Key Points
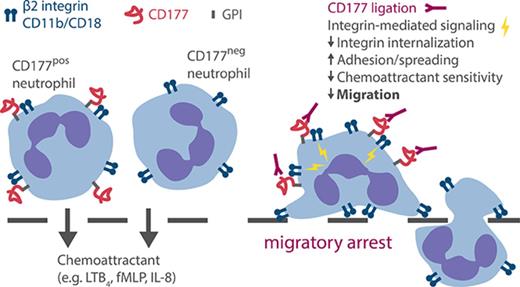
Ligation of CD177, a GPI-linked surface protein expressed selectively on neutrophils, blocks neutrophil migration independently of PECAM-1.
Blockade reflects activation through β2 integrins, immobilizing cells via stronger integrin attachments and impaired chemokine signaling.
Abstract
CD177 is a glycosylphosphatidylinositol (GPI)-anchored protein expressed by a variable proportion of human neutrophils that mediates surface expression of the antineutrophil cytoplasmic antibody antigen proteinase 3. CD177 associates with β2 integrins and recognizes platelet endothelial cell adhesion molecule 1 (PECAM-1), suggesting a role in neutrophil migration. However, CD177pos neutrophils exhibit no clear migratory advantage in vivo, despite interruption of in vitro transendothelial migration by CD177 ligation. We sought to understand this paradox. Using a PECAM-1-independent transwell system, we found that CD177pos and CD177neg neutrophils migrated comparably. CD177 ligation selectively impaired migration of CD177pos neutrophils, an effect mediated through immobilization and cellular spreading on the transwell membrane. Correspondingly, CD177 ligation enhanced its interaction with β2 integrins, as revealed by fluorescence lifetime imaging microscopy, leading to integrin-mediated phosphorylation of Src and extracellular signal-regulated kinase (ERK). CD177-driven cell activation enhanced surface β2 integrin expression and affinity, impaired internalization of integrin attachments, and resulted in ERK-mediated attenuation of chemokine signaling. We conclude that CD177 signals in a β2 integrin-dependent manner to orchestrate a set of activation-mediated mechanisms that impair human neutrophil migration.
Introduction
CD177 is a neutrophil surface molecule that was identified in 1971 as the target of alloimmune antibodies associated with fetal neutropenia.1 Its expression is restricted to a subset of neutrophils, averaging 45% to 65% of circulating cells, although 3% to 5% of healthy individuals express no CD177 through the introduction of a stop codon from a neighboring pseudogene, together with epigenetic silencing of at least 1 allele per cell.2-5 CD177 is expressed in developing neutrophils, beginning at the metamyelocyte stage, with mRNA peaking in band cells.6 The proportion of circulating neutrophils expressing CD177 is stable within an individual, independent of age, gender, or activation state, although the CD177pos fraction increases in pregnancy, under granulocyte-colony stimulating factor therapy, and in patients who acquire polycythemia rubra vera (PRV).7-9 Indeed, an alternative name for CD177 is PRV-1, reflecting an allele of CD177 identified in this hematoproliferative disease.9,10
The function of CD177 remains poorly understood. Lacking a transmembrane domain, CD177 is tethered to the neutrophil surface by a glycosylphosphatidylinositol anchor and correspondingly is concentrated in lipid rafts. Immunoprecipitation and binding studies, supported by confocal imaging, demonstrate physical association with β2 integrins, in particular Mac-1 (CD11b/CD18).11 Intriguingly, CD177 in turn anchors the neutrophil serine protease proteinase 3 (PR3), such that CD177pos neutrophils selectively express surface PR3.12-14 Anti-PR3 antibodies from patients with antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis recognize only CD177pos neutrophils and selectively activate these cells, as manifested in cell degranulation and superoxide production.11 This activation, mimicked by CD177 ligation with the anti-CD177 antibody MEM166, is inhibited by antibodies against CD11b and CD18, implicating Mac-1 as a signaling partner for CD177 in ANCA vasculitis.11
In addition to β2 integrins and PR3, CD177 recognizes platelet endothelial cell adhesion molecule 1 (PECAM-1), an immunoglobulin-family molecule expressed on endothelial cells as well as platelets and certain leukocytes, including neutrophils themselves. CD177-Fc precipitated PECAM-1 from human umbilical vein epithelial cells (HUVECs), a finding confirmed by affinity and transfection studies.15 The interaction between CD177 and PECAM-1 is mediated through PECAM-1 membrane proximal domain 6, and HUVEC cells bearing a common PECAM-1 polymorphism in this domain (S536) support more rapid neutrophil migration as well as enhanced PECAM-1 phosphorylation upon exposure to CD177-Fc.16,17 Correspondingly, CD177pos neutrophils migrate more efficiently than do CD177neg cells through a HUVEC layer in vitro, a system in which MEM166 inhibits migration.15,17,18 The migratory advantage of CD177pos cells in this setting may reflect the enzymatic effect of PR3, because a serine protease inhibitor also impairs transendothelial migration.18
However, the hypothesis that CD177 promotes neutrophil migration via PECAM-1 is discordant with other observations. In comparison with peripheral blood, no enrichment for CD177pos neutrophils was observed in inflamed synovial fluid, peritoneum, or oral cavity.14,19,20 CD177 does not promote binding to PECAM-1-expressing platelets or enhance migration across platelet-coated transwell membranes.17 Mice deficient in murine CD177, a protein with approximately 50% sequence homology to human CD177, exhibited no defect in neutrophil recruitment to the peritoneum, although a small decrease in early neutrophil recruitment to S. aureus-infected skin was observed, which was similar in magnitude to a reduction in circulating neutrophil count.4,21 Furthermore, in the HUVEC system, ligation of CD177 with MEM166 interrupted transmigration but not adhesion, which was at least as robust in MEM166-treated neutrophils as in isotype-treated neutrophils.18 Published data therefore leave considerable uncertainty about the role of CD177 in the migration of neutrophils.
To address this question, we tested the effect of CD177 ligation in an endothelium-free transwell migration system. In this PECAM-1-independent setting, CD177pos and CD177neg cells migrated equally, but MEM166 still preferentially inhibited migration of CD177pos cells. Rather than interfering with adhesion, CD177 ligation immobilized neutrophils at the transwell membrane. Neutrophils became activated, potentially through a dynamic cooperation between CD177 and β2 integrins, leading to the interruption of migration through a combination of mechanisms, including increased surface β2 integrin expression and affinity, reduced internalization of β2 integrin adhesions, and extracellular signal-regulated kinase (ERK)–mediated impairment of chemokine signaling. These findings define a PECAM-1-independent, β2 integrin–dependent pathway through which CD177 modulates the migration of human neutrophils.
Methods
Flow cytometry and immunofluorescence microscopy
Reagents and protocols for staining and analysis are described in the supplemental Methods section, available on the Blood Web site.
Isolation of human neutrophils from peripheral blood
Neutrophils were isolated from consenting healthy adult donors under Brigham and Women’s Hospital protocol 2006P001068 by red blood cell sedimentation with dextran 3%, followed by Ficoll-Hypaque density gradient centrifugation and hypotonic erythrocyte lysis, as has been described.22 Alternatively, neutrophils were isolated from EDTA-anticoagulated whole blood by using the EasySep Direct Human Neutrophil Isolation Kit (STEMCELL), according to the manufacturer's instructions. Cell viability was detected by trypan blue exclusion and exceeded 98%. The neutrophil percentage in the suspension was greater than 95% by flow cytometry. Cells were counted by hemocytometer.
Neutrophil activation
Isolated neutrophils suspended in RPMI 1640 medium, containing 0.1% heat-inactivated fetal bovine serum (FBS) at 106/mL, were exposed to anti-CD177 or isotype (10 µg/mL) in the absence or presence of leukotriene B4 (LTB4) 100 nM for 30 min at 37°C. Cells were immediately stained with fluorescence-activated cell sorter antibodies for activation markers on ice for 30 min, followed by flow cytometry analysis.
Neutrophil migration and adhesion in a transwell system
Isolated neutrophils suspended in RPMI 1640 medium containing 0.1% FBS at 106/mL were exposed to anti-CD177 or isotype (10 µg/mL) for 10 min at room temperature (RT). Thereafter, 100 µL of cells were placed in the upper compartment of a transwell chamber featuring uncoated polyester membrane with 3-µm pores (Corning), and 600 µL of 0.1% FBS RPMI 1640 medium containing LTB4 100 nM or vehicle was added to the bottom chamber. After incubation for 2 h at 37°C and 5% CO2, 500 µL were harvested from the lower chamber. Alternatively, the medium in the bottom chamber was harvested from human fibroblast-like synoviocytes (FLS) stimulated with tumor necrosis factor (TNF) (2 ng/mL) + interleukin-17 (IL-17) (5 ng/mL) (see supplemental Methods).23 For neutrophil adhesion, cells adherent on the membrane in the 3-µm-pore transwell system, or in some experiments using neutrophil-impermeable 0.4-µm-pore transwells, were detached in PBS with 5 mM EDTA at RT for 5 min and harvested by 2× PBS rinse. CD66+ singlets were enumerated using flow cytometry by comparison with counting beads added to each sample.
Dynamic adhesion assay
Microfluidic flow chambers (ibidi) were coated with 10 μg/mL recombinant human ICAM-1 (R&D Systems) and then blocked using 1% (weight-to-volume ratio) casein in PBS (Thermo Scientific). Flow chambers were attached to a syringe pump (Kent Scientific) to control the shear stress. Human neutrophils from healthy donor blood were purified by negative isolation (EasySep Direct, StemCell Technologies), suspended in Hanks Balanced Salt Solution containing isotype or anti-CD177 (10 µg/mL), and incubated at 37°C for 10 min prior to their introduction into the flow chamber. Neutrophils were allowed to settle and adhere for 3 min before initiation of flow at a shear stress of 8 dyn/cm2. The number of initially settled neutrophils that continued to adhere to the surface 1 min after initiation of flow was then quantified.
Neutrophil phagocytosis
Isolated neutrophils suspended in RPMI 1640 medium containing 0.1% FBS at 106/mL were exposed to anti-CD177 or isotype for 10 min at RT, then mixed with green fluorescent protein–Escherichia coli (1:10) or human complement sera opsonized 2-µm fluorescent latex beads (1:20) at 37°C for 30 min or 60 min. Cells were spun and washed extensively with PBS containing 5 mM EDTA, followed by staining with fluorescence-activated cell sorter antibodies on ice for 30 min. For green fluorescent protein–E coli phagocytosis, cells were incubated with PBS containing 5 mM EDTA and 0.1 mg/mL lysozyme on ice for 10 min before washing steps.
Integrin internalization
Isolated neutrophils suspended at 106/mL in 0.1% FBS RPMI 1640 medium containing anti-CD177 or isotype (10 µg/mL) were seeded in 12-well plates to allow adhesion for 15 min at 37°C. Adherent cells were washed with cold PBS, followed by incubation with anti-CD11b-PE in RPMI 1640 medium for 30 min at 4°C. Cells were rinsed twice with cold PBS, then incubated with prewarmed 0.1% FBS RPMI 1640 medium containing 100 nM LTB4. At desired time points, cells were immediately rinsed with cold PBS, followed by 2× acidic wash (0.3 M sodium acetate, pH 3). Cells were then detached and suspended with PBS 5 mM EDTA and applied to flow cytometry analysis.
Ca2+ flux
A flow cytometry protocol to determine the kinetics of cytoplasmic calcium was performed as described24 with slight modifications. Briefly, isolated human neutrophils were loaded with Fluo4 Ca2+ indicator according to the manufacturer's instruction, followed by incubation with anti-CD177 or isotype (10 µg/mL) for 20 min at RT in RPMI 1640 medium containing 0.1% FBS with 5 mM EGTA. Cell suspensions were analyzed by flow cytometry at low speed for 30 s to determine the baseline fluorescence for Fluo4 Ca2+ complex in the fluorescein isothiocyanate channel. Aspiration was then paused, LTB4 was added to the sample tube at a final concentration of 30 nM, and signal was recorded for an additional 150 s. For each separate sample, Fluo4-Ca2+-fluorescence was plotted versus time. Mean fluorescence intensity was recorded for each 5-s interval.
Fluorescence lifetime imaging microscopy (FLIM)
FLIM was performed as described, with specific combinations of donor and acceptor antibodies as detailed in the supplemental Methods.25
Intracellular signal transduction by western blotting
We exposed 1 × 106 isolated neutrophils suspended in RPMI 1640 medium containing 0.1% FBS to anti-CD177 (MEM166), anti-CD18 (MEM148), or isotype (10 µg/mL) in the absence or presence of 100 nM LTB4 at 37°C. At desired time points, cells were immediately rinsed with cold PBS and spun and lysed in 0.1 mL NP-40 lysis buffer containing protease and phosphatase inhibitor cocktails. Lysates were treated with 6× Laemmli sample buffer, followed by 10% gel sodium dodecyl sulfate–polyacrylamide gel electrophoresis and analyzed by immunoblotting with phosphorylation-specific antibodies.
Statistical analysis
Error bars depict standard errors of the mean (SEMs). P values were calculated using the Student t test, unless otherwise indicated in the figure legends. P < .05 was considered significant.
Results
CD177 ligation attenuates neutrophil migration in a PECAM-1-independent system
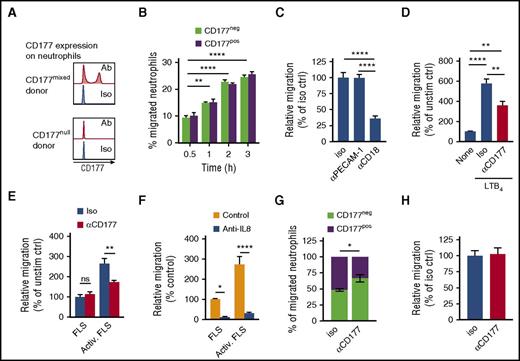
To better understand the role of CD177 in neutrophil migration, we adapted a simple transwell system.25 Neutrophils purified from human blood were allowed to migrate toward LTB4 through 3-µm pores. Cells harvested from the lower chamber were enumerated by flow cytometry. In contrast to in vitro transendothelial migration, in this system CD177pos and CD177neg cells migrated equally (Figure 1A,B). As was expected, PECAM-1 exhibited no role, but migration was instead primarily dependent on β2 integrins (Figure 1C). Nevertheless, CD177 ligation with the anti-CD177 antibody MEM166 still attenuated transmigration, implicating a PECAM-1-independent mechanism of migratory blockade (Figure 1D). Similar results were obtained when transwell membranes coated with fibrinogen and ICAM-1 were used or when N-formyl-methionyl-leucyl-phenylalanine was used as a chemoattractant (supplemental Figure 1). Anti-CD177 also inhibited migration toward chemoattractants generated from IL-17- and TNF-stimulated synovial fibroblasts, wherein blocking experiments implicated IL-8 as the key chemoattractant (Figure 1E,F). Inhibition mediated by CD177 ligation was partial, likely reflecting in part expression of CD177 in only a fraction of neutrophils. Correspondingly, the proportion of neutrophils in the lower chamber expressing CD177 was reduced after CD177 ligation (Figure 1G), and anti-CD177 had no effect on neutrophil migration in a CD177null donor (Figure 1H). Thus, attenuation of neutrophil migration by CD177 ligation in this system reflected a cell-intrinsic inhibitory process, active across several chemoattractants, rather than interference with a migratory advantage of CD177pos neutrophils.
CD177 ligation blocks migration in a PECAM-1-independent manner. Peripheral blood neutrophils were allowed to migrate through 3-µm pores toward LTB4 (B-D, G-H) or supernatants from resting or TNF/IL-17-activated FLS (E-F). (A) Representative histograms of CD177 expression by flow cytometry from a donor with both CD177pos and CD177neg cells (CD177mixed) or CD177neg cells only (CD177null). (B) Time course of migration of CD177pos and CD177neg neutrophils from donors with both populations, demonstrating equal intrinsic migratory efficiency. (C) Addition of blocking antibodies (10 µg/mL) to cells prior to introduction into the transwell demonstrates dependence of migration on CD18 but not PECAM-1. (D) CD177 ligation with MEM166 (10 µg/mL, termed αCD177) impedes neutrophil migration. (E) αCD177 attenuates neutrophil migration toward supernatants from activated FLS. TNF/IL-17-supplemented medium alone does not induce migration (data not shown). (F) Anti-IL-8 blocks the chemotactic effect of stimulated FLS. Data in panels B-F are normalized to no-chemoattractant migration to enable pooling of data from multiple donors. (G) αCD177 decreased the proportion of CD177pos neutrophils in the bottom chamber, indicating selective migratory blockade of CD177pos cells. (H) Migration of neutrophils obtained from a CD177null donor was unaffected by αCD177. All experiments reflect duplicate or triplicate wells per condition and are representative of 3 to 5 similar experiments. Means ± SEMs. *P < .05; **P < .01; ****P < .0001; by 2-way analysis of variance (ANOVA) (B-G) or 1-way ANOVA (C,D), followed by Tukey's multiple comparisons test. Ab, antibody; activ, activated; ctrl, control; iso, isotype; ns, not significant; unstim, unstimulated.
CD177 ligation blocks migration in a PECAM-1-independent manner. Peripheral blood neutrophils were allowed to migrate through 3-µm pores toward LTB4 (B-D, G-H) or supernatants from resting or TNF/IL-17-activated FLS (E-F). (A) Representative histograms of CD177 expression by flow cytometry from a donor with both CD177pos and CD177neg cells (CD177mixed) or CD177neg cells only (CD177null). (B) Time course of migration of CD177pos and CD177neg neutrophils from donors with both populations, demonstrating equal intrinsic migratory efficiency. (C) Addition of blocking antibodies (10 µg/mL) to cells prior to introduction into the transwell demonstrates dependence of migration on CD18 but not PECAM-1. (D) CD177 ligation with MEM166 (10 µg/mL, termed αCD177) impedes neutrophil migration. (E) αCD177 attenuates neutrophil migration toward supernatants from activated FLS. TNF/IL-17-supplemented medium alone does not induce migration (data not shown). (F) Anti-IL-8 blocks the chemotactic effect of stimulated FLS. Data in panels B-F are normalized to no-chemoattractant migration to enable pooling of data from multiple donors. (G) αCD177 decreased the proportion of CD177pos neutrophils in the bottom chamber, indicating selective migratory blockade of CD177pos cells. (H) Migration of neutrophils obtained from a CD177null donor was unaffected by αCD177. All experiments reflect duplicate or triplicate wells per condition and are representative of 3 to 5 similar experiments. Means ± SEMs. *P < .05; **P < .01; ****P < .0001; by 2-way analysis of variance (ANOVA) (B-G) or 1-way ANOVA (C,D), followed by Tukey's multiple comparisons test. Ab, antibody; activ, activated; ctrl, control; iso, isotype; ns, not significant; unstim, unstimulated.
Whereas migratory blockade was selective for CD177pos cells, it was not entirely specific. Although anti-CD177 elicited no migratory effect in CD177null donors, subtle impairment of CD177neg cell migration was observed in donors possessing both CD177pos and CD177neg neutrophils, indicating a “bystander effect” (supplemental Figure 2A). In confirmation, labeled CD177null donor neutrophils mixed with CD177pos neutrophils and exposed to anti-CD177 also exhibited partial migratory inhibition (supplemental Figure 2B). Impairment reflected at least in part soluble factors derived from CD177pos neutrophils, because cell-free supernatants from CD177pos/CD177neg donor neutrophils exposed to anti-CD177, but not anti-CD177-containing supernatants alone, replicated the inhibitory effect (supplemental Figure 2C). Thus, migratory blockade mediated by anti-CD177 reflects not only a direct effect on CD177pos neutrophils but also soluble mediators released by CD177pos cells that affect CD177neg bystanders.
Attenuated migration from CD177 ligation reflects immobilization at the membrane
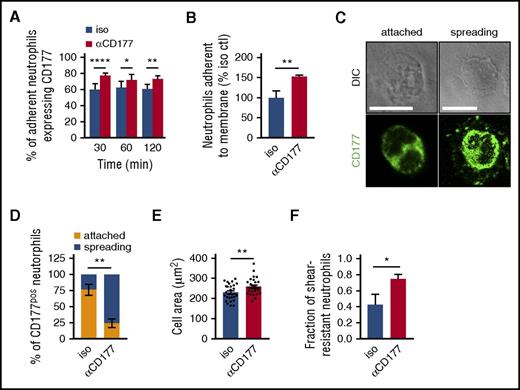
To assess the mechanism of migratory inhibition, we evaluated the locus of migratory blockade. Interestingly, as has been suggested from transendothelial studies,18 anti-CD177 enhanced cell adhesion to the transwell filter. In the 3-µm pore condition, CD177 ligation translated into an increased proportion of CD177pos neutrophils among residual adherent cells (Figure 2A). When the 3-µm pore transmembrane was replaced by a 0.4-µm pore membrane, through which neutrophils could not pass, adherent cells were also increased (Figure 2B). CD177 ligation enhanced adherence, spreading, and cellular area of LTB4-exposed CD177pos neutrophils on glass slides (Figure 2C-E). Concordant with this result, cell height measurement using atomic force microscopy showed that anti-CD177 decreased cell height as well as elasticity (supplemental Figure 3), whereas visualization of neutrophil adhesion under flow conditions showed an increase in shear-resistant attachment (Figure 2F). These findings indicate that failure to translocate reflects increased rather than decreased neutrophil adhesion.
Migratory blockade by CD177 ligation reflects enhanced adherence. (A) The proportion of neutrophils adherent to the 3-µm transwell filters that were CD177pos was quantitated by confocal microscopy in the presence of isotype control or αCD177. Anti-CD177 increased the proportion of adherent CD177pos cells. (B) Neutrophils were exposed to a migratory stimulus (LTB4 100 nM) across a barrier with pores too small to permit migration (0.4 µm), and the proportion of adherent neutrophils was assessed in the presence of isotype or αCD177. Anti-CD177 increased adherence to the membrane. Data for panels A and B represent triplicate wells per condition, representative of at least 2 to 3 similar experiments. (C) Neutrophils were incubated on glass coverslips in the presence of LTB4 100 nM for 15 min and washed, and CD177pos cells were characterized by paired differential interference contrast and immunofluorescence microscopy as attached (rounded shape with or without a trailing tail) or spreading (“halo” staining of CD177, lamellar protrusions, or both). Scale bars measure 10 µm. αCD177 markedly induced spreading morphology (D) and increased the cellular area of CD177pos neutrophils (E). (F) Treatment with anti-CD177 increases the fraction of shear stress resistant neutrophils. Data reflect 2 replicates enumerating 90 to 110 cells each (C-D), 25 to 30 cells each (E), and 3 independent experiments performed in duplicate, each replicate analyzing 10 to 50 cells per field of view (F). Means ± SEMs. *P < .05; **P < .01; ****P < .0001; by 2-way ANOVA (A,D) or unpaired t test (B,E,F). DIC, differential interference contrast. Ctl, control.
Migratory blockade by CD177 ligation reflects enhanced adherence. (A) The proportion of neutrophils adherent to the 3-µm transwell filters that were CD177pos was quantitated by confocal microscopy in the presence of isotype control or αCD177. Anti-CD177 increased the proportion of adherent CD177pos cells. (B) Neutrophils were exposed to a migratory stimulus (LTB4 100 nM) across a barrier with pores too small to permit migration (0.4 µm), and the proportion of adherent neutrophils was assessed in the presence of isotype or αCD177. Anti-CD177 increased adherence to the membrane. Data for panels A and B represent triplicate wells per condition, representative of at least 2 to 3 similar experiments. (C) Neutrophils were incubated on glass coverslips in the presence of LTB4 100 nM for 15 min and washed, and CD177pos cells were characterized by paired differential interference contrast and immunofluorescence microscopy as attached (rounded shape with or without a trailing tail) or spreading (“halo” staining of CD177, lamellar protrusions, or both). Scale bars measure 10 µm. αCD177 markedly induced spreading morphology (D) and increased the cellular area of CD177pos neutrophils (E). (F) Treatment with anti-CD177 increases the fraction of shear stress resistant neutrophils. Data reflect 2 replicates enumerating 90 to 110 cells each (C-D), 25 to 30 cells each (E), and 3 independent experiments performed in duplicate, each replicate analyzing 10 to 50 cells per field of view (F). Means ± SEMs. *P < .05; **P < .01; ****P < .0001; by 2-way ANOVA (A,D) or unpaired t test (B,E,F). DIC, differential interference contrast. Ctl, control.
CD177 ligation enhances the interaction of CD177 with β2 integrins
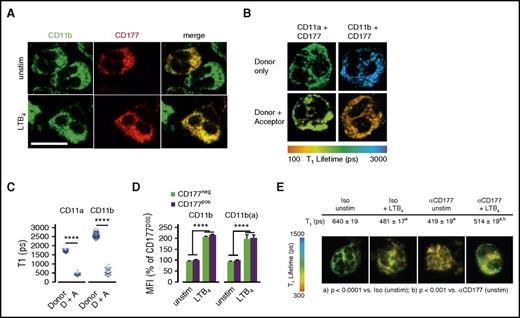
Previous studies found that CD177 associates closely with β2 integrins.11 We confirmed this observation for CD11b by using confocal microscopy (Figure 3A) and for both CD11b and CD11a by using FLIM, an imaging strategy based on fluorescence resonance energy transfer that establishes spatial interaction within 10 nm, consistent with direct intermolecular contact (Figure 3B). Furthermore, CD177-Fc could immunoprecipitate recombinant CD11b/CD18 (αMβ2) (supplemental Figure 4). Therefore, we explored whether the interaction of CD177 with integrins mediated the immobilization of neutrophils resulting from CD177 ligation.
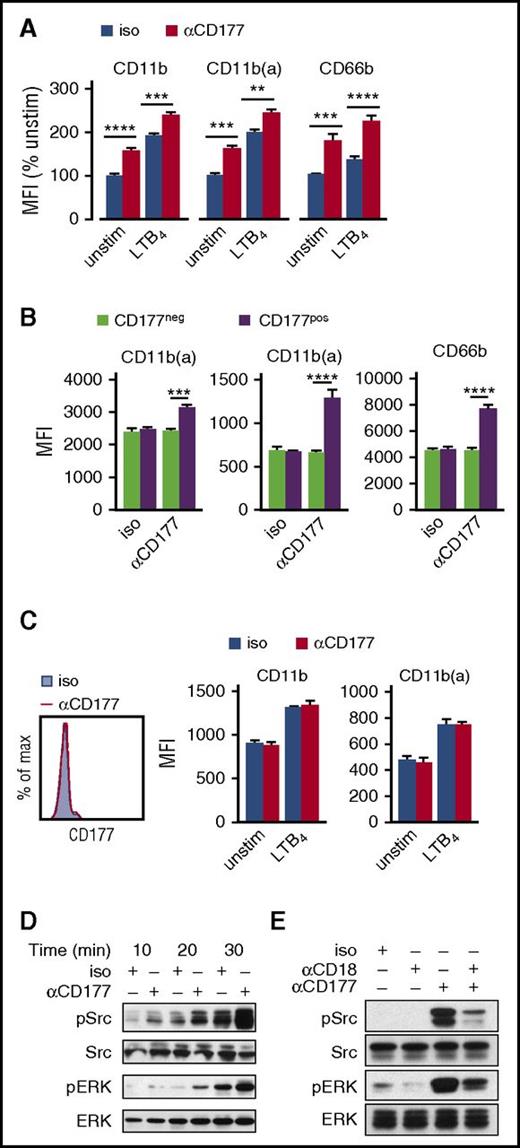
CD177 interacts with β2 integrins but does not alter integrin expression or affinity. (A) Immunofluorescence microscopy was performed to assess colocalization of CD177 and CD11b on human peripheral blood neutrophils. Scale bar, 10 µm. (B) Pseudocolored FLIM demonstrating molecular interaction between CD177 and CD11b, with reduction in τ1 indicative of fluorescence resonance imaging transfer quenching. (C) Quantitation of τ1 for interaction between CD11b or CD11a as donor and CD177 as acceptor. Data shown reflect at least 2 experiments each. (D) Flow cytometry was used to assess surface expression of total and active surface CD11b on freshly isolated peripheral neutrophils or after activation with LTB4 100 nM for 30 min. Similar results were obtained for CD11a, CD18, ICAM-1 binding, and integrin-dependent phagocytosis and with endogenously activated neutrophils obtained from inflamed joints (supplemental Figure 5A-D). Integrin data are pooled from 5 independent donors and at least 3 replicates. (E) CD177 ligation enhances the spatial proximity of CD177 with β2 integrins. Reduction in τ1 by FLIM upon CD177 ligation with MEM166 reflects enhanced intermolecular interaction with CD11b. Data were pooled from 3 separate experiments, including 60 to 90 pixels from the surface membrane used to calculate values. Donor-only τ1 was 1290 ± 9. Means ± SEMs. ****P < .0001, by unpaired t test (C,E) or 2-way ANOVA (D). A, acceptor; D, donor; MFI, mean fluorescence intensity; ps, picoseconds.
CD177 interacts with β2 integrins but does not alter integrin expression or affinity. (A) Immunofluorescence microscopy was performed to assess colocalization of CD177 and CD11b on human peripheral blood neutrophils. Scale bar, 10 µm. (B) Pseudocolored FLIM demonstrating molecular interaction between CD177 and CD11b, with reduction in τ1 indicative of fluorescence resonance imaging transfer quenching. (C) Quantitation of τ1 for interaction between CD11b or CD11a as donor and CD177 as acceptor. Data shown reflect at least 2 experiments each. (D) Flow cytometry was used to assess surface expression of total and active surface CD11b on freshly isolated peripheral neutrophils or after activation with LTB4 100 nM for 30 min. Similar results were obtained for CD11a, CD18, ICAM-1 binding, and integrin-dependent phagocytosis and with endogenously activated neutrophils obtained from inflamed joints (supplemental Figure 5A-D). Integrin data are pooled from 5 independent donors and at least 3 replicates. (E) CD177 ligation enhances the spatial proximity of CD177 with β2 integrins. Reduction in τ1 by FLIM upon CD177 ligation with MEM166 reflects enhanced intermolecular interaction with CD11b. Data were pooled from 3 separate experiments, including 60 to 90 pixels from the surface membrane used to calculate values. Donor-only τ1 was 1290 ± 9. Means ± SEMs. ****P < .0001, by unpaired t test (C,E) or 2-way ANOVA (D). A, acceptor; D, donor; MFI, mean fluorescence intensity; ps, picoseconds.
We first tested the hypothesis that membrane CD177 directly enhances β2 integrin expression or function. In healthy donors with both CD177pos and CD177neg neutrophils, circulating neutrophils of both groups exhibited comparable CD11b and active-conformation CD11b (abbreviated CD11b(a)) (Figure 3D), as well as CD18, CD11a, ICAM-1 binding, and integrin-dependent phagocytosis (supplemental Figure 5A-C), whether unmanipulated or after activation with LTB4. Similarly, CD177pos and CD177neg neutrophils from fresh inflammatory arthritis synovial fluids (ie, endogenously transmigrated and activated) exhibited comparable integrin expression and function (supplemental Figure 5D). Thus, consistent with the integrin-dependent transwell system, we find no evidence that CD177 directly promotes altered β2 expression or function.
We then tested the effect of CD177 ligation on β2 integrins. FLIM showed that CD177 ligation triggered a decrease in fluorescence lifetime τ1, reflecting closer interaction between domains of CD11b and CD177, labeled by donor and receptor antibodies, respectively (Figure 3E). We therefore investigated the impact of CD177 ligation on integrin function.
CD177 ligation activates CD177pos neutrophils through β2 integrins
The “bystander effect” of CD177 ligation on CD177neg neutrophils implied activation of CD177pos neutrophils, resulting in release of soluble mediators (supplemental Figure 2). Prior studies had already established that CD177 ligation elicits neutrophil degranulation and superoxide generation, an effect mediated through β2 integrins as the signaling partner of CD177.11 If CD177 ligation activates neutrophils, then it could also enhance β2 integrin expression and affinity. Indeed, we found this to be the case. Anti-CD177 enhanced neutrophil surface expression of CD11b, CD11b(a), and CD66b (Figure 4A). In a mixed population of CD177pos and CD177neg neutrophils, these changes affected only CD177pos cells, indicating a cell-intrinsic mechanism without bystander effect (Figure 4B). Correspondingly, no changes were observed in neutrophils from a CD177null donor (Figure 4C). CD177 ligation activated downstream pathways including Src and ERK (Figure 4D,E). These effects did not require CD177 crosslinking, because they could be replicated by MEM166 Fab fragments (supplemental Figure 6). Consistent with the findings of Jerke et al,11 the blocking anti-CD18 antibody MEM148 abrogated CD177-mediated activation, disrupting CD177-driven phosphorylation of Src and ERK (Figure 4E). These results show that CD177 ligation activates CD177pos neutrophils, likely signaling through β2 integrins.
CD177 ligation activates neutrophils in a β2 integrin-dependent manner. (A) Neutrophils were incubated with αCD177 or isotype (10 µg/mL × 30 min), with or without LTB4 100 nM, followed by assessment of surface activation by flow cytometry. (B) Quantitation of change in surface activation markers in CD177pos versus CD177neg cells. Panels A and B reflect pooled data from 3 individual donors. (C) Activation of neutrophils from a CD177null donor was not affected by αCD177. Data are pooled from 2 replicate experiments. (D) Peripheral blood neutrophils were incubated with αCD177 or isotype 10 µg/mL ± LTB4 100 nM at 37°C for times as are indicated, detecting Src and ERK activation by western blot. (E) Phosphorylation of Src and ERK by αCD177 is inhibited by contemporaneous addition of blocking antibody against CD18 (MEM148, 10 µg/mL). Panels D and E are representative of 3 experiments in neutrophils from 2 donors. Means ± SEMs. **P < .01; ***P < .001; ****P < .0001; by 2-way ANOVA (A-B). Max, maximum; Min, minimum.
CD177 ligation activates neutrophils in a β2 integrin-dependent manner. (A) Neutrophils were incubated with αCD177 or isotype (10 µg/mL × 30 min), with or without LTB4 100 nM, followed by assessment of surface activation by flow cytometry. (B) Quantitation of change in surface activation markers in CD177pos versus CD177neg cells. Panels A and B reflect pooled data from 3 individual donors. (C) Activation of neutrophils from a CD177null donor was not affected by αCD177. Data are pooled from 2 replicate experiments. (D) Peripheral blood neutrophils were incubated with αCD177 or isotype 10 µg/mL ± LTB4 100 nM at 37°C for times as are indicated, detecting Src and ERK activation by western blot. (E) Phosphorylation of Src and ERK by αCD177 is inhibited by contemporaneous addition of blocking antibody against CD18 (MEM148, 10 µg/mL). Panels D and E are representative of 3 experiments in neutrophils from 2 donors. Means ± SEMs. **P < .01; ***P < .001; ****P < .0001; by 2-way ANOVA (A-B). Max, maximum; Min, minimum.
CD177 ligation enhances adhesion through impaired integrin internalization
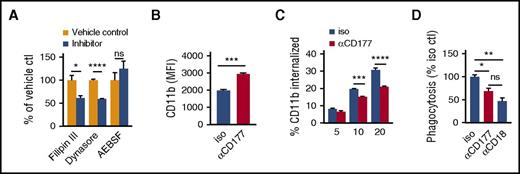
Completion of translocation requires release of β2 integrin attachments at the trailing edge, a step that could potentially be mediated through enzymatic cleavage by the CD177-associated protease PR3 and through integrin internalization.26,27 We tested the effect of inhibitors of serine proteases (4-benzenesulfonyl fluoride hydrochloride [AEBSF]) as well as of endocytic vesicle formation (Filipin III and Dynasore), finding that only the latter modulated migration (Figure 5A; for evidence in support of the efficacy of AEBSF inhibition on CD177-bound PR3, see supplemental Figure 7). Correspondingly, anti-CD177 translated into higher levels of surface CD11b on neutrophils detached from transwell filters by vigorous mechanical washing (Figure 5B). Surface-labeling studies showed that anti-CD177 attenuated integrin internalization, whereas integrin-mediated phagocytosis of complement-opsonized beads was impaired (Figure 5C,D). Thus, in addition to activation-mediated enhancement of integrin surface expression and activation state, anti-CD177 ligation contributed to cell immobilization not through PR3 blockade but instead through impaired internalization of integrin-mediated neutrophil attachments.
CD177 ligation impairs integrin internalization. (A) Peripheral blood neutrophils were incubated with Filipin III (inhibitor of calveolin-mediated endocytosis, 3 µg/mL), Dynasore (inhibitor of dynamin, required for clathrin or calveolin-mediated endocytosis, 80 µM), AEBSF (pan-serine protease inhibitor, 10 µM), or corresponding vehicles and allowed to migrate across a 3-µm pore transwell toward LTB4 100 nM × 2 h. Effect on migration is expressed as a proportion of corresponding vehicle control. Inhibition of endocytosis but not proteolytic cleavage impairs migration. (B) Neutrophils were preincubated with αCD177 or isotype 10 µg/mL RT for 10 min and then added to a transwell apparatus with a 0.4-µm pore membrane that was impermeable to neutrophil transit. After 2 h of attraction toward LTB4 100nM in the bottom chamber, attached neutrophils were liberated from the membrane using EDTA and assessed for CD11b expression. (C) Neutrophils exposed to isotype immunoglobulin G (IgG) or αCD177 10 µg/mL for 15 min at RT were allowed to adhere to 6-well plates and then incubated for with anti-CD11b-phycoerythrin (1 µg/mL, 30 min) at 4°C. After washing, cells were incubated at 37°C in prewarmed media containing 100 nM LTB4, as is indicated, acid-washed to strip off the surface signal, and then detached using EDTA for analysis of internalized fluorescence by flow cytometry. (D) Neutrophils exposed to isotype IgG, αCD177 10 µg/mL, or MEM148 for 10 min at RT were incubated with complement-opsonized 2-µm fluorescent latex beads for 1 h at 37°C, followed by washing in PBS 5-mM EDTA to remove adherent beads. Internalized fluorescence was then analyzed by flow cytometry. Each experiment is representative of at least 3 replicates. Means ± SEMs. *P < .05; **P < .01; ***P < .001; ****P < .0001; by unpaired t test (A-B), 2-way ANOVA (C) or 1-way ANOVA (D).
CD177 ligation impairs integrin internalization. (A) Peripheral blood neutrophils were incubated with Filipin III (inhibitor of calveolin-mediated endocytosis, 3 µg/mL), Dynasore (inhibitor of dynamin, required for clathrin or calveolin-mediated endocytosis, 80 µM), AEBSF (pan-serine protease inhibitor, 10 µM), or corresponding vehicles and allowed to migrate across a 3-µm pore transwell toward LTB4 100 nM × 2 h. Effect on migration is expressed as a proportion of corresponding vehicle control. Inhibition of endocytosis but not proteolytic cleavage impairs migration. (B) Neutrophils were preincubated with αCD177 or isotype 10 µg/mL RT for 10 min and then added to a transwell apparatus with a 0.4-µm pore membrane that was impermeable to neutrophil transit. After 2 h of attraction toward LTB4 100nM in the bottom chamber, attached neutrophils were liberated from the membrane using EDTA and assessed for CD11b expression. (C) Neutrophils exposed to isotype immunoglobulin G (IgG) or αCD177 10 µg/mL for 15 min at RT were allowed to adhere to 6-well plates and then incubated for with anti-CD11b-phycoerythrin (1 µg/mL, 30 min) at 4°C. After washing, cells were incubated at 37°C in prewarmed media containing 100 nM LTB4, as is indicated, acid-washed to strip off the surface signal, and then detached using EDTA for analysis of internalized fluorescence by flow cytometry. (D) Neutrophils exposed to isotype IgG, αCD177 10 µg/mL, or MEM148 for 10 min at RT were incubated with complement-opsonized 2-µm fluorescent latex beads for 1 h at 37°C, followed by washing in PBS 5-mM EDTA to remove adherent beads. Internalized fluorescence was then analyzed by flow cytometry. Each experiment is representative of at least 3 replicates. Means ± SEMs. *P < .05; **P < .01; ***P < .001; ****P < .0001; by unpaired t test (A-B), 2-way ANOVA (C) or 1-way ANOVA (D).
CD177-mediated ERK phosphorylation attenuates neutrophil migration
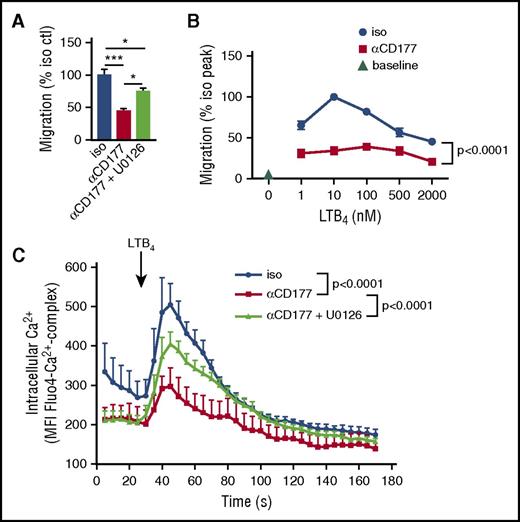
The partial effect of endocytosis blockers suggests that impaired integrin internalization contributes only a part of the migratory blockade resulting from CD177 ligation. Mitogen-activated protein kinases lie downstream of β2 integrins, and activation of ERK is known to impair migration through GRK2-mediated desensitization of chemokine receptors.28 To test whether this pathway contributed to CD177-mediated migratory blockade, we evaluated the effect of ERK blockade with the selective inhibitor U0126. Indeed, this inhibitor partially reversed inhibition of migration by CD177 ligation (Figure 6A). We proceeded to test the effect of CD177 ligation on chemoreceptor sensitization in 2 ways. First, we assessed the effect of MEM166 on the migratory dose response of neutrophils to LTB4 in the transwell setting. Although suppression of migration limited the resolution of this method, the data suggested a shift in migratory peak from LTB4 10 nM to 100 nM (Figure 6B). Second, we tested calcium flux. Neutrophils were loaded with the Ca-sensitive marker Fluo4 to allow interrogation of the timecourse of Ca flux by flow cytometry. Consistent with the expected ERK effect, CD177 ligation markedly dampened LTB4-mediated Ca flux, and this effect was partially reversed by U0126 (Figure 6C). Thus, CD177 ligation contributed to the immobilization of neutrophils via ERK-mediated attenuation of chemoreceptor signaling.
CD177-mediated ERK activation impairs chemoreceptor signaling. (A) Neutrophils were pretreated with MEK inhibitor U0126 (1 µM, 15 min), exposed to αCD177, and then allowed to migrate through a 3-µm pore transwell toward LTB4 for 2 h. ERK inhibition partially rescued migration from CD177 ligation-mediated blockade. (B) Neutrophils were preincubated with αCD177 or isotype (10 µg/mL, 10 min RT) and allowed to migrate across 3- µm transwells for 2 h in response to increasing doses of LTB4. (C) Neutrophils were loaded with the Ca2+-sensitive dye Fluo4 and stimulated with LTB4 ± preincubation with U0126 (1 µM, 15 min), demonstrating suppression of Ca2+ flux by CD177 ligation. Each experiment is representative of at least 2 replicates. Means ± SEMs. *P < .05; **P < .01; ***P < .001; ****P < .0001; by 1-way ANOVA (A) or 2-way ANOVA (B-C).
CD177-mediated ERK activation impairs chemoreceptor signaling. (A) Neutrophils were pretreated with MEK inhibitor U0126 (1 µM, 15 min), exposed to αCD177, and then allowed to migrate through a 3-µm pore transwell toward LTB4 for 2 h. ERK inhibition partially rescued migration from CD177 ligation-mediated blockade. (B) Neutrophils were preincubated with αCD177 or isotype (10 µg/mL, 10 min RT) and allowed to migrate across 3- µm transwells for 2 h in response to increasing doses of LTB4. (C) Neutrophils were loaded with the Ca2+-sensitive dye Fluo4 and stimulated with LTB4 ± preincubation with U0126 (1 µM, 15 min), demonstrating suppression of Ca2+ flux by CD177 ligation. Each experiment is representative of at least 2 replicates. Means ± SEMs. *P < .05; **P < .01; ***P < .001; ****P < .0001; by 1-way ANOVA (A) or 2-way ANOVA (B-C).
Discussion
CD177 is expressed on a subpopulation of human neutrophils in most individuals, but its function remains poorly understood. Here we show that CD177 ligation orchestrates a set of mechanisms that promote neutrophil immobilization during transmigration, likely mediated through β2 integrins as a signaling partner. These mechanisms include enhanced integrin expression and affinity, reduced integrin internalization, and ERK-mediated impairment of chemoattractant signals. These results establish CD177 as a potentially important modulator of human neutrophil migration via an array of pathways mediated through cell activation.
The mechanisms identified here help to explain the otherwise puzzling discordance between the in vitro observation that CD177 ligation attenuates transendothelial migration and the in vivo observation that CD177pos and CD177neg cells migrate equally to inflamed joints, peritoneum, and oral cavity. Migratory blockade by anti-CD177 results in enhancement of rather than in blocking of adhesion, a mechanism reminiscent of the leukadherins, small molecules that immobilize neutrophils through integrin activation.29 This effect offers a potential explanation for the anti-CD177-driven adhesion of neutrophils to HUVEC cells observed by others, a finding that is not an obvious consequence of interference with PECAM-1 binding.18 Interestingly, partial migratory blockade was also observed for “bystander” CD177neg cells, although upregulation of integrins was restricted to cells directly ligated by anti-CD177 antibodies. Whereas we observed no neutrophil crawling under the conditions of our dynamic adhesion studies (Figure 2F), defining the role of CD177 ligation on this aspect of integrin-dependent neutrophil migration will be of interest going forward.30
These results notwithstanding, enhanced migration of unmanipulated CD177pos neutrophils across a HUVEC layer, as a function of the HUVEC PECAM-1 genotype, indicates that CD177 provides a PECAM-1-dependent migratory advantage under some circumstances.16,17 The adhesion molecules required for neutrophil egress vary with vascular bed and with chemoattractant stimulus.31-33 It is possible therefore that future studies will identify locations and disease states under which CD177 plays a role in promoting neutrophil entry into tissues.
Our results develop the understanding of β2 integrins as signaling partners of CD177. Using FLIM molecular imaging, we show not only that CD177 and β2 integrins directly interact but that CD177 ligation with anti-CD177 further approximates these molecules. The precise nature of this enhanced proximity, and its implication for signaling, remains to be determined. Integrin signaling is complex, but we show that this clustering activates both Src as well as the MAP kinase ERK.34 The latter renders CD177-stimulated cells less susceptible to chemoattractant signals, potentially via desensitization of chemokine receptors.28 Integrin activation can initiate a cascade resulting in accelerated internalization.35 CD177 ligation reduces this internalization, likely rendering neutrophils less able to release their trailing edges. How the CD177-integrin interaction mediates impairment of internalization remains unknown.
An endogenous disease process that may engage these pathways is ANCA-associated vasculitis (AAV). The most severe AAV, granulomatous polyangiitis, is most commonly associated with antibodies against PR3, a protease that adheres to the neutrophils’ surface via CD177.12-14 Correspondingly, the proportion of neutrophils expressing CD177 is higher in patients with ANCA vasculitis than in healthy donors.36 Antibodies against PR3 mediate neutrophil activation in vitro, and murine studies with antibodies against the alternative ANCA antigen myeloperoxidase suggest that antibody-mediated neutrophil activation contributes to vascular injury in vivo.37 Jerke et al demonstrated that PR3-ANCA antibodies activate neutrophils via CD177 in a β2 integrin-dependent manner.11 Here we show that CD177 ligation results in migratory arrest, an effect that may contribute potentially to endothelial injury as observed in leukocytoclastic vasculitis. A corollary of this observation is that blockade of CD177 might attenuate ANCA-mediated neutrophil activation and so offer a new therapeutic approach to this vasculitis.
Although our results provide insight into CD177 function, the physiological relevance of the striking variability of CD177 expression among human neutrophils remains obscure.38 The proportion of CD177pos neutrophils is a direct correlate of the number of CD177 alleles with an uninterrupted open reading frame. In most cases, the CD177null phenotype arises through replacement of exons of both copies of CD177 with those of the nearby pseudogene CD177P1 that encodes a stop codon. Subjects with a low or moderate proportion of CD177pos cells usually have 1 intact and 1 interrupted CD177 allele, and individuals with more than 50% to 60% CD177pos neutrophils typically have 2 intact copies.4 This genetic heterogeneity translates into cell-to-cell variation through epigenetic silencing of 1 parental allele.5 How this occurs is incompletely understood, in particular because the proportion of CD177pos cells is rarely 100%, even in individuals homozygous for intact CD177. No distinct physiological roles for CD177pos and CD177neg cells have been defined, although some recent data have suggested a possible role for CD177pos cells as an effector in inflammatory bowel disease.39 No in vivo circumstance has yet been described in which these neutrophils migrate differentially. Surface expression and in vitro studies have so far failed to distinguish differences between CD177pos and CD177neg neutrophils, whereas CD177null individuals and CD177-deficient mice appear generally healthy.21,40 Therefore the selective pressures that have supported both the preservation of CD177 across mammalian species as well as its variable expression remain to be determined.
The present data highlight how ligation of CD177 triggers functional consequences distinct from those that result from surface expression. Mechanisms beyond PECAM-1 interference translate CD177 ligation into cell-intrinsic migratory blockade, including alterations in integrin expression and internalization as well as reduced chemoattraction. These mechanisms are relevant not only to CD177 subpopulations but also to ANCA-associated vasculitis, wherein anti-PR3 antibodies may activate similar pathways, potentially contributing to vascular injury and end-organ disease.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was funded by the National Institutes of Health (NIH), National Institute of Arthritis and Musculoskeletal and Skin Diseases (awards R01 AR065538 and P30 AR070253) and by the Fundación Bechara (P.A.N.). R.G.-B. was supported by an MD Fellowship from Boehringer Ingelheim Fonds and a JBC Microgrant. J.W. was supported by a grant from the Arthritis National Research Foundation. O.H. was supported by the NIH, National Institute of Allergy and Infectious Diseases (T32 AI007512) and a JBC Microgrant. P.C. was supported by a JBC Microgrant.
Authorship
Contribution: M.B. and R.G.-B. designed the research, performed research, analyzed data, and contributed to writing the paper; J.W. designed research, performed research, and analyzed data; A.B.S., Z.S.W., and C.T.L. performed research and analyzed data; L.Z., O.H., M.D.G., H.N.N., A.L., and P.C. performed research; R.J.S. analyzed data; and P.A.N. conceptualized and designed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peter A. Nigrovic, Brigham and Women’s Hospital, Building for Transformative Medicine 6002-L, 60 Fenwood Rd, Boston, MA 02115; e-mail: pnigrovic@bwh.harvard.edu.
References
Author notes
M.B. and R.G.-B. contributed equally to this study.