an IV bolus caused seizures and sudden death. The predicted Cmax was only 528 ng/mL, elimination half-life 7.9 hours, and the AUC was 5.98 µg/mL·h, which is >300-fold less than what could be achieved with the C61-LNP formulation 25A (Figure 3A3). These PK parameters of C61-salt for BALB/c mice were similar to those previously reported for CD-1 mice.10
In vivo pharmacokinetics and antileukemic efficacy of 25A. (A1-A2) show the plasma concentration–time profile of the C61-LNP formulation 25A in BALB/c mice after a single IV bolus injection (dose: 80 mg/kg). (A1) depicts the time-dependent change of the plasma C61 concentration (log scale) over the first 3 hours after injection. (A2) depicts the time-dependent change of the plasma C61 concentration (linear scale) over a 48-hour time period after injection. (A3) shows the plasma concentration–time profile of C61-salt formulation in BALB/c mice after a single IV bolus injection (dose: 40 mg/kg). C61-salt was used as a control and administered as a 40-mg/kg IV bolus dose because an 80 mg/kg dose level caused seizures and sudden death. (B-C) Shown are EFS curves of NOD/SCID mice challenged with xenograft cells derived from primary LLPCs of 3 patients with B-precursor ALL. 25A treatments administered every other day for 3 days (2 cases) or 10 days (1 case) significantly improved the EFS outcome. In the control group of 34 mice that all developed fatal leukemia, the spleens measured 3.56 ± 0.09 cm (mean ± standard error [SE]) and contained 4.88 ± 0.44 × 108 cells (mean ± SE). The results for the control group were very similar in each of the 3 independent experiments performed: experiment 1: N = 7, 3.14 ± 0.4 cm/3.33 ± 0.73 × 108 cells; experiment 2: N = 15, 3.70 ± 0.05 cm/6.31 ± 0.79 × 108 cells; experiment 3: N = 12, 3.63 ± 0.05cm/4.00 ± 0.22 × 108 cells. 25A was well tolerated and did not cause any morbidity or mortality in any of the 26 NOD/SCID mice treated. Development of overt leukemia was significantly delayed by 25A treatments. Of the long-term surviving mice in the 25A-treatment group, 6 were electively euthanized on day 241 while in healthy condition. None of these 6 mice had splenomegaly, and their bone marrow, livers, kidneys, or brains did not contain any leukemic infiltrates. The average (mean ± SE) spleen size was 1.8 ± 0.1 cm, and the average spleen cell count was 7.2 ± 2.1 × 106 cells per spleen. The spleen size of the combined control group was significantly larger than the spleen size of the 25A-treated mice euthanized healthy on day 241 (difference = 1.7 cm, Student t test, unequal variances, T = 16.3, degrees of freedom = 32.1, P = 5.1 × 10−17), and their spleen cell count was significantly higher (log10 transformed difference = 1.8, Student t test, unequal variances, T = 12.4, degrees of freedom = 11.1, P = 8.1 × 10−8). t1/2, half-life.
In vivo pharmacokinetics and antileukemic efficacy of 25A. (A1-A2) show the plasma concentration–time profile of the C61-LNP formulation 25A in BALB/c mice after a single IV bolus injection (dose: 80 mg/kg). (A1) depicts the time-dependent change of the plasma C61 concentration (log scale) over the first 3 hours after injection. (A2) depicts the time-dependent change of the plasma C61 concentration (linear scale) over a 48-hour time period after injection. (A3) shows the plasma concentration–time profile of C61-salt formulation in BALB/c mice after a single IV bolus injection (dose: 40 mg/kg). C61-salt was used as a control and administered as a 40-mg/kg IV bolus dose because an 80 mg/kg dose level caused seizures and sudden death. (B-C) Shown are EFS curves of NOD/SCID mice challenged with xenograft cells derived from primary LLPCs of 3 patients with B-precursor ALL. 25A treatments administered every other day for 3 days (2 cases) or 10 days (1 case) significantly improved the EFS outcome. In the control group of 34 mice that all developed fatal leukemia, the spleens measured 3.56 ± 0.09 cm (mean ± standard error [SE]) and contained 4.88 ± 0.44 × 108 cells (mean ± SE). The results for the control group were very similar in each of the 3 independent experiments performed: experiment 1: N = 7, 3.14 ± 0.4 cm/3.33 ± 0.73 × 108 cells; experiment 2: N = 15, 3.70 ± 0.05 cm/6.31 ± 0.79 × 108 cells; experiment 3: N = 12, 3.63 ± 0.05cm/4.00 ± 0.22 × 108 cells. 25A was well tolerated and did not cause any morbidity or mortality in any of the 26 NOD/SCID mice treated. Development of overt leukemia was significantly delayed by 25A treatments. Of the long-term surviving mice in the 25A-treatment group, 6 were electively euthanized on day 241 while in healthy condition. None of these 6 mice had splenomegaly, and their bone marrow, livers, kidneys, or brains did not contain any leukemic infiltrates. The average (mean ± SE) spleen size was 1.8 ± 0.1 cm, and the average spleen cell count was 7.2 ± 2.1 × 106 cells per spleen. The spleen size of the combined control group was significantly larger than the spleen size of the 25A-treated mice euthanized healthy on day 241 (difference = 1.7 cm, Student t test, unequal variances, T = 16.3, degrees of freedom = 32.1, P = 5.1 × 10−17), and their spleen cell count was significantly higher (log10 transformed difference = 1.8, Student t test, unequal variances, T = 12.4, degrees of freedom = 11.1, P = 8.1 × 10−8). t1/2, half-life.
We next examined the in vivo antileukemic activity of 25A in 3 separate xenograft models derived from the primary leukemia cells of 2 pediatric patients with relapsed B-precursor ALL (xenograft case numbers 5 and 10) and 1 pediatric patient with newly diagnosed B-precursor ALL (xenograft case number 3). All 34 control mice challenged with an IV inoculum of xenograft cells (1 × 106 cells per mouse from xenograft case number 5; 2 × 106 cells per mouse from xenograft case numbers 3 and 10) isolated from the spleens of leukemic NOD/SCID mice that had been inoculated with patients’ primary leukemia cells and then left untreated (N = 9), treated with 5% dextrose in water (N = 12), or treated with the drug-free control LNP formulation 25B (N = 13) either died or were killed in moribund condition due to their advanced leukemia within 120 days, with a median event-free survival (EFS) time of only 50 days (Figure 3C). In contrast, the median EFS time was prolonged to 226 days for the 26 test mice that were treated every other day with IV injections of C61-LNP formulation 25A (80 mg/kg every other day × 3 doses [xenograft case numbers 5 and 10] or 10 doses [xenograft case number 3]) starting on the day of leukemic cell inoculation. Thus, the 25A treatment regimen resulted in a significant improvement of the EFS outcome in NOD/SCID mice challenged with an invariably fatal dose of patient-derived ALL xenograft cells (P value < .0001) (Figure 3C). We next set out to determine if the cells that caused leukemia in 25A-treated mice vs 25B-treated mice differed in their SYK expression levels. Leukemia cells isolated from the spleens of 25A- as well as 25B-treated mice had abundant SYK expression with no evidence of a reduced SYK expression in leukemia cells from 25A-treated mice (supplemental Figure 13). Thus, the cause of leukemia in 25A-treated mice did not appear to be the selection of a SYK-negative leukemic B-cell precursor clone.
Discussion
The experiments presented herein demonstrate the clinical potential of targeting the SYK-dependent antiapoptotic blast cell survival machinery of B-precursor ALL cells with C61-LNP 25A, a unique nanoscale pharmaceutical composition. 25A (1) exhibited a favorable pharmacokinetic and safety profile in mice, (2) induced apoptosis in primary B-precursor ALL blast cells taken directly from patients as well as B-precursor ALL xenograft cells, (3) killed in vivo clonogenic B-precursor ALL xenograft cells capable of causing overt leukemia in NOD/SCID mice, and (4) exhibited at nontoxic dose levels potent in vivo antileukemic activity against patient-derived ALL cells in xenograft models of aggressive B-precursor ALL. Our study provides the preclinical proof of concept for the use of an NLF of a SYK inhibitor as an antileukemic agent against B-precursor ALL.
Because of the similarities of the adenosine triphosphate (ATP) pocket structures among different kinases, most inhibitors of the ATP binding sites of tyrosine kinases, including SYK inhibitors, affect multiple tyrosine kinases and have off-target activities.17-20 Unlike available inhibitors of SYK targeting the ATP binding site, such as R788, C61 targets the tyrosine kinase substrate binding site of SYK.9 It thereby provides a unique opportunity to selectively target the SYK-dependent antiapoptotic blast cell survival machinery that is controlled by the tyrosine kinase activity of SYK, especially via tyrosine phosphorylation events leading to activation of STAT3 and phosphatidylinositol 3-kinase.8 Inhibitors, such as C-61, that target the substrate binding sites of tyrosine kinases are hoped to have enhanced specificity and potency.21,22 The availability of both ATP binding site inhibitors (eg, R788 and its active form R-406) and P-site inhibitors (eg, C61) of SYK may be helpful in patients with leukemia who have SYK mutations that might influence inhibitor binding to ATP and/or substrate binding sites.18,19
We previously reported that infant pro-B ALL is characterized by defective SYK expression23 and C61 does not cause apoptosis in SYK-negative infant pro-B ALL cells.10 Therefore, we were interested in knowing if delayed leukemia in 25A-treated mice was caused by SYK-negative leukemic subclones. The cause for the development of overt leukemia despite treatment with C61-LNP did not appear to be the selection and outgrowth of a SYK-negative clone. Nevertheless, it is possible that we might observe such a phenomenon when we encounter ALL cases with more heterogeneous populations of leukemic cells that may include SYK-negative clonogenic cells.
The treatment schedule for C61-LNP remains to be optimized, and its effects on the antileukemic potency of standard chemotherapy regimens remain to be studied. Based on the PK profile of this formulation and its favorable safety profile of 25A, a 4-week daily administration schedule will also be considered in our future investigational new drug–enabling preclinical studies. C61 was previously shown to be capable of causing apoptosis in primary leukemia cells from relapsed patients with B-precursor ALL who are resistant to multiple standard chemotherapy drugs, including vincristine, dexamethasone, doxorubicin, vinorelbine, methotrexate, cytarabine, fludarabine, etoposide, and 2-CdA.10 The lack of crossresistance to C61 in chemotherapy-resistant leukemic cells provides the opportunity to explore new combination strategies with several different classes of chemotherapy drugs. Further development of the liposomal C61 formulation, reported here, or other selective SYK inhibitors as part of reinduction or consolidation regimens may provide the basis for new and more effective treatment strategies against therapy-refractory or relapsed B-precursor ALL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ms Catherine Yao (University of Illinois at Urbana-Champaign) for preparation of the cartoon image of 25A. The authors thank Drs Stuart Siegel and Dorothea E. Myers for a critical review of the manuscript. The authors further thank Mrs Parvin Izadi of the Children's Hospital, Los Angeles Bone Marrow Laboratory, Mrs Tsen-Yin Lin of the CHLA fluorescence activated cell sorting Core, Dr Nickolas Chelyapov of the University of Southern California NanoBiophysics Core Facility, as well as Ernesto Barron and Anthony Rodriguez of the USC Norris Comprehensive Cancer Center Cell and Tissue Imaging Core for their assistance.
This work was supported in part by Department of Health and Human Services grants (P30CA014089, U01-CA-151837, R01CA-154471, and R21-CA-164098) (F.M.U.) from the National Cancer Institute, and the National Institute of Health’s Director’s New Innovator Award (1DP2OD007246) (J.C.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. This work was also supported in part by a 2011 V Foundation Translational Research Award (F.M.U., P.G., A.T.), Nautica Triathlon and its producer Michael Epstein (F.M.U.), Ronald McDonald House Charities of Southern California (F.M.U.), Couples Against Leukemia Foundation (F.M.U.), a William Lawrence & Blanche Hughes Foundation grant (F.M.U.), 2011 and 2012 Saban Research Institute Merit Awards (F.M.U.), and the Turkish Academy of Sciences (K.S.).
Authorship
Contribution: S.Y. designed, prepared, and partially characterized the physicochemical characteristics of C61-loaded LNP formulation 25A, conceived the scale-up of LNP preparation for preclinical experiments, and performed the C61 measurements on blood samples from mice analyzed in the PK experiments; S.Q. performed the statistical analyses and PK parameter determinations; I.C. and A.S. performed multiple experiments and collected data; K.S. and I.O. performed the toxicity evaluations in mice and rats; Q.Y. and J.C. performed the ζ potential measurements on the C61-LNP; P.G. and A.T. evaluated pediatric leukemia patients for study eligibility and obtained bone marrow/blood specimens; and F.M.U. was the NIH-funded principal investigator who designed, directed, and supervised this study and wrote the final manuscript. All authors have made significant and substantive contributions to the study. All authors reviewed and revised the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Fatih M. Uckun, Children’s Hospital Los Angeles, MS#160, Los Angeles, CA 90027-0367; e-mail: uckun@usc.edu; and Seang Yiv, Children’s Hospital Los Angeles, MS#160, Los Angeles, CA 90027-0367; e-mail: syiv@chla.usc.edu.

![Figure 3. In vivo pharmacokinetics and antileukemic efficacy of 25A. (A1-A2) show the plasma concentration–time profile of the C61-LNP formulation 25A in BALB/c mice after a single IV bolus injection (dose: 80 mg/kg). (A1) depicts the time-dependent change of the plasma C61 concentration (log scale) over the first 3 hours after injection. (A2) depicts the time-dependent change of the plasma C61 concentration (linear scale) over a 48-hour time period after injection. (A3) shows the plasma concentration–time profile of C61-salt formulation in BALB/c mice after a single IV bolus injection (dose: 40 mg/kg). C61-salt was used as a control and administered as a 40-mg/kg IV bolus dose because an 80 mg/kg dose level caused seizures and sudden death. (B-C) Shown are EFS curves of NOD/SCID mice challenged with xenograft cells derived from primary LLPCs of 3 patients with B-precursor ALL. 25A treatments administered every other day for 3 days (2 cases) or 10 days (1 case) significantly improved the EFS outcome. In the control group of 34 mice that all developed fatal leukemia, the spleens measured 3.56 ± 0.09 cm (mean ± standard error [SE]) and contained 4.88 ± 0.44 × 108 cells (mean ± SE). The results for the control group were very similar in each of the 3 independent experiments performed: experiment 1: N = 7, 3.14 ± 0.4 cm/3.33 ± 0.73 × 108 cells; experiment 2: N = 15, 3.70 ± 0.05 cm/6.31 ± 0.79 × 108 cells; experiment 3: N = 12, 3.63 ± 0.05cm/4.00 ± 0.22 × 108 cells. 25A was well tolerated and did not cause any morbidity or mortality in any of the 26 NOD/SCID mice treated. Development of overt leukemia was significantly delayed by 25A treatments. Of the long-term surviving mice in the 25A-treatment group, 6 were electively euthanized on day 241 while in healthy condition. None of these 6 mice had splenomegaly, and their bone marrow, livers, kidneys, or brains did not contain any leukemic infiltrates. The average (mean ± SE) spleen size was 1.8 ± 0.1 cm, and the average spleen cell count was 7.2 ± 2.1 × 106 cells per spleen. The spleen size of the combined control group was significantly larger than the spleen size of the 25A-treated mice euthanized healthy on day 241 (difference = 1.7 cm, Student t test, unequal variances, T = 16.3, degrees of freedom = 32.1, P = 5.1 × 10−17), and their spleen cell count was significantly higher (log10 transformed difference = 1.8, Student t test, unequal variances, T = 12.4, degrees of freedom = 11.1, P = 8.1 × 10−8). t1/2, half-life.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/21/10.1182_blood-2012-11-470633/4/m_4348f3.jpeg?Expires=1769151315&Signature=Ao9IKrkodrQ8dBPwzqGs0h3n7DzVPpHIfQ8QrwuVp3fDdihjJwAoXXNPqmAbQiPSKt25WMW9OhLbdUPZdnawqYKN48tEnKypMoe2qOjSjAQ-7oqgQPj1nxAP~xBkrZWyYgOTz4dc0r5c0avM7JsxRNXIpaesNV6e-pG09VrI7yvILJgCP~NncFhqjLoQcUUk8UH4GkBTm2Zghd7VlTSymSUzquLvkzo~5ljWZBJmC6HOn93rTZtIXktggmBbaRBEEsZnCdJVPIvUTScvwR8UZT4p65ZNHwm1ak08yeDlCAX66VnAlMgd2cQr3L~ylmIlPJWlNy6xeW5G6BICJlarIA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)