Key Points
Clonal chromosomal abnormalities in Ph− metaphases not including –Y predict decreased FFS, EFS, TFS, and OS in patients with CML.
They affect TFS independently of baseline variables, although this is abrogated by inclusion of early response in multivariate models.
Abstract
Clonal chromosomal abnormalities in Philadelphia chromosome-negative (CCA/Ph−) metaphases emerge as patients with chronic phase chronic myeloid leukemia (CP-CML) are treated with tyrosine kinase inhibitors (TKIs). We assessed the characteristics and prognostic impact of 598 patients with CP-CML treated on clinical trials with various TKIs. CCA/Ph− occurred in 58 patients (10%); the most common were −Y in 25 (43%) and trisomy 8 in 7 patients (12%). Response to TKI therapy was similar for patients with CCA/Ph− and those without additional chromosomal abnormalities (ACAs). We further categorized CCA/Ph− into those in which –Y was the only clonal abnormality, and all others. We found that patients with non –Y CCA/Ph− had worse failure-free survival (FFS), event-free survival (EFS), transformation-free survival (TFS), and overall survival (OS) compared with those without ACAs with the following 5-year rates: FFS (52% vs 70%, P = .02), EFS (68% vs 86%, P = .02), TFS (76% vs 94%, P < .01), and OS (79% vs 94%, P = .03). In a multivariate analysis, non –Y CCA/Ph− increased the risk of transformation or death when baseline characteristics were considered with a hazard ratio of 2.81 (95% confidence interval, 1.15-6.89; P = .02). However, this prognostic impact was not statistically significant when achieving BCR-ABL <10% at 3 months was included in the analysis. In conclusion, non –Y CCA/Ph− are associated with decreased survival when emerging in patients with chronic-phase CML across various TKIs. This trial was registered at www.clinicaltrials.gov as #NCT00048672, #NCT00038649, and #NCT00050531 (imatinib); #NCT00254423 (dasatinib); #NCT00129740 (nilotinib); and NCT01570868 (ponatinib).
Introduction
Chronic myeloid leukemia (CML) originates from a neoplastic clonal proliferation of a pluripotent hematopoietic stem cells.1 It is driven by the fusion of the Abelson oncogene (ABL) with the breakpoint cluster region (BCR) most commonly manifested as a translocation t(9;22)(q34.1;q11.2) or Philadelphia chromosome (Ph).2,3 The successful use of tyrosine kinase inhibitors (TKIs) targeting the BCR-ABL fusion oncogene has reshaped the clinical and therapeutic landscape of CML, turning it from a life-threatening disease into a chronic condition 4-7 ; thus, it is estimated that the prevalence of CML will double in 20 years.8 During the course of treatment, some patients develop other chromosomal abnormalities in addition to Ph. Appearance of clonal chromosomal abnormalities in the Ph+ cells (CCA/Ph+), known as cytogenetic clonal evolution, is considered a marker of disease progression.9 In some patients, additional chromosomal abnormalities emerge in the Ph− metaphases as patients respond to treatment.10,11 The significance of these clonal chromosomal abnormalities in Ph− (CCA/Ph−) cells remains unclear. The National Comprehensive Cancer Network guidelines and the European LeukemiaNet recommendations suggest that the overall prognosis of CCA/Ph− seems to be good and dependent on response to imatinib, with the exception of chromosome 7 abnormalities.12,13 We and others have reported cases in which chromosome 7 abnormalities lead to the emergence of a new malignant clone resulting in myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML).14,15 In addition, we have previously reported the adverse prognostic impact of CCA/Ph− in patients treated with imatinib 14 ; however, there is very limited information on the occurrence and significance of this phenomenon among patients treated with second-generation TKI. We conducted a retrospective analysis of patients treated on prospective, single-institution frontline trials of first-, second-, or third-generation TKI to further characterize those abnormalities and determine their clinical impact.
Methods
Patients and treatments
Between July 2000 and September 2014, 598 adult patients with previously untreated chronic phase chronic myeloid leukemia (CP-CML) were treated with frontline TKI on prospective clinical trials at our institution. All patients signed an informed consent form for clinical trial participation, and all trials were approved by the institutional review board of The University of Texas MD Anderson Cancer Center. The eligibility criteria for these studies have been previously reported.16-19 A total of 279 patients (46%) were treated with imatinib (72 patients at 400 mg daily and 207 at 800 mg daily), 126 (21%) with nilotinib, 143 (23%) with dasatinib, and 50 (8%) with ponatinib. Of note, patients with accelerated-phase (AP) criteria at the time of diagnosis were eligible for the nilotinib trial and were excluded from this analysis. Patients with clonal chromosomal abnormalities in the Ph+ metaphases at diagnosis were also excluded (Figure 1; supplemental Table 12, available on the Blood Web site).
Flowchart outlining the selection of cases in this study. ACAs, additional chromosomal abnormalities.
Flowchart outlining the selection of cases in this study. ACAs, additional chromosomal abnormalities.
Cytogenetic analysis
Conventional cytogenetic analysis was done in bone marrow cells at baseline, every 3 months during the first year, and then every 6 to 12 months. Cytogenetic studies were performed with the standard G-banding technique at MD Anderson Cancer Center’s cytogenetic laboratory. Real-time polymerase chain reaction for BCR-ABL was performed at baseline and every 3 months for the first year and every 6 months thereafter.
Karyotypes were interpreted with the International System for Human Cytogenetic Nomenclature.20 Evaluation required a complete analysis of at least 20 metaphases with good-quality banding. Clonal ACAs were identified as abnormalities present in ≥2/20 metaphases or if the abnormalities were present in 1 metaphase in ≥2 assessments.
Response and outcome definitions
Hematologic and cytogenetic response criteria were as previously described.21 Molecular responses were defined as following: major molecular response (MMR) defined as a BCR-ABL1/ABL1 ratio ≤0.1% by international scale22 and molecular response with a 4.5-log reduction (MR4.5) as a BCR-ABL1/ABL1 ratio ≤0.0032% by international scale. Early response to therapy was determined by assessing BCR-ABL1/ABL1 ratio <10% at 3 months.
Event-free survival (EFS) was measured from the start of treatment to the date of any of the following events while on therapy: loss of complete hematologic remission, loss of major cytogenetic response (MCyR), progression to accelerated (defined as blasts ≥15%, blasts + promyelocytes ≥30%, basophils ≥20%, platelets <100 × 109/L, unrelated to therapy, or cytogenetic clonal evolution), blast phase (defined as blasts ≥30% or extramedullary disease), or death from any cause at any time while on study.23 Transformation-free survival (TFS) was measured from the start of therapy to the date of transformation to AP (including acquisition of CCA/Ph+) or blast phase while on therapy or deaths on study (progression to AML/MDS was not considered an event for TFS). Failure-free survival (FFS) was measured from the start of treatment to the date of any of the events defined in EFS with the addition of treatment discontinuation for any other reason as an event. Overall survival (OS) was measured from the time treatment was started to the date of death from any cause at any time or date of last follow-up.
Statistical analysis
The differences between variables were analyzed by the chi-square test and the Kruskal-Wallis test for categorical and continuous variables. Survival probabilities were estimated by the Kaplan-Meier method and the log-rank test was used for comparisons. Univariate and multivariate analyses were performed to identify whether presence of CCA/Ph− can predict for survival outcomes. Age, gender, baseline laboratory variables, Sokal score, BCR-ABL transcript type, type of TKI, and cytogenetic categories were all included in the univariate analysis. Achieving BCR-ABL <10% at 3 months was later added to the model as a marker of early response. Variables with P ≤ .05 in the univariate analysis were entered into a multivariate model and analyzed using the Cox proportional hazard regression. A reduced multivariate model was also performed using the backward elimination method (supplemental Tables 1-12 and Figures 1-4). Computations were performed using Stata/SE, version 14.1 (Stata Corp. LP, College Station, TX), and GraphPad Prism (version 6.07) statistical programs.
Results
Description of the chromosomal abnormalities
Among the 598 evaluable patients with CP-CML, 108 (18%) had ACAs appearing in the Ph− metaphases after cytogenetic response to TKIs. Of these patients, 58 (10%) had CCA/Ph− (Figure 1; supplemental Table 1 for list of CCA/Ph−; supplemental Table 10 for nonclonal ACAs). The incidence and type of ACAs in the Ph− metaphases were mostly similar across TKI groups, albeit with a slightly higher incidence trend in the nilotinib and ponatinib groups: among the 279 patients who received imatinib, 46 had ACAs (16%), compared with 20 of 143 patients on dasatinib (14%, P = .3), 31 of 126 on nilotinib (25%, P = .07), and 11 of 50 on ponatinib (22%, P = .4). Four patients had ACAs in the Ph+ metaphases at diagnosis and later developed other ACAs in Ph− metaphases. Those patients had no other criteria for accelerated phase and were excluded from the outcomes analysis (Figure 1).
Most ACAs appeared within the first year of the start of the TKI (median, 6 months; range, 3-78 months); first occurrence after 12 months of therapy happened in only 23 patients. CCA/Ph− was transient and detected in only ≤2 time points in 36 of the 58 cases (62%); this persisted >4 time points in 11/58 cases (20%) (Figure 2A,C). The median number of Ph− metaphases containing CCA/Ph− per analysis was 5/20 (range, 1 to 20) with an average of 7/20 (Figure 2B). The most commonly detected CCA/Ph− was loss of the Y chromosome in 25 patients (43%) and trisomy 8 in 7 patients (12%). There were 23 patients (39%) with loss of chromosome Y as the sole CCA/Ph−. In 2 other cases (3%), loss of Y was detected in addition to another clonal ACA. CCA/Ph− consistent with a complex karyotype defined as ≥3 chromosomal abnormalities was detected in 4 cases (7%) (Figure 2C). Various translocations, not including t(9;22) or t(v;22), were detected in 10 cases (17%). These translocations were seen in only 1 metaphase and were included in the analysis when associated with other ACAs. Only 2 patients had translocations that were detected in ≥2 metaphases: t(5;19)(p15.2;q10) and t(7;14)(p15;q22) (supplemental Table 1). One patient had an additional chromosomal abnormality in the Ph+ metaphase, which was the same CCA/Ph− that had previously appeared after initial response to TKI; this was a complex karyotype: 45,XY, add(1)(p36.1), add(3)(q21), add(9)(q34), der(13;21)(q10;q10), add22 (q11.2) [12]/46,XY [8].
Landscape of CCA/Ph− in patients with chronic-phase chronic myeloid leukemia. (A) Distribution of CCA/Ph− according to the year after treatment start it is first detected. (B) Number of times CCA/Ph− is detected in independent cytogenetic analyses per patient, done every 3 months during the first year, then every 6 to 12 months while on study. (C) Maximum number of metaphases containing CCA/Ph− in a cytogenetic analysis per patient. (D) Distribution of CCA/Ph− by chromosome. Each row represents the chromosomes numbered from 1 to 22 with additional rows for the sex chromosomes and a row labeled “C” for patients with a complex karyotype defined as ≥3 chromosomal abnormalities in 1 metaphase. Each column represents a patient with CCA/Ph−. Red. deletion or loss; blue. gain; D, dasatinib; I, imatinib; N, nilotinib; P, ponatinib. Some of the CCA/Ph− metaphases were translocations not shown in this figure. Additional details are provided in supplemental Table 1.
Landscape of CCA/Ph− in patients with chronic-phase chronic myeloid leukemia. (A) Distribution of CCA/Ph− according to the year after treatment start it is first detected. (B) Number of times CCA/Ph− is detected in independent cytogenetic analyses per patient, done every 3 months during the first year, then every 6 to 12 months while on study. (C) Maximum number of metaphases containing CCA/Ph− in a cytogenetic analysis per patient. (D) Distribution of CCA/Ph− by chromosome. Each row represents the chromosomes numbered from 1 to 22 with additional rows for the sex chromosomes and a row labeled “C” for patients with a complex karyotype defined as ≥3 chromosomal abnormalities in 1 metaphase. Each column represents a patient with CCA/Ph−. Red. deletion or loss; blue. gain; D, dasatinib; I, imatinib; N, nilotinib; P, ponatinib. Some of the CCA/Ph− metaphases were translocations not shown in this figure. Additional details are provided in supplemental Table 1.
Clinical and hematologic presentation
Baseline characteristics are summarized in Table 1. Patients who developed CCA/Ph− were significantly older than those without ACAs (median age, 59 vs 47; P < .001). Specifically, patients with –Y CCA/Ph− were the oldest (median age, 64; P < .001), followed by those with non −Y CCA/Ph− (median age, 52; P = .003) when compared with those without ACAs (median age, 47). The CCA/Ph− group had more men than women compared with the group without ACAs (81% vs 57% males; P < .001), given that loss of Y was a common abnormality. Other baseline characteristics were mostly similar between the 2 groups, but the group without ACAs tended to have a higher proportion of patients with a low Sokal score (70% vs 50%, P = .01), in part because of to the comparative younger age of this group. The distribution of patients with and without CCA/Ph− according to transcript type (e13a2 and e14a2) was mostly the same. Similarly, there was no statistical difference when comparing the distribution of patients between the 2 groups according to the TKI used.
Baseline characteristics of patients with CP-CML achieving cytogenetic response after TKI
| Characteristic . | No ACAs (n = 473) . | CCA/Ph−(n = 54) . | P . |
|---|---|---|---|
| Median age, y (range) | 47 (15-86) | 59 (25-82) | <.001 |
| Female, no. (%) | 205 (43) | 10 (19) | <.001 |
| Male, no. (%) | 268 (57) | 44 (81) | |
| WBC, median ×109/L (range) | 28 (0.8-342) | 37 (2.5-277) | .71 |
| Hb, median g/L (range) | 12.2 (6.2-16.7) | 12.4 (8.5-16.7) | .22 |
| Platelets, median ×109/L (range) | 331 (28-2928) | 340 (73-1540) | .55 |
| Basophils, median % (range) | 3 (0-19) | 2 (0-16) | .51 |
| Blasts in BM, median % (range) | 2 (0-14) | 1 (0-8) | .89 |
| Type of BCR-ABL transcript, no. (%) | .83 | ||
| e13a2 | 195 (41) | 22 (41) | |
| e14a2 | 189 (40) | 21 (39) | |
| e13a2 and e14a2 | 84 (18) | 10 (18) | |
| Other | 5 (1) | 1 (2) | |
| Sokal score, no. (%) | .01 | ||
| Low | 330 (70) | 27 (50) | |
| Intermediate | 112 (24) | 20 (37) | |
| High | 31 (6) | 7 (13) | |
| Treatment, no. (%) | .04 | ||
| Imatinib 400 mg | 51 (11) | 12 (22) | |
| Imatinib 800 mg | 173 (37) | 15 (28) | |
| Dasatinib | 118 (25) | 8 (15) | |
| Nilotinib | 92 (19) | 15 (28) | |
| Ponatinib | 39 (8) | 4 (7) |
| Characteristic . | No ACAs (n = 473) . | CCA/Ph−(n = 54) . | P . |
|---|---|---|---|
| Median age, y (range) | 47 (15-86) | 59 (25-82) | <.001 |
| Female, no. (%) | 205 (43) | 10 (19) | <.001 |
| Male, no. (%) | 268 (57) | 44 (81) | |
| WBC, median ×109/L (range) | 28 (0.8-342) | 37 (2.5-277) | .71 |
| Hb, median g/L (range) | 12.2 (6.2-16.7) | 12.4 (8.5-16.7) | .22 |
| Platelets, median ×109/L (range) | 331 (28-2928) | 340 (73-1540) | .55 |
| Basophils, median % (range) | 3 (0-19) | 2 (0-16) | .51 |
| Blasts in BM, median % (range) | 2 (0-14) | 1 (0-8) | .89 |
| Type of BCR-ABL transcript, no. (%) | .83 | ||
| e13a2 | 195 (41) | 22 (41) | |
| e14a2 | 189 (40) | 21 (39) | |
| e13a2 and e14a2 | 84 (18) | 10 (18) | |
| Other | 5 (1) | 1 (2) | |
| Sokal score, no. (%) | .01 | ||
| Low | 330 (70) | 27 (50) | |
| Intermediate | 112 (24) | 20 (37) | |
| High | 31 (6) | 7 (13) | |
| Treatment, no. (%) | .04 | ||
| Imatinib 400 mg | 51 (11) | 12 (22) | |
| Imatinib 800 mg | 173 (37) | 15 (28) | |
| Dasatinib | 118 (25) | 8 (15) | |
| Nilotinib | 92 (19) | 15 (28) | |
| Ponatinib | 39 (8) | 4 (7) |
BM, bone marrow; Hb, hemoglobin; WBC, white blood cell count. Other types of BCR-ABL included e1a2, e13a3 and 1 patient with an unknown transcript type.
Cytogenetic and molecular responses
By definition, CCA/Ph− can be identified only when patients with CML achieve a cytogenetic response. However, considering the high overall cytogenetic response rate with TKI in this setting, all patients assigned to the corresponding cohorts (no ACAs, n = 473; CCA/Ph−, n = 54) were considered evaluable for response in an intention to treat analysis. The cumulative cytogenetic and molecular response rates comparing the CCA/Ph− group with the control group without ACAs were mostly similar (Table 2). Cumulative response rates according to presence or absence of CCA/Ph− were as follows: MCyR for CCA/Ph− was 98% and 93% for those without ACAs (P = .15) and complete cytogenetic response (CCyR) rate was 87% and 91%, respectively (P = .38). Cumulative molecular response rates were not significantly different (CCA/Ph− vs no ACAs; MMR 78% vs 85%, P = .16; MR4.5 70% vs 68%, P = .73). However, the time to response (TTR) for MCyR was shorter for patients without ACAs compared with those with CCA/Ph− (median, TTR 2.9 [range, 2-15 months] vs 3.0 [range, 3-24 months], respectively; P = .01). The median times to achieve CCyR, MMR, and MR4.5 were also somewhat longer in the CCA/Ph− group compared with the group without ACAs but not statistically significant. We also analyzed the rate of early molecular response (ie, BCR-ABL <10% at 3 months).24,25 In the CCA/Ph− group, 74% had a BCR-ABL <10% at 3 months compared with 81% of those without ACAs (P = .21). However, among patients with CCA/Ph−, those with non −Y abnormalities tended to have a rate of BCR-ABL <10% at 3 months (68% vs 81%, P = .06) compared with those with –Y who had a rate almost similar to patients without ACAs (83% vs 81%, P = .8).
Responses of patients with CP-CML achieving cytogenetic response after TKI
| Cumulative response . | No ACAs (n = 473) . | CCA/Ph−(n = 54) . | P* . | ||
|---|---|---|---|---|---|
| N (%) . | Median TTR, months (range)† . | N (%) . | Median TTR, months (range)† . | ||
| MCyR | 441 (93) | 2.9 (2-15)† | 53 (98) | 3.0 (3-24)† | .15 |
| CCyR | 429 (91) | 3.1 (2-36) | 47 (87) | 3.1 (3-31) | .38 |
| MMR | 402 (85) | 5.7 (2-74) | 42 (78) | 5.8 (3-76) | .16 |
| MR4.5 | 322 (68) | 11.8 (3-145) | 38 (70) | 12.1 (3-136) | .73 |
| BCR-ABL <10% at 3 mo | 384 (81) | — | 40 (74) | — | .21 |
| Cumulative response . | No ACAs (n = 473) . | CCA/Ph−(n = 54) . | P* . | ||
|---|---|---|---|---|---|
| N (%) . | Median TTR, months (range)† . | N (%) . | Median TTR, months (range)† . | ||
| MCyR | 441 (93) | 2.9 (2-15)† | 53 (98) | 3.0 (3-24)† | .15 |
| CCyR | 429 (91) | 3.1 (2-36) | 47 (87) | 3.1 (3-31) | .38 |
| MMR | 402 (85) | 5.7 (2-74) | 42 (78) | 5.8 (3-76) | .16 |
| MR4.5 | 322 (68) | 11.8 (3-145) | 38 (70) | 12.1 (3-136) | .73 |
| BCR-ABL <10% at 3 mo | 384 (81) | — | 40 (74) | — | .21 |
P values shown are the results of comparing the corresponding response rates.
The only statistically significant difference in TTR was for MCyR with P = .01; the rest, not shown, were not significant.
Survival
The median follow-up for survivors in this cohort was 7.6 years (range, 0.2-14.7 years). Survival rates were generally lower in the CCA/Ph− cohort compared with those without ACAs (supplemental Figure 1). There was a nonstatistically significant trend for inferior FFS and EFS for the CCA/Ph− patients compared with the control group. The corresponding 5-year FFS rates were 62% vs 69% (P = .34), and 5-year EFS rates were 74% and 86% (P = .09), respectively. However, patients with CCA/Ph− had worse TFS and OS rates compared with patients without ACAs (supplemental Figure 1). The 5-year TFS rates were 81% vs 94% (P = .03); the 5-year OS rates were 79% and 94% (P = .02), respectively, with the hazard ratio (HR) for risk of death in patients with CCA/Ph− of 2.06 (95% confidence interval [CI], 1.1-5.9; P = .02). In contrast, survival for nonclonal ACAs appearing in the Ph− cells was similar to the control group without ACAs (P values using log-rank test for FFS, EFS, TFS, and OS were 0.57, 0.32, 0.98, and 0.77, respectively).
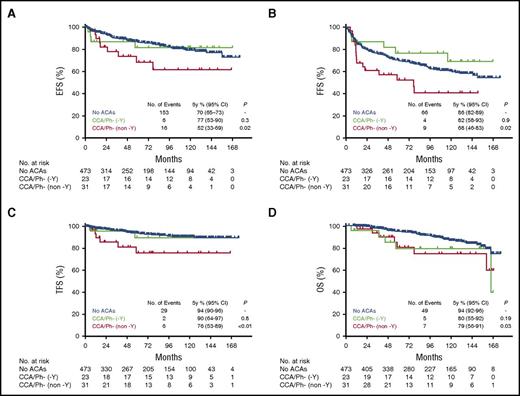
Given that loss of chromosome Y had been associated with a good prognosis among cytogenetic abnormalities constituting clonal evolution,21,26 CCA/Ph− were further categorized between patients with and without −Y as the only clonal abnormality. Patients with non −Y CCA/Ph− had the worst comparative outcomes (Figure 3). The 5-year FFS rate for patient with −Y as the only CCA/Ph− was 77% compared with 70% for patients without ACAs (P = .3), whereas patients with non -Y CCA/Ph− had a significantly inferior 5-year FFS rate of 52% (P = .02) (Figure 3A). Similarly the 5-year EFS was the worst for patients with non −Y CCA/Ph− compared with patients without ACAs (68% vs 86%, respectively; P = .02) (Figure 3B). There was a nonstatistically significant trend for inferior TFS for patients with –Y as the only CCA/Ph− compared with patients without ACAs with a 5-year TFS rate of 90% vs 94% (P = .8). Those patients also tended to have worse OS compared with patients without ACAs with a 5-year OS rate of 80% vs 94% (P = .19) (Figure 3B). However, patients with non −Y abnormalities or other abnormalities in addition to loss of chromosome Y had worse TFS and OS compared with patients without ACAs with 5-year TFS and OS rates of 76% vs 94% (P < .01) and 79% vs 94% (P = .03), respectively (Figure 3C-D). Among patients without ACAs, 15 progressed to AP or blast phase CML vs 4 among those with CCA/Ph−. None of the patients with CCA/Ph+ at diagnosis progressed. Accounting for death at any time as a competing risk, the cumulative incidence of transformation in patients with non –Y CCA/Ph− was significantly higher when compared with patients without ACAs with a 5-year rate of 13% (95% CI 4 27) vs 3% (95% CI, 2-4) respectively (Gray’s test: P < .01) (supplemental Figure 2). The HR for the risk of death in patients with non –Y CCA/Ph− was 2.2 (95% CI, 1.0 −5.0, P = .03), almost similar to the risk of death seen in patients who develop CCA/Ph+ with a HR of 2.9 (95% CI, 1.8-22.3, P = .004) when both are compared with patients without ACAs (supplemental Table 11; supplemental Figure 3).
Survival of patients with chronic phase chronic myeloid leukemia by presence and type of CCA/Ph−metaphases. (A) FFS. (B) EFS. (C) TFS. (D) OS. P value is determined by comparing survival of patients with CCA/Ph− with those without ACAs using the log-rank test. −Y, loss of chromosome Y.
Survival of patients with chronic phase chronic myeloid leukemia by presence and type of CCA/Ph−metaphases. (A) FFS. (B) EFS. (C) TFS. (D) OS. P value is determined by comparing survival of patients with CCA/Ph− with those without ACAs using the log-rank test. −Y, loss of chromosome Y.
Patients were stratified by each type of CCA/Ph−; however, the relatively small numbers in each group precluded meaningful conclusions. Survival was the worst in patients with −7 (4 patients) as the sole CCA/Ph− (5-year OS 37% [95% CI, 1-80], P < .0001), with a trend toward adverse outcomes in 4 patients with complex CCA/Ph−, when compared with those without ACAs. In contrast, patients with trisomy 8 as the sole CCA/Ph− (4 patients) tended to have their prognosis similar to those without ACAs (supplemental Figure 4).
Two of the 54 patients with CCA/Ph− developed MDS or AML, both with chromosome 7 abnormalities as their additional abnormality (supplemental Table 2). One of them had monosomy 7 appear 9 months from the start of ponatinib in 9/20 metaphases and it persisted. He had dysplasia at the time this abnormality was detected. He was taken off ponatinib because of pancytopenia and subsequently received bosutinib, achieved and maintained a CCyR, but a high-risk MDS was documented approximately 1 year after appearance of the −7 clone. There were no driver mutations detected by the clinical laboratory using limited targeted sequencing (panel containing 28 genes frequently affected in myeloid disorders). He was started on decitabine and achieved a partial cytogenetic response for MDS. Another patient in the imatinib cohort with −7 developed secondary AML (CCyR for CML) and died of a multiple organ failure after allogeneic stem cell transplant from a 1 antigen-mismatched unrelated donor. No patients without ACAs developed MDS or AML.
Multivariate analysis for survival
We conducted a multivariate analysis to determine whether presence of CCA/Ph− is an independently adverse prognostic factor affecting survival. The CCA/Ph− group was separated in this analysis between –Y as the only clonal abnormality (N = 23) and all other patients termed CCA/Ph− (non –Y) (N = 31). The analysis was performed including all baseline characteristics and another analysis included BCR-ABL <10% at 3 months as an additional variable.
The univariate analysis showed that the CCA/Ph− (non –Y) group had comparatively worse FFS, EFS, TFS and OS (supplemental Tables 3-6). The HR for decreased FFS was 1.78 (95% CI 1.06-2.97; P = .02) for the CCA/Ph− (non –Y) group compared with the group without ACAs. Similarly, the HR for decreased EFS, TFS and OS were 2.22 (95% CI 1.11-4.46; P = .02), 3.30 (95% CI 1.37-7.94; P = .008), and 2.29 (95% CI 1.03-5.07, P = .04), respectively, for patients with CCA/Ph− (non −Y). Presence of –Y as the only clonal abnormality in Ph− metaphases did not significantly impact survival outcomes, however patients with –Y CCA/Ph− tended to have worse overall survival compared with patients without ACAs though not statistically significant with a HR of 1.84 (95% CI 0.73-4.62, P = .19).
In the multivariate analysis, the age at diagnosis was significantly and independently associated with worse FFS, EFS, TFS, and OS (Table 3; supplemental Tables 7-9). When analyzing the prognostic impact of CCA/Ph− on survival, the presence of non –Y abnormalities adversely affected the risk for transformation or death independently of all baseline characteristics with a HR of 2.81 (95% CI, 1.15-6.89; P = .02) compared with no ACAs (Table 3). This adverse effect was not statistically significant in the multivariate analysis of FFS, EFS, and OS. However, when achieving a BCR-ABL level <10% at 3 months was added as a variable, the statistically significant adverse impact on risk of transformation or death of non –Y abnormalities was abrogated. Indeed, not achieving a BCR-ABL level <10% at 3 months was found to be the strongest predictor of worse FFS, EFS, TFS, and OS. Those findings were confirmed in the reduced model using backward elimination (supplemental Tables 7-9). In addition, a high blast percentage in the bone marrow was associated with a decreased TFS when all variables were considered with a HR of 1.18 (95% CI, 1.03-1.37; P = .01). All other variables did not have a statistically significant affect in this model.
Multivariate analysis for risk of transformation or risk of death in patients with CP-CML
| BCR-ABL <10% at 3 mo . | Not included in MVA . | Included in MVA . | ||
|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | |
| TFS | ||||
| Age | 1.03 (1.00-1.05) | .03 | 1.03 (1.01-1.06) | .01 |
| Blasts in BM (%) | 1.15 (1.00-1.33) | .05 | 1.18 (1.03-1.37) | .01 |
| Basophils in BM (%) | 1.07 (0.95-1.20) | .28 | 1.05 (0.90-1.16) | .74 |
| CCA/Ph− (−Y)* | 0.80 (0.18-3.44) | .76 | 0.93 (0.21-4.04) | .94 |
| CCA/Ph− (non −Y)* | 2.81 (1.15-6.89) | .02 | 2.17 (0.86-5.50) | .10 |
| BCR-ABL <10% at 3 mo† | — | — | 3.96 (1.80-8.73) | <.01 |
| OS | ||||
| Age | 1.06 (1.03-1.08) | <.01 | 1.06 (1.03-1.08) | <.01 |
| Sokal score‡ | ||||
| Intermediate | 1.01 (0.55-1.86) | .96 | 1.03 (0.56-1.89) | .92 |
| High | 1.74 (0.78-3.90) | .17 | 1.65 (0.73-3.70) | .22 |
| CCA/Ph− (−Y)* | 0.91 (0.35-2.34) | .84 | 1.03 (0.40-2.68) | .94 |
| CCA/Ph− (non −Y)* | 1.62 (0.55 −3.63) | .24 | 1.39 (0.61-3.15) | .43 |
| BCR-ABL <10% at 3 mo† | — | — | 2.39 (1.24-4.60) | <.01 |
| BCR-ABL <10% at 3 mo . | Not included in MVA . | Included in MVA . | ||
|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | |
| TFS | ||||
| Age | 1.03 (1.00-1.05) | .03 | 1.03 (1.01-1.06) | .01 |
| Blasts in BM (%) | 1.15 (1.00-1.33) | .05 | 1.18 (1.03-1.37) | .01 |
| Basophils in BM (%) | 1.07 (0.95-1.20) | .28 | 1.05 (0.90-1.16) | .74 |
| CCA/Ph− (−Y)* | 0.80 (0.18-3.44) | .76 | 0.93 (0.21-4.04) | .94 |
| CCA/Ph− (non −Y)* | 2.81 (1.15-6.89) | .02 | 2.17 (0.86-5.50) | .10 |
| BCR-ABL <10% at 3 mo† | — | — | 3.96 (1.80-8.73) | <.01 |
| OS | ||||
| Age | 1.06 (1.03-1.08) | <.01 | 1.06 (1.03-1.08) | <.01 |
| Sokal score‡ | ||||
| Intermediate | 1.01 (0.55-1.86) | .96 | 1.03 (0.56-1.89) | .92 |
| High | 1.74 (0.78-3.90) | .17 | 1.65 (0.73-3.70) | .22 |
| CCA/Ph− (−Y)* | 0.91 (0.35-2.34) | .84 | 1.03 (0.40-2.68) | .94 |
| CCA/Ph− (non −Y)* | 1.62 (0.55 −3.63) | .24 | 1.39 (0.61-3.15) | .43 |
| BCR-ABL <10% at 3 mo† | — | — | 2.39 (1.24-4.60) | <.01 |
This multivariate model included covariates with P < .05 in the univariate analysis. Another model using the backward elimination method was also used and led to similar results as detailed in supplemental Tables 1-12 and Figures 1-4.
MVA, multivariate analysis.
CCA/Ph− groups were compared with the control group without additional chromosomal abnormalities.
There were 46 patients in which the BCR-ABL level at 3 mo was not available and was included as a separate group in the univariate analysis as detailed in the supplemental Tables 1-12. Not achieving a BCR-ABL <10% at 3 mo was compared with the group in which this level was achieved.
Low Sokal score group was used as a reference for the other Sokal score groups.
Discussion
In this study, we investigated the clinical characteristics and prognostic impact of CCA/Ph− occurring during the course of initial therapy with TKI for patients with CP-CML. Our analysis demonstrates that CCA/Ph− occur in about 10% of CP-CML cases, are most commonly represented by loss of chromosome Y, and more frequently occur in older patients. Considering the relatively young median age of our population, it is possible that the incidence might be higher in the general CML population. In this regard, it is important to underscore that loss of chromosome Y has been identified as an age-related phenomenon. However, its incidence in hematological malignancies is higher than in age-matched cases without malignancies, indicating a possible implication in the malignant process.27 The involvement in the leukemic or treatment process in our cohort is suggested by the fact that –Y was not present at the time of diagnosis and was only detected usually shortly after the treatment started. CCA/Ph− were mostly transient, appearing during treatment with all TKI used, with a slight trend for higher incidence with nilotinib and ponatinib. Whether this trend is real or a figment of small cohort sizes requires confirmation.
We did not find significantly different cytogenetic response rates comparing patients with CCA/Ph− with those without ACAs. Because these abnormalities can only be assessed in the presence of Ph− metaphases, it is not unexpected that response to therapy is not affected. Furthermore, there was no significant difference in molecular response rates comparing patients with CCA/Ph− with those without ACAs. The impact of CCA/Ph− on prognosis varied depending on the type of abnormality, similar to what has been shown when present in the Ph+ metaphases.9,26 Indeed, presence of non –Y CCA/Ph− was associated with worse FFS, EFS, TFS, and OS. In fact, in a multivariate analysis including the baseline characteristics, presence of non –Y CCA/Ph− independently increased the risk of transformation or death almost threefold compared with patients without ACAs. However, this prognostic impact was abrogated when achieving BCR-ABL <10% at 3 months (a response with a tendency to occur less frequently among patients with CCA/Ph−) was added to the model. Risk of development of MDS or AML has been reported in the literature among patients with these abnormalities.14,15,28,29 In our present series, 2 of 4 patients with monosomy 7 developed AML or MDS, confirming the risk of this occurrence.
To our knowledge, this is the largest study to investigate the prognostic impact of CCA/Ph− emerging in patients with CP-CML across various TKI modalities. This is a single-institution investigation leveraging carefully annotated clinical variables and responses from various prospective clinical trials. In addition, this is the first study investigating the relative prognostic impact of these abnormalities in a multivariate analysis. Based on our findings, presence of non −Y CCA/Ph− should alert clinicians about adverse survival outcomes, perhaps warranting a closer monitoring. Outcomes in our analysis showed a trend for inferiority for patients with CCA/Ph− for most measures of response and long-term survival end points. The multivariate analysis showed again the strong prognostic impact of early response assessment such as BCR-ABL <10% at 3 months. This response was somewhat less likely to occur among patients with CCA/Ph− (74% vs 81%). Whether the lack of statistical significance is a product of the small sample size or a true insignificant variation requires additional confirmation. If there is indeed a real difference, the biologic features that underlie this lower response rate, which in turn translates into poorer long-term outcomes, should be discerned.
This study is limited by the retrospective nature of the analysis (albeit all studies and evaluations were conducted prospectively) and the relatively small cohort size that may not allow firm conclusions of the trends observed. This also makes it difficult to analyze the relative impact of individual types of chromosomal abnormalities, similar to what has been done when they appear in the Ph+ metaphases.30 Still, the trends observed further support the inclusion of the occurrence of these abnormalities as a warning sign as per the European LeukemiaNet recommendations for CML. It also suggests that performing conventional cytogenetics during the early stages of treatment of patients with CML still holds value despite the increasing reliance on polymerase chain reaction for monitoring. Because few abnormalities occur for the first time after 12 months of therapy the value of cytogenetic assessment perhaps decreases after this time point for patients with a stable deep response to therapy.
The identification of this cohort of patients with CP-CML gaining chromosomal abnormalities in the Ph− metaphases with relatively adverse clinical outcomes despite successful targeting of the Ph+ clone sheds some light on residual CML cells when treated with TKI. Though the older age of patients is associated with an increased probability of developing CCA/Ph− and older age itself can be a major risk factor leading to the adverse outcomes, presence of non –Y CCA/Ph− was found to be an independent prognostic factor in the multivariate analysis. These abnormalities could be arising from preleukemic clones made evident now by the successful treatment.31 Another hypothesis could be that some of these residual cells are nonleukemic, but contribute to the malignant transformation through modification of the microenvironment leading to reemergence of the malignant clone.32 Finally, presence of CCA/Ph− could be a reflection of genomic instability, either inherently present in some cases or induced by treatment with TKIs.33 In this regard, it is worth noting that all patients with CCA/Ph− had worse OS. This is reminiscent of the reported impact clonal hematopoiesis of indeterminate potential has on all-cause mortality including a possible increased risk of cardiovascular disease.34-36 Further studies are needed to better elucidate the molecular landscape present during the appearance of CCA/Ph−.
In conclusion, the appearance of nondeletion Y CCA/Ph− during treatment with various TKI is associated with decreased FFS, EFS, TFS, and OS, and a low but significant risk of developing MDS or AML for those with monosomy 7. Although the adverse prognostic impact associated with this group was not significant when the rate of early molecular response was considered, there is a trend for a lower rate of such responses in this patient population. Further studies are needed to define the biologic determinants and long-term implications of this phenomenon.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was funded by MD Anderson Cancer Center support grant (CA016672) and National Institutes of Health, National Cancer Institute (P01 CA049639).
Authorship
Contribution: G.C.I. designed the study, collected the data, and wrote the manuscript; J.E.C. designed the study, treated the patients, conducted the clinical trials, and wrote the manuscript. S.D. collected the data. G.N.G., K.S., S.D., G.C.I., and J.E.C. analyzed the data. H.M.K., G.B., W.W., F.R., T.K., A.F., G.G.-M., E.J., and J.E.C. treated patients; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: J.E.C. received research support from and is a consultant for Ariad, BMS, Novartis, and Pfizer. The remaining authors declare no competing financial interests.
Correspondence: Jorge E. Cortes, Department of Leukemia, MD Anderson Cancer Center, 151 Holcombe Blvd, Box 428, Houston, TX 77030; e-mail: jcortes@mdanderson.org.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal