In this issue of Blood, we review 2 studies investigating neutrophil integrin activation. Morikis et al describe the molecular dynamics of mechanotransduction, which underlie E-selectin–mediated neutrophil rolling and adhesion. Bai et al investigate CD177-induced integrin activation in regulating adhesion and transmigration.1,2
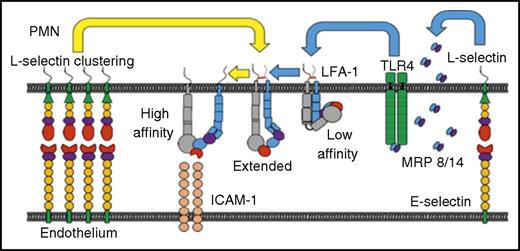
E-selectin cross-linking activates the release of myeloidrelated protein (MRP) 8/14 and extension of β2 integrin to a high-affinity state. PMN, polymorphonuclear leukocyte; TLR, Toll-like receptor. See Figure 6A in the article by Morikis et al that begins on page 2101.
E-selectin cross-linking activates the release of myeloidrelated protein (MRP) 8/14 and extension of β2 integrin to a high-affinity state. PMN, polymorphonuclear leukocyte; TLR, Toll-like receptor. See Figure 6A in the article by Morikis et al that begins on page 2101.
Neutrophil recruitment can be broadly conceptualized as 3 sequential steps: rolling, adhesion, and transmigration. The interaction between endothelial cell selectins (E-selectin) and glycosylated neutrophil ligands (L-selectin, P-selectin glycoprotein ligand-1 [PSGL-1], sialylated glycosphingolipids) promotes neutrophil rolling along the vascular endothelium through catch-bonds and slip-bonds. Adhesion is established through the interaction of neutrophil integrins LFA-1 (CD11a/CD18a) and Mac-1 (CD11b/CD18) with their endothelial cell counterreceptor, ICAM-1. Neutrophil transmigration then occurs at interendothelial junctions requiring associations between LFA-1 and Mac-1 with ICAM-1, ICAM-2, and JAM family members, and the contributions of CD99 and PECAM-1.3 The spatial-temporal regulation of integrin activation plays a critical role in all steps of neutrophil recruitment.
In contrast to mouse neutrophils, human neutrophils can transition LFA-1 to the high-affinity state via rolling over E-selectin, even in the absence of G protein–coupled receptor signaling.4,5 Human neutrophils incorporate N-glycans with the tetrasaccharide carbohydrate sialyl Lewisx (sLex) onto L-selectin. This difference allows human neutrophils to bind and cluster E-selectin, leading to the release of cytosolic calcium, activation of Src-family kinases, and β2-integrin activation. Although previous studies have demonstrated that blocking antibodies to E-selectin can prevent human neutrophil arrest under shear conditions, it is still unclear how E-selectin mediates outside-in signaling to promote a shift in β2 integrins from the intermediate to high-affinity conformation.6
Using Rivipansel, an sLex tetrasaccharide structure mimic that disrupts L-selectin interactions, Morikis and colleagues explored the role of E-selectin in mediating outside-in signaling of neutrophil β2 integrin. The authors found that Rivipansel significantly inhibited neutrophil arrest and migration across interleukin 1β (IL-1β)–stimulated endothelial cells. Because Rivipansel is recognized by all 3 selectins, the authors used blocking antibodies to known E-selectin ligands (CD44, PSGL-1, L-selectin) to determine the relative contributions of each to integrin activation in neutrophil rolling and following E-selectin cross-linking. All blocking antibodies inhibited E-selectin binding; however, only anti-L-selectin antibodies inhibited high-affinity CD18 activation in response to E-selectin cross-linking. Under shear flow conditions, blocking L-selectin interactions also inhibited neutrophil arrest on E-selectin, results similar to those with anti-β2-integrin blocking antibodies. In contrast, PSGL-1 blocking antibodies were unable to disrupt neutrophil arrest.
E-selectin catch-bond formation allows for an increasing force to convert short-lived tethers into strong, longer-lived binding interactions (see figure). To investigate the bond mechanics between sLex and E-selectin, the authors observed neutrophil capture under variable shear stress conditions. Both Rivipansel and the PSGL-1 glycopeptide mimetic, GSnP-6, increased neutrophil mean rolling velocity; however, only Rivipansel inhibited neutrophil arrest. It is known that both L-selectin and PSGL can form catch-bonds when tether force and wall shear stress are increased. However, the authors report that tether duration and efficiency were significantly decreased when L-selectin interactions were blocked, as compared with blocking PSGL-1. In addition, the authors observed L-selectin clustering and increased focal adhesion on neutrophils under shear conditions, which were blocked when Rivipansel was used to disrupt L-selectin interactions. Such clustering has previously been associated with Src kinase activity. Indeed, the authors found that Rivipansel inhibited activation of the Src kinase, Lck, and thereby high-affinity β2-integrin expression. Interestingly, the ligation of E-selectin also resulted in the concomitant release of MRP8, which appears to contribute to a shift in β2 integrin to an intermediate-affinity state.
Morikis et al reveal additional insight into the evolutionary differences between mouse and human neutrophils, and demonstrate that efficient high-affinity β2-intergrin expression associated with E-selectin mechanotransduction may require a second signal, stressing the importance of temporal regulation in neutrophil integrin activation. Understanding the contribution of these signaling pathways in neutrophil recruitment will require future investigations. This study also describes the therapeutic utility of Rivipansel for treating vascular occlusive disease and invites the possibly of its use in other conditions to regulate neutrophil recruitment.
Bai and colleagues investigate the next step in neutrophil recruitment: the transition from cell adhesion to migration. For most individuals, CD177 is expressed on a subset of neutrophils (45%-65%), although a small population of individuals (3%-5%) do not express CD177 on any circulating neutrophils (CD177null). CD177 is the counterreceptor for PECAM-1 and has been shown to play a role in neutrophil transmigration.7 CD177 has also been reported to anchor the neutrophil serine protease proteinase 3 to the neutrophil surface.8 CD177 has been shown to physically associate with β2 integrins, particularly Mac-1 (CD11b/CD18), but the role of this interaction is unclear.9 Although several studies have demonstrated a migratory advantage for CD177pos neutrophils, other studies have shown that this enrichment does not occur in vivo.10 Therefore, the role of CD177 in neutrophil recruitment is an area of continued investigation.
Using a PECAM-1–independent transwell system, Bai and colleagues report that CD177 ligation (using a cross-linking CD177 antibody) disrupted CD177pos neutrophil migration in response to IL-8 or fMLP. Ligation of CD177 was found to enhance CD177pos neutrophil adherence and spreading but, paradoxically, ligation of CD177 inhibited neutrophil translocation. The authors found that ligation of CD177 enhanced the expression of CD11a, CD11bA (the active confirmation of CD11b), CD66, and activated Src and extracellular signal-regulated kinase (ERK) signaling. Strikingly, the ERK inhibitor U0126 was found to partially restore neutrophil migration of CD177pos neutrophils following ligation.
Previous studies have reported that CD177 associates closely with β2 integrins.9 Using fluorescence lifetime imaging microscopy, the authors confirmed close spatial interactions between CD11b and CD177 and report that ligation inhibited integrin internalization. This suggests that CD177 may be able to arrest cell migration not only through integrin activation, but also by inhibiting integrin internalization and recycling.
The ligation of CD177 is most likely to occur when neutrophils are transmigrating, contacting PECAM-1 at endothelial cell junctions during transmigration, a location where arresting neutrophil transmigration would be undesirable. Therefore, if CD177 inhibits neutrophil migration, then we would expect other factors to compensate for this by promoting integrin recycling or uncoupling trailing integrin associations. Whether this includes neutrophil serine proteases or other soluble factors will be an intriguing area for future investigations.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal