Key Points
Neutrophils rolling on E-selectin form catch-bonds with L-selectin that mechanosignal β2-integrin bond formation with intracellular adhesion molecule 1.
Rivipansel blocks E-selectin recognition of sLex on L-selectin, thereby antagonizing outside-in signaling of high-affinity β2-integrin.
Abstract
E-selectin extends from the plasma membrane of inflamed endothelium and serves to capture leukocytes from flowing blood via long-lived catch-bonds that support slow leukocyte rolling under shear stress. Its ligands are glycosylated with the tetrasaccharide sialyl Lewisx (sLex), which contributes to bond affinity and specificity. E-selectin-mediated rolling transmits signals into neutrophils that trigger activation of high-affinity β2-integrins necessary for transition to shear-resistant adhesion and transendothelial migration. Rivipansel is a glycomimetic drug that inhibits E-selectin-mediated vaso-occlusion induced by integrin-dependent sickle–red blood cell–leukocyte adhesion. How Rivipansel antagonizes ligand recognition by E-selectin and blocks outside-in signaling of integrin-mediated neutrophil arrest while maintaining rolling immune-surveillance is unknown. Here, we demonstrate that sLex expressed on human L-selectin is preferentially bound by E-selectin and, on ligation, initiates secretion of MRP8/14 that binds TLR4 to elicit the extension of β2-integrin to an intermediate affinity state. Neutrophil rolling over E-selectin at precise shear stress transmits tension and catch-bond formation with L-selectin via sLex, resulting in focal clusters that deliver a distinct signal to upshift β2-integrins to a high-affinity state. Rivipansel effectively blocked formation of selectin catch-bonds, revealing a novel mechanotransduction circuit that rapidly converts extended β2-integrins to high-affinity shear-resistant bond clusters with intracellular adhesion molecule 1 on inflamed endothelium.
Introduction
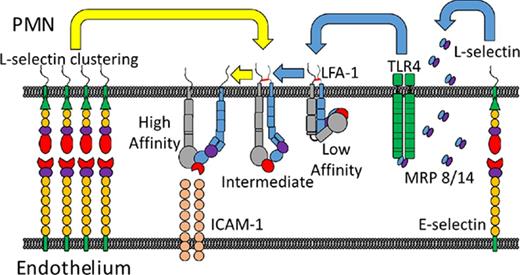
Selectins are C-type lectin glycoproteins that initiate leukocyte recruitment at sites of inflammation.1,2 Each selectin contains a lectin-EGF domain that is conserved across mammals that recognizes tetrasaccharide sialyl Lewisx (sLex) expressed on glycoprotein and glycolipid ligands, including P-selectin glycoprotein ligand-1 (PSGL-1), CD44, and E-selectin ligand-1 (ESL-1) on mouse polymorphonuclear leukocytes (PMNs). Human E-selectin recognizes PSGL-1, L-selectin, and sialylated glycosphingolipids.3,4 E-selectin binding to its ligands on PMNs supports slow rolling that facilitates interrogation of the vasculature during inflammation. E-selectin ligation of L-selectin and PSGL-1 receptors induces their redistribution into membrane clusters that elicits release of cytosolic calcium and activation of Src-family tyrosine kinases.5 This process, denoted outside-in signaling, activates an upshift in β2-integrin binding affinity, which on bond formation with endothelial intracellular adhesion molecules (ICAMs) leads to PMN arrest on inflamed endothelium.5-8 L-selectin expressed on murine PMNs is not recognized by E-selectin; they lack the fucosyltransferase (FUT9) to link fucose onto sLex presenting O- and N-linked amino acids on L-selectin.9,10 In mouse, the mechanism for outside-in signaling during PMN rolling on inflamed venules is attributed to E-selectin engagement of PSGL-1, which associates with L-selectin at the tips of microvilli.11-13 It is important to distinguish the mechanism of ESL recognition and receptor clustering between mouse and human, as this may account for the species difference in mechanosignaling via selectins.6
After colocalization of PSGL-1/L-selectin by E-selectin, a signaling complex forms by interaction with Src-family tyrosine kinases on the cytosolic side of membrane rafts.14 This in turn phosphorylates the immunoreceptor tyrosine-based activation motif, leading to extension of the β2-integrin LFA-1 (CD11a/CD18) into an intermediate conformation that supports slow rolling on ICAM-1.15-17 Subsequent transition of LFA-1 to a high-affinity state that supports PMN arrest on inflamed mouse venules requires chemokine signaling through G protein-coupled receptor (GPCR) ligation.9,12,18,19 Unlike mouse PMNs, human PMNs can transition LFA-1 to a high-affinity state via rolling over E-selectin in the absence of GPCR signaling.20-22 Human PMNs have evolved a means of decorating N-glycans with sLex on L-selectin that facilitates clustering and its capacity to signal the conversion of β2-integrins to high affinity.23,24 Pruenster et al24 demonstrated a distinct pathway for activating β2-integrin. They showed that mouse PMNs rolling on E-selectin result in secretion of myeloid-related protein (MRP)8/14 that signals RAP1-GTPase activation of β2-integrin via ligation of toll-like receptor-4 (TLR4).24 However, the events associated with E-selectin-mediated outside-in signaling of a shift in β2-integrin conformation from intermediate to high affinity have not been established. We address how E-selectin recognition of PSGL-1 and L-selectin under tensile force leads to distinct bond mechanics that support mechanotransduction of signals that activate β2-integrin.
The species difference between human and mouse ligand recognition by E-selectin has significant ramifications for efficient targeting of glycomimetics to intervene in inflammatory PMN recruitment. Rivipansel is a selectin antagonist in clinical trials for the treatment of vascular occlusive crisis associated with sickle cell disease.25 It mimics the sLex tetrasaccharide structure and contains an extended sulfate domain that is recognized by all 3 selectins.26-28 GSnP-6 is another pan-selectin glycomimetic that structurally resembles the N-terminal domain of PSGL-1 and binds with highest affinity to P-selectin.29 Given that Rivipansel functions as an sLex mimetic and GSnP-6 as a PSGL-1 mimetic, we employed them to discriminate the relative importance of E-selectin recognition of sLex on L-selectin versus PSGL-1 during PMN recruitment.
To enable efficient recruitment, E-selectin is capable of forming catch-bonds with its ligands on PMNs. E-selectin in complex with sLex under force is hypothesized to adopt a high-affinity extended conformation during catch-bond formation, which prolongs bond lifetime and transfers force that pulls membrane tethers during PMN slow rolling.30-35 Above a threshold in shear force, catch-bonds transition to slip-bonds characterized by a shorter bond lifetime and higher rolling velocities under flow.36 We previously reported that E-selectin binding to sLex on L-selectin and PSGL-1 on a rolling PMN drives colocalization of these receptors on membrane tethers at the trailing edge, resulting in activation of β2-integrin-mediated cell arrest.6,7 In this report, we employ real-time immunofluorescence total internal reflection fluorescent microscopy (TIRF) imaging of PMNs to examine the molecular dynamics of selectin-ligand receptor engagement during the transition from cell tethering and rolling to arrest on recombinant E-selectin and ICAM-1 in a microfluidic channel. We demonstrate that Rivipansel blocks E-selectin recognition of sLex on L-selectin and antagonizes formation of catch-bonds necessary for focal membrane clustering and outside-in signaling. These data highlight a species difference in sLex ligand recognition by E-selectin that accounts for its capacity to mechanotransduce via L-selectin the activation of high-affinity β2-integrin-dependent arrest in human and not mouse PMNs.
Materials and methods
Study subjects
Rivipansel is a selectin antagonist that mimics the sLex tetrasaccharide structure, currently in clinical trials for the treatment of vascular occlusive crisis in patients with sickle-cell disease (SCD). Phase 1/2 clinical trials were conducted on adults with sickle cell disease.25,28 Rivipansel was intravenously infused (20 mg/kg) followed by a 10 mg/kg dose 10 hours later. Blood samples were collected, and PMN adhesion to recombinant E-selectin/ICAM-1 (E/I) substrates assembled on glass coverslips beneath PDMS microfluidic channels21 were measured.
TIRF and qDF
Protein A/G coated coverslips were coated with E-selectin and/or ICAM-1 (1 μg/mL), as previously described, and assembled on microfluidic devices.21 TIRF and quantitative dynamic footprinting (qDF)37,38 were employed to record fluorescently tagged antibodies targeting L-selectin (DREG55) and PSGL-1 (PL2) cluster area and frequency during rolling of isolated PMNs over E- or P-selectin in the presence or absence of glycomimetic inhibition. DREG55 did not alter L-selectin’s recognition by E-selectin or β2-integrin activation (supplemental Figure 2A-B, available on the Blood Web site).39 High-affinity CD18 clusters were quantified using TIRF on PMNs that rolled to arrest in the presence of mAb24.
PMN bead collision assay
Protein G-coated beads (diameter = 1 μm) were derivitized with E-selectin-IgG, as per manufacturer’s instructions. Beads were then treated as described by Edmondson et al.40 PMNs were treated with blocking antibodies to PSGL-1 (KPL-1) and Mac-1 (M1/7041 ) before perfusion. Adhesive interactions were identified as collisions that showed a pause in PMN motion for 1 frame with velocities below the hydrodynamic velocity.
Additional information in supplemental Data.
Results
Rivipansel inhibits neutrophil arrest and migration across inflamed endothelium
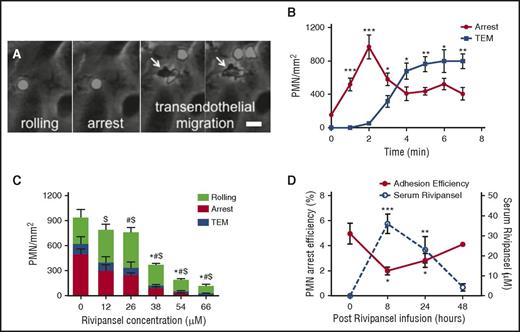
E-selectin mediates slow rolling of PMNs and triggers integrin-mediated deceleration, as evidenced by blocking E-selectin or ICAM-1 with antibodies that abrogate transition to arrest.6,42-45 This prompted us to examine recruitment under shear flow in microfluidic channels (supplemental Figure 1A) of PMNs isolated from healthy subjects and from blood samples obtained from patients with SCD participating in phase 2 clinical trials.25,28 On IL-1β inflamed endothelium, PMNs transitioned from rolling to arrest, and within minutes, a majority (∼60%) adopted a polarized shape before transmigrating underneath the monolayer (Figure 1A-B). We examined the dose-dependent effect of Rivipansel on the multistep process of PMN recruitment on stimulated human umbilical vein endothelial cells. Rivipansel exhibited a 50% inhibitory concentration (IC50) ∼26 μM for antagonizing the transition to arrest and a slightly lower IC50 of ∼17 μM in blocking transmigration of PMNs obtained from healthy subjects (Figure 1C; supplemental Figure 1B). It is noteworthy that Rivipansel exerted a greater inhibitory effect on signaling the transition from rolling to arrest than on PMN capture and rolling (Figure 1C). Blood samples from patients with SCD were assessed ex vivo for serum levels of drug along with the capacity for PMNs to roll to arrest over an E/I substrate (Figure 1D; supplemental Table 1). PMN arrest efficiency decreased for all patients over the course of 8 hours of Rivipansel infusion, before rising in a manner inversely correlated with drug serum levels (Figure 1D; supplemental Table 1). We examined the effect of Rivipansel on healthy African American subjects whose blood was left untreated or doped in vitro at concentrations measured in patients with SCD. PMN arrest efficiency was equivalent between ethnic controls and patients, as was the efficacy of inhibition for rolling to arrest (supplemental Figure 1C). These data demonstrate that PMN recruitment from blood is initiated by stable adhesion signaled through E-selectin and inhibited in a dose-dependent manner by Rivipansel.
PMN arrest and transmigration on inflamed endothelium is inhibited by Rivipansel. Isolated human PMNs were perfused over IL-1β-stimulated human umbilical vein endothelial cell monolayers in a microfluidic flow chamber at physiological shear stress of 2 dynes/cm2. (A) PMN rolling to arrest and transmigration across human umbilical vein endothelial cell monolayers are superimposed. PMNs are phase bright until migration below endothelial monolayer, then become phase dark (marked by white arrows; scale bar, 10 μm). (B) PMN density, adherent on inflamed human umbilical vein endothelial cells measured after 2 minutes of shear flow. Arrest and TEM averaged from 5 fields of view represents mean ± SEM for 40 cells (n = 3 separate experiments). Significant differences are indicated for PMN arrest and TEM compared with stimulation and no shear condition. ***P < .001; **P < .01; *P < .05. (C) PMN rolling at 3 minutes, arrest, and TEM density on human umbilical vein endothelial cells at 7 min in the presence and absence of Rivipansel inhibition are superimposed. Data points represent mean ± SEM from 5 fields per condition (n = 4 separate experiments). Rolling (*), arrest ($), and TEM (#) significance (at P < .01) compared with untreated reported. (D) PMN arrest efficiency (number of PMNs captured/count in blood) of whole blood sheared over recombinant E-selectin/ICAM-1 for 4 patients treated for sickle cell disease before (t = 0) and after Rivipansel infusion (left y-axis) and average serum concentrations of Rivipansel after infusion (right y-axis). Each data point is the mean ± SEM of 5 to 11 fields of view along the centerline of the channel. ***P < .001; **P < .005; *P < .01 denote significance from baseline at t = 0). TEM, transendothelial migration.
PMN arrest and transmigration on inflamed endothelium is inhibited by Rivipansel. Isolated human PMNs were perfused over IL-1β-stimulated human umbilical vein endothelial cell monolayers in a microfluidic flow chamber at physiological shear stress of 2 dynes/cm2. (A) PMN rolling to arrest and transmigration across human umbilical vein endothelial cell monolayers are superimposed. PMNs are phase bright until migration below endothelial monolayer, then become phase dark (marked by white arrows; scale bar, 10 μm). (B) PMN density, adherent on inflamed human umbilical vein endothelial cells measured after 2 minutes of shear flow. Arrest and TEM averaged from 5 fields of view represents mean ± SEM for 40 cells (n = 3 separate experiments). Significant differences are indicated for PMN arrest and TEM compared with stimulation and no shear condition. ***P < .001; **P < .01; *P < .05. (C) PMN rolling at 3 minutes, arrest, and TEM density on human umbilical vein endothelial cells at 7 min in the presence and absence of Rivipansel inhibition are superimposed. Data points represent mean ± SEM from 5 fields per condition (n = 4 separate experiments). Rolling (*), arrest ($), and TEM (#) significance (at P < .01) compared with untreated reported. (D) PMN arrest efficiency (number of PMNs captured/count in blood) of whole blood sheared over recombinant E-selectin/ICAM-1 for 4 patients treated for sickle cell disease before (t = 0) and after Rivipansel infusion (left y-axis) and average serum concentrations of Rivipansel after infusion (right y-axis). Each data point is the mean ± SEM of 5 to 11 fields of view along the centerline of the channel. ***P < .001; **P < .005; *P < .01 denote significance from baseline at t = 0). TEM, transendothelial migration.
E-selectin binds to sLex on L-selectin during transition from rolling to arrest on ICAM-1
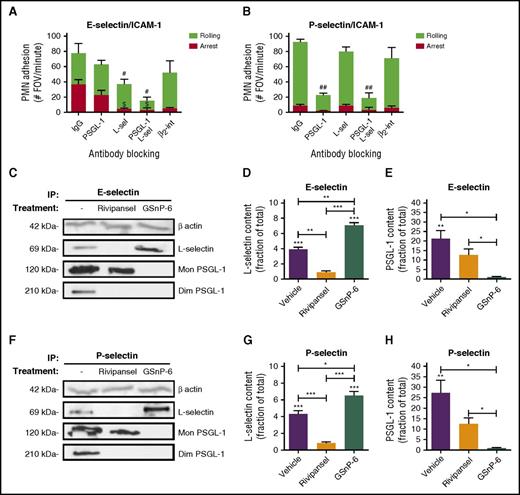
In mouse, P-selectin and E-selectin upregulated on inflamed endothelium bind to PSGL-1 as the predominant sLex expressing ligand on PMNs, and activation of β2-integrin to the extended conformation is mediated through its colocalization with L-selectin.12,23,46,47 To determine the relative contributions of known human ESLs in PMN binding and activation via E-selectin, assays were performed in the presence of antibodies to CD44, PSGL-1, and L-selectin. Anti-PSGL-1 resulted in a ∼40% decrease compared with an IgG control antibody, whereas anti-L-selectin elicited a ∼25% decrease in E-selectin binding (supplemental Figure 2A). Anti-CD44 resulted in a ∼20% decrease in E-selectin binding, and no additional inhibition was observed in conjunction with anti-L-selectin and/or anti-PSGL-1. To examine the role of these ESLs in outside-in signaling of β2-integrin activation, E-selectin-IgG was bound to PMNs and cross-linked with the same blocking antibodies (supplemental Figure 2B). Only anti-L-selectin showed a significant decrease in high-affinity CD18 activation. This indicates that CD44 plays a marginal role in E-selectin binding and activation of PMNs. We next assessed the relative contributions of L-selectin and PSGL-1 to recruitment of human PMNs under shear flow over E/I or P/I substrates (Figure 2A-B). Blocking L-selectin abrogated the transition to arrest on E-selectin, similar to the absence of ICAM-1, or blocking β2-integrin function with antibody42,44 (supplemental Figure 2C). Blocking PSGL-1 did not diminish arrest efficiency, but in conjunction with anti-L-selectin, abrogated rolling on E-selectin (Figure 2A). Shearing PMNs on a substrate of P-selectin revealed a similar frequency of cell capture and rolling, but arrest efficiency did not rise above baseline (Figure 2B; supplemental Figure 2D). We conclude that PSGL-1 functions as an efficient ligand for PMN capture and rolling, but does not activate the transition to arrest under shear stress on P- or E-selectin. To directly compare the capacity of PMNs isolated from mouse versus human blood to roll and activate on E-selectin, a competitive recruitment assay over human or mouse E/I was performed between fluorescent (LysM-EGFP) murine PMNs and DiI-labeled human PMNs (supplemental Figure 2E-F). PMNs from either species captured and rolled with equivalent efficiency on E-selectin; however, arrest efficiency was markedly higher for human PMNs rolling on human E-selectin than on mouse (∼48% on human E-selectin/human ICAM-1, ∼33% on mouse E-selectin/human ICAM-1). These data indicate that E-selectin recognition of L-selectin on human PMNs is necessary and sufficient to activate arrest via β2-integrin-ICAM-1 bonds.
E-selectin binds sLexon L-selectin and inhibition with Rivipansel. Isolated human PMNs were treated with IgG, anti-PSGL-1 antibody (KPL-1), anti-L-selectin (DREG56), the combination, or anti-β2-integrin (IB4) antibody and perfused over (A) E/I or (B) P/I substrate in a microfluidic chamber at 2 dynes/cm2. PMN rolling (#P < .05; ##P < .001) and arrest ($P < .01) significance compared with IgG control, subtracting background adhesion. Values depicted are mean ± SEM for n = 3 separate experiments. L-selectin and PSGL-1 were immunoprecipitated from isolated human PMNs with recombinant E-selectin or P-selectin treated with vehicle, or in the presence of Rivipansel (100 μM), or GSnP-6 (120 μM). Western blot protein content of (C) E-selectin-IgG pulldown and quantitation of the ratio of (D) L-selectin (∼69 kDa) or (E) PSGL-1 (∼120 kDa + ∼210 kDa) to untreated immunoprecipitation of total protein. (F) P-selectin pulldown of (G) L-selectin and (H) PSGL-1 relative to total protein pulldown. Relative density of L-selectin and PSGL-1, respectively, was compared between mean ± SEM, as depicted (n = 3 separate experiments). *P < .05; **P < .01; ***P < .001.
E-selectin binds sLexon L-selectin and inhibition with Rivipansel. Isolated human PMNs were treated with IgG, anti-PSGL-1 antibody (KPL-1), anti-L-selectin (DREG56), the combination, or anti-β2-integrin (IB4) antibody and perfused over (A) E/I or (B) P/I substrate in a microfluidic chamber at 2 dynes/cm2. PMN rolling (#P < .05; ##P < .001) and arrest ($P < .01) significance compared with IgG control, subtracting background adhesion. Values depicted are mean ± SEM for n = 3 separate experiments. L-selectin and PSGL-1 were immunoprecipitated from isolated human PMNs with recombinant E-selectin or P-selectin treated with vehicle, or in the presence of Rivipansel (100 μM), or GSnP-6 (120 μM). Western blot protein content of (C) E-selectin-IgG pulldown and quantitation of the ratio of (D) L-selectin (∼69 kDa) or (E) PSGL-1 (∼120 kDa + ∼210 kDa) to untreated immunoprecipitation of total protein. (F) P-selectin pulldown of (G) L-selectin and (H) PSGL-1 relative to total protein pulldown. Relative density of L-selectin and PSGL-1, respectively, was compared between mean ± SEM, as depicted (n = 3 separate experiments). *P < .05; **P < .01; ***P < .001.
To examine the importance of sLex for selectin ligand recognition, PMNs were lysed and immunoprecipitated with human E-selectin or P-selectin conjugated beads. Rivipansel abrogated pull down of L-selectin by E- or P-selectin to baseline (Figure 2C-H), and reduced by ∼50% binding of PSGL-1. GSnP-6, which mimics the N-terminal domain of PSGL-1, abrogated pull-down of PSGL-1 by either E- or P-selectin. Unexpectedly, GSnP-6 promoted a significant increase in L-selectin pull-down by E- and P-selectin, perhaps through antagonizing the interaction between L-selectin and PSGL-1. These data indicate that E- and P-selectin binding to L-selectin occurs predominantly via recognition of sLex that is effectively blocked by Rivipansel. PSGL-1 binding is partially inhibited by Rivipansel, but pull-down is abrogated by GSnP-6, which efficiently antagonizes binding of sLex along with the sulfotyrosine-amino acid motif important for its recognition.48
sLex glycomimetics alter E-selectin bond mechanics
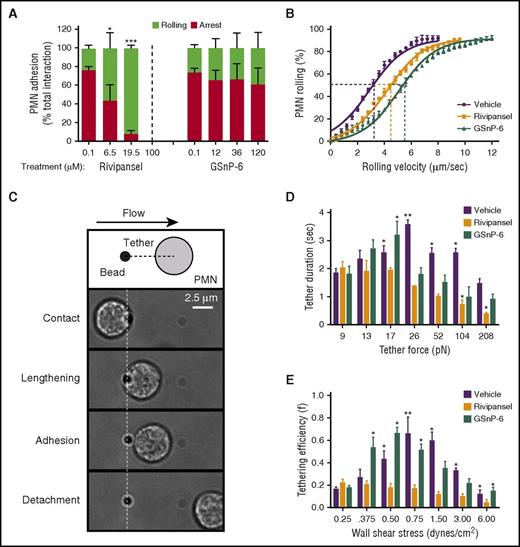
Slow rolling of PMNs on E-selectin involves catch-bond formation with ESLs.30,49,50 The efficiency of tether formation and the mechanical properties that dictate the duration to bond rupture are unique for each ESL.36,50-52 To assess the influence of glycomimetic dose on the inhibition of PMNs rolling and transition to arrest, PMNs were perfused over a substrate of E/I. GSnP-6 decreased the frequency of PMN capture on E-selectin, but did not reduce the transition to arrest (Figure 3A). Rivipansel inhibited arrest by ∼60% at a dose of ∼6.5 μM, which matched its IC50 in blocking activation of high-affinity β2-integrin signaled through cross-linking bound E-selectin-IgG (supplemental Figure 3). We next examined the influence of sLex recognition on rolling velocity over an E-selectin substrate. PMNs rolled at a mean velocity of 3.0 μm/s and variance of 2.83 μm2/s2 (Figure 3B). Treatment with Rivipansel or GSnP-6 elevated mean rolling velocity by 33% and 75%, respectively, and also increased the variance (4.57 and 7.20 μm2/s2, respectively). These data are consistent with glycomimetic disruption of sLex recognition decreasing the number and/or strength of E-selectin bonds, and with PSGL-1 predominately mediating rolling velocity, whereas L-selectin is necessary to activate β2-integrin arrest on ICAM-1.
sLexglycomimetics alter rolling to arrest on E-selectin and bond mechanics. (A) PMNs rolling to arrest over E/I substrates (<0.4 μm/sec) treated with vehicle (PBS), Rivipansel, or GSnP-6 was measured and normalized to total number of interacting PMNs; data are reported as mean ± SEM (n = 3 separate experiments; *P < .05; ***P < .001 compared with vehicle). (B) PMN rolling velocity was quantified over an E-selectin substrate treated with vehicle, Rivipansel (6.5 μM), or GSnP-6 (12 μM) and binned at intervals of 0.4 μm/sec. Comparison of GMI-1070 showed *significance and GSnP-6 showed ***significance over untreated controls (n = 3 separate experiments; *P < .05 and ***P < .001). (C) Schematic depicts dynamic interaction between PMNs and recombinant E-selectin-coated protein-G beads recorded at 50 frames per second in the flow channel. Isolated human PMNs were treated with an anti-Mac-1 and anti-PSGL-1 blocking antibodies and then perfused through flow chambers. PMNs pivot over beads and pull a membrane tether at defined wall shear stress. Adhesive interactions were identified as collisions that had a visible pause in PMN motion for at least 1 frame, along with velocities below the hydrodynamic velocity. (D) Tether duration was compared with step-wise increases in calculated tether forces. (E) Tether efficiency (collisions resulting in adhesion divided by the total collisions observed) as shear stress was ramped in a stepwise manner. Data were reported as mean ± SEM (n = 3 separate experiments; **P < .01; *P < .05 glycomimetics compared with 9 pN tether force and 0.25 dynes/cm2 wall shear stress).
sLexglycomimetics alter rolling to arrest on E-selectin and bond mechanics. (A) PMNs rolling to arrest over E/I substrates (<0.4 μm/sec) treated with vehicle (PBS), Rivipansel, or GSnP-6 was measured and normalized to total number of interacting PMNs; data are reported as mean ± SEM (n = 3 separate experiments; *P < .05; ***P < .001 compared with vehicle). (B) PMN rolling velocity was quantified over an E-selectin substrate treated with vehicle, Rivipansel (6.5 μM), or GSnP-6 (12 μM) and binned at intervals of 0.4 μm/sec. Comparison of GMI-1070 showed *significance and GSnP-6 showed ***significance over untreated controls (n = 3 separate experiments; *P < .05 and ***P < .001). (C) Schematic depicts dynamic interaction between PMNs and recombinant E-selectin-coated protein-G beads recorded at 50 frames per second in the flow channel. Isolated human PMNs were treated with an anti-Mac-1 and anti-PSGL-1 blocking antibodies and then perfused through flow chambers. PMNs pivot over beads and pull a membrane tether at defined wall shear stress. Adhesive interactions were identified as collisions that had a visible pause in PMN motion for at least 1 frame, along with velocities below the hydrodynamic velocity. (D) Tether duration was compared with step-wise increases in calculated tether forces. (E) Tether efficiency (collisions resulting in adhesion divided by the total collisions observed) as shear stress was ramped in a stepwise manner. Data were reported as mean ± SEM (n = 3 separate experiments; **P < .01; *P < .05 glycomimetics compared with 9 pN tether force and 0.25 dynes/cm2 wall shear stress).
To further assess sLex recognition on the strength and duration of selectin bonds, a PMN–bead collision assay was performed under defined shear stress. E-selectin-IgG-coated beads were annealed to the substrate of microfluidic channels and served as targets to capture PMNs from the flow field to measure durable membrane tethers and survival until bond rupture (Figure 3C; supplemental Figure 4).30,40,53 PMNs treated with anti-L-selectin and/or anti-PSGL-1 revealed that either ligand is capable of forming catch-bonds, and in combination abrogated tether formation (supplemental Figure 4). PMN suspensions were blocked with anti-PSGL-1 to isolate tethering via L-selectin, and glycomimetics were infused (Figure 3D). Tether duration increased with tether force to a threshold of 26pN with vehicle and 17pN with GSnP-6 before decreasing at higher shear stress (Figure 3D). Increased tether duration was attributed to E-selectin/L-selectin catch-bond formation, as it was not altered by GSnP-6. In the presence of Rivipansel, catch-bond behavior via L-selectin was abrogated, as evidenced by decreased tether duration with increased force (Figure 3D). Catch-bond behavior was also evident in vehicle control and GSnP-6 samples, where tether efficiency increased from 20% to 65% up to a shear threshold of 0.75 and 0.50 dynes/cm2, respectively (Figure 3E). Rivipansel treatment abrogated the rise in tether efficiency, which dropped with increased shear stress from a maximum of 20% at 0.25 dynes/cm2. We conclude that E-selectin recognition of sLex on L-selectin is necessary for catch-bond formation that promotes PMN capture and slow rolling over a characteristic range of shear stress-mediated tensile bond force.
Rivipansel inhibits L-selectin clustering and mechanosignaling of high-affinity β2-integrin
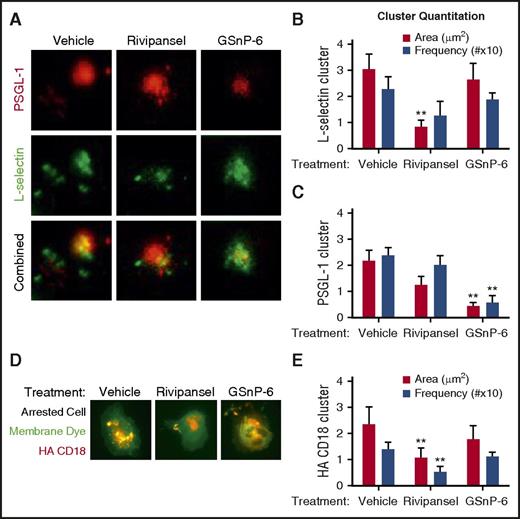
E-selectin recognition of sLex on L-selectin and PSGL-1 elicits receptor coclustering on rolling PMNs, but their relative roles in signaling arrest is unresolved.12,14,42 We employed TIRF imaging to quantify their redistribution during rolling to arrest on E/I substrates.37 Clusters of ESL assembled within seconds of PMN capture and rolling on E-selectin (Figure 4A; supplemental Video 1). Treatment with Rivipansel antagonized the buildup in area of receptor clusters by ∼60% compared with vehicle or GSnP-6, and the frequency of contacts was lowered (Figure 4B; supplemental Video 2). GSnP-6 effectively antagonized ∼70% of the formation of PSGL-1 receptor clusters, but had no effect on L-selectin cluster assembly (Figure 4C). High-affinity β2-integrin cluster and bond formation on ICAM-1 was imaged using TIRF as PMNs transitioned to arrest (Figure 4D). Within 10 seconds of arrest, high-affinity β2-integrin clusters increased in frequency and area. Rivipansel inhibited the area increase by ∼50% and the frequency of focal adhesions by ∼38% compared with vehicle control or GSnP-6 treatment (Figure 4E). We conclude that mechanosignaling via sLex-dependent clustering of L-selectin rapidly led to the activation and assembly of high-affinity β2-integrin focal adhesions on ICAM-1.
Glycomimetic antagonism of selectin mediated β2-integrin activation. (A) PMN rolling on a substrate of E-selectin in the presence of vehicle control, Rivipansel (6.5 μM), or GSnP-6 (12 μM) was dynamically imaged using qDF to detect L-selectin (AF488 anti-human DREG55) and PSGL-1 (PE anti-human PL-1) engagement in the plane of adhesive contact. (B) L-selectin (FITC DREG-55) and (C) PSGL-1 (PE PL-2) receptor cluster area and frequency were determined and reported as mean ± SEM (n = 3 separate experiments; **P < .01 compared with vehicle). (D) PMNs imaged by TIRF were pretreated with a membrane dye (DiI) and HA β2-integrin reporter antibody (mAb24) and perfused over E/I substrates treated with vehicle, Rivipansel (6.5 μM), or GSnP-6 (12 μM). (E) HA β2-integrin cluster number (MFI > 10 pixels above background more than ∼0.1 μm in area) in plane of contact were quantified using qDF in real time and compared between fixed cells that had rolled to arrest between the 3 conditions (n = 3 separate experiments; **P < .01 compared with vehicle).
Glycomimetic antagonism of selectin mediated β2-integrin activation. (A) PMN rolling on a substrate of E-selectin in the presence of vehicle control, Rivipansel (6.5 μM), or GSnP-6 (12 μM) was dynamically imaged using qDF to detect L-selectin (AF488 anti-human DREG55) and PSGL-1 (PE anti-human PL-1) engagement in the plane of adhesive contact. (B) L-selectin (FITC DREG-55) and (C) PSGL-1 (PE PL-2) receptor cluster area and frequency were determined and reported as mean ± SEM (n = 3 separate experiments; **P < .01 compared with vehicle). (D) PMNs imaged by TIRF were pretreated with a membrane dye (DiI) and HA β2-integrin reporter antibody (mAb24) and perfused over E/I substrates treated with vehicle, Rivipansel (6.5 μM), or GSnP-6 (12 μM). (E) HA β2-integrin cluster number (MFI > 10 pixels above background more than ∼0.1 μm in area) in plane of contact were quantified using qDF in real time and compared between fixed cells that had rolled to arrest between the 3 conditions (n = 3 separate experiments; **P < .01 compared with vehicle).
E-selectin engagement with sLex on clustered L-selectin elicits phosphorylation of Lck
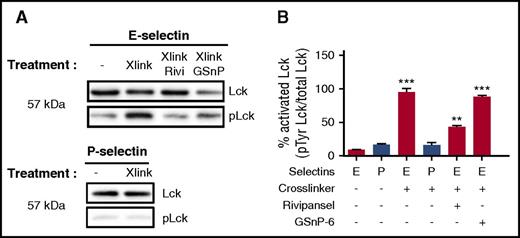
Lck is a Src family protein tyrosine kinase that interacts with the cytoplasmic tail of L-selectin during outside-in signaling after ligation and clustering by E-selectin or sulfatides.5,6,54 We examined Lck activation by quantifying the fraction of phosphorylated to total immunoprecipitated Lck after cross-linking of E- or P-selectin-IgG bound to PMNs. Addition of E- or P-selectin-IgG alone did not elicit significant Lck phosphorylation (Figure 5A). An 8-fold increase in phosphor-Lck was detected in the presence of cross-linked E-selectin-IgG, but not P-selectin-IgG. Rivipansel inhibited activation by ∼70%, whereas GSnP-6 registered no effect (Figure 5B). We conclude that E-selectin recognition of sLex presented on L-selectin requires not only ligation but also membrane clustering to phosphorylate Lck via a process that is independent of PSGL-1 recognition.
E-selectin engagement of sLexon L-selectin requires cross-linking to induce phospho-Lck. (A) E-selectin or P-selectin cross-linked by the addition of polyclonal secondary antibody were immunoprecipitated in the absence and presence of glycomimetics. Western blot was applied to detect Lck along with its tyrosine phosphorylated state. (B) Activation of phospho-Lck was normalized to total protein to quantify the increase after E-selectin or P-selectin cross-linking in the presence of Rivipansel (6.5 μM) or GSnP-6 (12 μM). No significant increase was quantified for cross-linked P-selectin. Data presented as mean ± SEM for cross-linked E-selectin were significant compared with E-selectin alone (n = 3 separate experiments; **P < .01; ***P < .001).
E-selectin engagement of sLexon L-selectin requires cross-linking to induce phospho-Lck. (A) E-selectin or P-selectin cross-linked by the addition of polyclonal secondary antibody were immunoprecipitated in the absence and presence of glycomimetics. Western blot was applied to detect Lck along with its tyrosine phosphorylated state. (B) Activation of phospho-Lck was normalized to total protein to quantify the increase after E-selectin or P-selectin cross-linking in the presence of Rivipansel (6.5 μM) or GSnP-6 (12 μM). No significant increase was quantified for cross-linked P-selectin. Data presented as mean ± SEM for cross-linked E-selectin were significant compared with E-selectin alone (n = 3 separate experiments; **P < .01; ***P < .001).
E-selectin ligation of L-selectin elicits release of MRP8/14, whereas clustering induces high-affinity β2-integrin
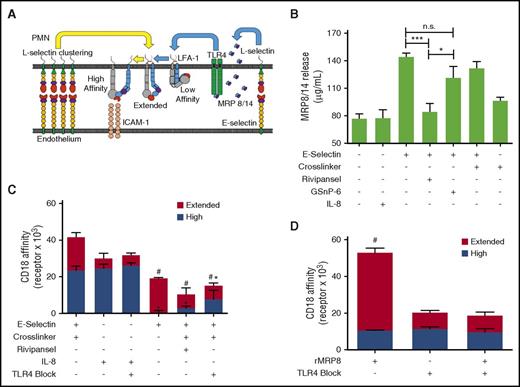
It was recently reported that murine PMNs rolling on E-selectin elicits secretion of MRP8/14 via engagement of PSGL-1, which in turn binds TLR4 that signals the activation of β2-integrin.24 We assessed whether E-selectin recognition of sLex on L-selectin elicits MRP8/14 release and subsequent activation of β2-integrin on human PMNs (Figure 6A). Addition of E-selectin to PMNs in suspension was sufficient to elicit maximum extracellular release of MRP8/14, which was blocked in the presence of Rivipansel (Figure 6B). We next employed β2-integrin reporter antibodies to detect the extended and high-affinity conformations after E-selectin ligation and cross-linking.17,55 Ligation of E-selectin-IgG elicited the extension of ∼20 000 KIM127 sites, representative of ∼25% of total β2-integrin receptors expressed on untreated PMNs (Figure 6C).56 Cross-linking E-selectin-IgG induced activation of an additional ∼25 000 mAb24 bound high-affinity sites, whereas ∼20 000 were maintained in the extended conformation. This level of high-affinity β2-integrin induction equaled maximal activation via CXCR1/2 ligation by IL-8 (Figure 6C). Rivipansel abrogated E-selectin-mediated activation of high-affinity β2-integrin and reduced by half the number of intermediate KIM127 sites (supplemental Figure 3B). To examine the capacity of MRP8/14 to activate PMNs in the absence of E-selectin ligation, we added recombinant MRP8 to PMNs in suspension. This resulted in the extension of ∼35 000 KIM127 sites, which was abrogated by pretreatment with anti-TLR4. MRP8 ligation did not activate high-affinity β2-integrin, but did induce twice the number of intermediate affinity β2-integrin, as activated by ligation of E-selectin (Figure 6D). To discriminate the signaling dynamics in conversion from intermediate- to high-affinity β2-integrin during rolling to arrest on E-selectin, PMNs were sheared on a substrate of E-selectin with mAb24 or KIM127, whereas MRP8/14 signaling was blocked in the presence of anti-TLR4. Capture efficiency via E-selectin was equivalent on substrates of mAb24 or KIM127, consistent with the finding that it activates both extended and high-affinity β2-integrin (supplemental Figure 5A). Direct activation of PMNs in suspension via recombinant MRP8 before shearing across a substrate of E-selectin and CD18 reporter antibodies resulted in a ∼4-fold higher arrest efficiency on KIM127 compared with mAb24, which was blocked with anti-TLR4 (supplemental Figure 5B). These data indicate that ligation of E-selectin and/or the extracellular release of MRP8/14 and its ligation of TLR4 activate an upshift of β2-integrin to an intermediate affinity state. A second distinct signal transduced through E-selectin clustering of L-selectin activates the conversion to high-affinity β2-integrin.
E-selectin cross-linking activates release of MRP8/14 and TLR4 signaled extension of β2-integrin with upshift to a high-affinity state. (A) Diagram of outside-in mechanosignaling of β2-integrin activation via engagement of E-selectin and subsequent clustering of L-selectin on human PMNs. E-selectin binding to L-selectin induces extracellular release of MRP8/14 that then binds TLR4, which activates upshift from low-affinity to an extended intermediate-affinity state of β2-integrin. Further clustering of E-selectin bound L-selectin activates high-affinity β2-integrin. (B) MRP8/14 release by human PMNs treated with E-selectin and cross-linked in the absence or presence of Rivipansel (100 μM) or activation with IL-8 (10 nM). Release into supernatant measured by ELISA as mean ± SEM (n = 3 separate experiments; **P < .01; ***P < .001 significance, as indicated). (C) Affinity state of β2-integrin detected on PMNs in suspension by flow cytometry. β2-integrin receptors bound by KIM127 (extended intermediate affinity) versus mAb24 (high affinity) is shown for PMNs treated with E-selectin with or without cross-linker in the absence or presence of Rivipansel (100 μM), TLR4 blocking antibody, or IL-8 (10 nM; n = 3 separate experiments; #analysis of extended, *high-affinity with P < .01 compared with cross-linked E-selectin). (D) MRP8/14 (0.8 ng/mL) activation and extension of β2-integrin analyzed by flow cytometric detection of KIM127 and mAb24 receptor number in the absence and presence of TLR4 blocking antibody. Data shown as mean ± SEM (n = 3 separate experiments; #P < .05 compared with unstimulated control or TLR4 blocked samples).
E-selectin cross-linking activates release of MRP8/14 and TLR4 signaled extension of β2-integrin with upshift to a high-affinity state. (A) Diagram of outside-in mechanosignaling of β2-integrin activation via engagement of E-selectin and subsequent clustering of L-selectin on human PMNs. E-selectin binding to L-selectin induces extracellular release of MRP8/14 that then binds TLR4, which activates upshift from low-affinity to an extended intermediate-affinity state of β2-integrin. Further clustering of E-selectin bound L-selectin activates high-affinity β2-integrin. (B) MRP8/14 release by human PMNs treated with E-selectin and cross-linked in the absence or presence of Rivipansel (100 μM) or activation with IL-8 (10 nM). Release into supernatant measured by ELISA as mean ± SEM (n = 3 separate experiments; **P < .01; ***P < .001 significance, as indicated). (C) Affinity state of β2-integrin detected on PMNs in suspension by flow cytometry. β2-integrin receptors bound by KIM127 (extended intermediate affinity) versus mAb24 (high affinity) is shown for PMNs treated with E-selectin with or without cross-linker in the absence or presence of Rivipansel (100 μM), TLR4 blocking antibody, or IL-8 (10 nM; n = 3 separate experiments; #analysis of extended, *high-affinity with P < .01 compared with cross-linked E-selectin). (D) MRP8/14 (0.8 ng/mL) activation and extension of β2-integrin analyzed by flow cytometric detection of KIM127 and mAb24 receptor number in the absence and presence of TLR4 blocking antibody. Data shown as mean ± SEM (n = 3 separate experiments; #P < .05 compared with unstimulated control or TLR4 blocked samples).
Discussion
Neutrophil surveillance of the microcirculation in most inflamed tissues involves rolling on E-selectin.57 An immune adaption that humans and not mouse have evolved is the capacity to transition to arrest via outside-in signaling of L-selectin, independent of GPCR-mediated inside-out signaling.6,7,42 Here we show that recognition of sLex on L-selectin by E-selectin during PMN rolling initiates shear force-resistant catch-bonds that facilitate tether formation, bond clustering, and cell arrest. Rivipansel mimics the sLex moiety and targets the lectin domain on E-selectin, abrogating the formation of L-selectin catch-bonds required for receptor clustering and signaling of integrin activation.27,31 GSnP-6, a novel PSGL-1 mimetic that blocks its recognition by E-selectin or P-selectin elicited a doubling in PMN rolling velocity, but had no effect on L-selectin outside-in signaling of β2-integrin.29 This differential glycomimetic inhibition of PMN rolling versus signaling reveals a novel mechanosignaling circuit in which E-selectin transmitted tension on L-selectin induces clustering at the adhesive interface that results in high-affinity β2-integrin/ICAM-1 bonds. We report that the therapeutic efficacy of Rivipansel in treatment of vascular occlusive crisis may involve antagonism of sLex recognition by E-selectin that selectively blocks catch-bond mediated L-selectin mechanosignaling, along with MRP8/14 release and TLR4 signaling of integrin activation.
Although L-selectin plays a crucial role on mouse and human PMNs for induction of integrin activation, a key difference is in the presentation of sLex to the lectin domain of E-selectin. Clues to the specific glycosylation of ESLs on human PMNs come from studies using CRISPR-Cas9 disruption of glycan construction in HL-60 cells.58 O-glycans contribute to initial capture and rolling of PMNs, and when knocked out, result in reduced rolling frequency with no effect on transition to arrest. N-glycans are involved in both PMN recruitment and activation of arrest on E-selectin. L-selectin predominantly presents N-glycan-linked sLex, in contrast to PSGL-1, which is decorated with O-linked sLex.59 GSnP-6, by design, is a glycosulfopeptide that effectively blocks E-selectin binding to sLex expressed on PSGL-1, as well as its docking to the EGF domain29,60 In contrast, Rivipansel occludes docking of sLex to the lectin domain independent of amino acid sequence of ESLs. This may explain its specificity in antagonism of L-selectin and dimeric PSGL-1 recognition and its minimal influence in increasing PMN rolling velocity. Antibody to L-selectin blocked signaling as effectively as Rivipansel, suggesting ancillary ESLs, such as glycosphingolipids and PSGL-1, play a role in L-selectin clustering within lipid raft domains enriched in signaling kinases.54,58 Binding via E-selectin is the critical event in transmission of mechanical force necessary for outside-in signaling.
Binding of L-selectin by its cognate ligands under shear force results in catch-bond behavior.30 Unlike human PMNs, Mouse L-selectin is not glycosylated with sLex, and consequently is not bound by E-selectin. Structural details of how shear-resistant bonds form between E-selectin and L-selectin and the importance of sLex docking by its lectin domain provide a basis for catch-bond behavior. X-ray scattering measurements of E-selectin’s lectin domain in conjunction with a molecular dynamics model predict that tensile force can induce a 10-Å shift in receptor headpiece angle that promotes strong engagement with sLex.30 Our data revealed efficient catch-bond formation within a narrow regime of shear between ∼1 and 2 dynes/cm2, whereby tensile forces on the order of 25pN supported L-selectin bond clusters capable of mechanotransducing outside-in signaling.61 We observed β2-integrins convert from a low- or intermediate- to a high-affinity state during rolling, as measured by capture on a substrate coexpressing E-selectin-IgG and mAb24 under shear flow. A different result was recently reported by Kuwano et al, who observed that a majority of PMNs sheared in microfluidic channels at 6 dynes/cm2 were not activated to arrest on ICAM-1 and were captured on a substrate of E-selectin and KIM127, but not on mAb24.17 They concluded that signaling via E-selectin clustered PSGL-1 activates only the extended- and not high-affinity conformation of β2-integrin. This apparent discrepancy may be explained by the fact that selectins display slip-bond behavior at shear stresses beyond 3 dynes/cm2, a regime in which tethers form less efficiently and dissociate more rapidly. This highlights a fundamental mechanism by which hydrodynamics regulate PMN adhesive signaling to optimize recruitment at appropriate shear stress, while minimizing arrest and vaso-occlusion at very low flow rates.
L-selectin clustering induces effector activities before its downregulation by ectodomain shedding.52 It promotes phospho-Lck activation at the cytoplasmic tail, which in turn activates the Ras pathway and an upshift in β2-integrin affinity.54,62 Rivipansel inhibited activation of phospho-Lck by antagonizing E-selectin catch-bond engagement necessary for L-selectin clustering and signaling. Our data support a mechanism in which Rivipansel antagonizes E-selectin interaction with L-selectin more efficiently than with PSGL-1 on human PMNs. Our finding of a 4-fold higher dose of Rivipansel required to abrogate PMN rolling to arrest than to block induction of high-affinity β2-integrin supports a 2-step mechanism of outside-in signaling. Initial ESL ligation was sufficient for MRP8/14 release and of β2-integrin extension via TLR4. A distinct signal resulted from cross-linking and focal clustering of L-selectin during cell rolling, which triggered phospho-Lck and activation of high-affinity β2-integrin via Rap-1-GTPase. Thus, induction of high-affinity CD18 is cooperative but independent of β2-integrin extension signaled via MRP8/14/TLR4. It is noteworthy that the superposition of selectin-mediated outside-in signaling and ligation of CXCR to induce GPCR signaling can effectively amplify the number of high-affinity β2-integrin by ∼100-fold.63 Thus, PMN recruitment involves temporal cooperativity between selectins and CXC receptors to regulate the number and affinity state of β2-integrin.
In summary, we reveal that ligation of L-selectin induces MRP8/14 and TLR4 signaling that primes β2-integrin in an extended conformation that supports slow rolling of human PMNs. Shear-resistant arrest requires signaling through clustered E-selectin/L-selectin bonds that result in Lck phosphorylation and the rapid activation of β2-integrin to a high-affinity state capable of shear-resistant bond formation with ICAM-1. We demonstrate that catch-bond behavior via sLex recognition on L-selectin is critical for outside-in signaling by E-selectin, thereby revealing a novel aspect of inhibition with a new class of glycomimetic anti-inflammatories. Rivipansel antagonizes outside-in mechanosignaled PMN arrest while leaving intact rolling dependent immunosurveillance within the microcirculation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Minsoo Kim at the University of Rochester for providing anti-β2-integrin antibody KIM127 and Ely Lilly for providing the 327c antibody.
The work was supported by the National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases grant 2R01AI047294 (S.I.S.); NIH, National Institute of Diabetes and Digestive and Kidney Diseases grant R01DK107405 (E.L.C.); NIH, National Institute of General Medical Sciences grant U01GM116196 (E.L.C); and NIH, National Heart, Lung, and Blood Institute grant R01HL128237 (E.L.C.).
Authorship
Contribution: V.A.M. performed research, analyzed data, wrote the manuscript, and designed the research; S.C. performed research and analyzed data; T.W. designed the research; E.L.C. contributed vital new reagents; J.L.M. contributed vital reagents; S.I.S. designed the research and edited each draft of the manuscript; and all authors contributed to editing the manuscript.
Conflict-of-interest disclosure: J.L.M. has declared a financial interest in Glycomimetics Inc., whose drug GMI-1070 is presently under license and clinical trials with Pfizer. Although J.L.M. contributed reagents, no additional funds were provided for these studies. The remaining authors declare no competing financial interests.
Correspondence: Scott I. Simon, Department of Biomedical Engineering, University of California, Davis, Davis, CA 95616; e-mail: sisimon@ucdavis.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal