Key Points
A coagulation initiating pathway is revealed in which the TF-FVIIa-nascent FXa complex activates FVIII apart from thrombin feedback.
Direct activation of the intrinsic pathway by TF may preserve hemostasis under anticoagulant therapy targeting thrombin amplification.
Safe and effective antithrombotic therapy requires understanding of mechanisms that contribute to pathological thrombosis but have a lesser impact on hemostasis. We found that the extrinsic tissue factor (TF) coagulation initiation complex can selectively activate the antihemophilic cofactor, FVIII, triggering the hemostatic intrinsic coagulation pathway independently of thrombin feedback loops. In a mouse model with a relatively mild thrombogenic lesion, TF-dependent FVIII activation sets the threshold for thrombus formation through contact phase-generated FIXa. In vitro, FXa stably associated with TF-FVIIa activates FVIII, but not FV. Moreover, nascent FXa product of TF-FVIIa can transiently escape the slow kinetics of Kunitz-type inhibition by TF pathway inhibitor and preferentially activates FVIII over FV. Thus, TF synergistically primes FIXa-dependent thrombin generation independently of cofactor activation by thrombin. Accordingly, FVIIa mutants deficient in direct TF-dependent thrombin generation, but preserving FVIIIa generation by nascent FXa, can support intrinsic pathway coagulation. In ex vivo flowing blood, a TF-FVIIa mutant complex with impaired free FXa generation but activating both FVIII and FIX supports efficient FVIII-dependent thrombus formation. Thus, a previously unrecognized TF-initiated pathway directly yielding FVIIIa-FIXa intrinsic tenase complex may be prohemostatic before further coagulation amplification by thrombin-dependent feedback loops enhances the risk of thrombosis.
Introduction
Blood clotting in response to tissue injury is key for hemostasis1 and innate immunity2 but can cause vascular thrombosis, leading to serious diseases.1,3 In the current coagulation scheme (Figure 1A), the extrinsic pathway initiation complex of tissue factor (TF) with active factor VIIa (FVIIa) promotes a cascade of proteolytic reactions yielding FXa; this combines with FVa in the prothrombinase complex, converting prothrombin to thrombin. This initially generated thrombin activates the FVIII and FV cofactors in feedback reactions that amplify coagulation,1 but active cofactor generation prior to significant thrombin production is also possible, and indeed, FXa is now viewed as the relevant FV activator during coagulation initiation.4 Whether FXa5,-7 or TF-FVIIa8,9 contribute to coagulation initiation through direct FVIII activation remains unclear.
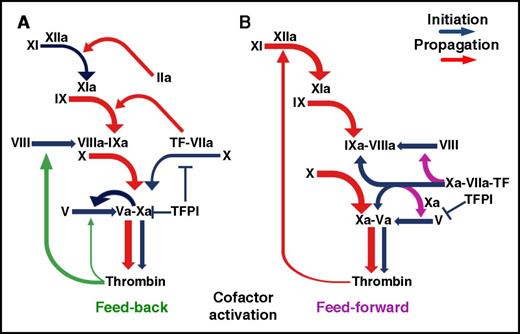
Schematic representation of coagulation initiation and amplification. (A) In the current paradigm, activation of the FVIII and FV cofactors is considered to be primarily a thrombin-mediated feedback reaction, although a key role of FXa in relation to thrombin has been demonstrated for FV activation. (B) The coagulation scheme supported by the present studies highlights in addition the newly identified selective activation of FVIII by nascent FXa within the extrinsic TF-FVIIa-FXa initiation complex prior to inhibition by TFPI. By concurrently activating FIX, the extrinsic TF-FVIIa complex can initiate directly the FVIIIa-FIXa antihemophilic pathway. The 2 depicted mechanisms of coagulation activation may be variably integrated in response to different stimuli.
Schematic representation of coagulation initiation and amplification. (A) In the current paradigm, activation of the FVIII and FV cofactors is considered to be primarily a thrombin-mediated feedback reaction, although a key role of FXa in relation to thrombin has been demonstrated for FV activation. (B) The coagulation scheme supported by the present studies highlights in addition the newly identified selective activation of FVIII by nascent FXa within the extrinsic TF-FVIIa-FXa initiation complex prior to inhibition by TFPI. By concurrently activating FIX, the extrinsic TF-FVIIa complex can initiate directly the FVIIIa-FIXa antihemophilic pathway. The 2 depicted mechanisms of coagulation activation may be variably integrated in response to different stimuli.
Extrinsic coagulation initiation is controlled by the TF pathway inhibitor (TFPI), which by inactivating FVIIa and FXa within a quaternary complex with TF10,11 attenuates thrombosis.12 Moreover, a positively charged TFPIα carboxyl terminal region interacts with a specific acidic sequence in partially processed FV interfering with FXa formation of active prothrombinase.13,14 These mechanisms reducing direct thrombin generation are compensated for by TF-FVIIa activating the intrinsic pathway FIX15 in a kinetically favored reaction in the presence of physiologic plasma inhibitors.16 Alternatively, FIXa is generated by FXIa activated in a thrombin feedback loop,17 also promoting vascular inflammation,18 or by contact phase FXIIa.19 In mouse models,20 FXIIa contributes to amplified thrombin generation in experimental thrombosis but, consistent with human data,21 has no role in hemostasis.
The current coagulation paradigm, with the expanded function of TFPI tightly controlling both TF-dependent initiation and prothrombinase generation, cannot readily explain how initially produced thrombin can be the origin of FVIIIa cofactor for FIXa produced by the contact phase pathway or by TF-FVIIa itself. Here, we outline a novel function of the extrinsic coagulation initiation complex whereby nascent FXa associated with TF-FVIIa directly activates FVIII, resisting inhibitor control by TFPI. Such a mechanism may be relevant for the function of TF in hemostasis and provides new perspectives for interpreting the distinct roles of coagulation reactions in physiologic and pathologic thrombus formation.
Materials and methods
Blood perfusion experiments
TF-coated glass coverslips were perfused with venous blood at a wall shear rate of 300 s−1 (see the supplemental Materials and methods, available on the Blood Web site). A Zeiss Axiovert 135M/LSM 410 microscope with a Plan-Apochromat ×40/1.40-NA oil immersion objective (Carl Zeiss AG, Oberkochen, Germany) was used to visualize platelets/leukocytes and fibrin stained with mepacrine and a specific antibody, respectively. Image analysis to calculate volumes was performed as has been described.22
Thrombin generation (TG) analysis in human native or reconstituted platelet-rich plasma
TG in platelet-rich plasma (PRP) or reconstituted PRP was evaluated as has been described.23 Platelets in PRP were adjusted to 180 ⋅ 103 per microliter with homologous platelet-poor plasma (PPP), and corn trypsin inhibitor (CTI) was added at 30-50 μg/mL. Reconstituted PRP was prepared with washed platelets resuspended at 180 ⋅ 103 per microliter into PPP. TG was initiated by adding recombinant TF (rTF), FIXa, or both at defined concentrations with 18 mM CaCl2 into microtiter-plate wells containing 360 µM benzyloxycarbonyl-glycyl-glycyl-l-arginine coupled to fluorogenic 7-amido-4-methylcoumarin (Bachem Americas, Torrance, CA) as thrombin substrate. The rate of fluorescence intensity increase (measured at 355/460 nm excitation/emission) as a function of time (dF/dt) was calculated with Turbo Delphi 2006 (Borland Software Corporation, Austin, TX) and converted to thrombin-equivalent concentration (nM) using a calibration curve. TG was also measured with a discontinuous 2-stage assay with a detection limit of 5 pM. In this assay, reactions incubated for up to 11 min at 37°C were terminated with 20 mM EDTA, and generated thrombin was measured using the sensitive thrombin substrate H-D-CHA-Ala-Arg-AMC⋅2AcOH (Pefafluor TH, Pentapharm, Basel, Switzerland) at 50 µM.
Analysis of FVIII and FV activation by immunoblotting
Coagulation products were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis under reducing conditions; only reactions containing anti-FVIIa monoclonal antibody (MoAb) were processed under nonreducing conditions to avoid confounding effects from immunoglobulin G (IgG) heavy chains with a molecular mass comparable to FVIII A1 domain. Proteins transferred to polyvinylidene fluoride membranes were probed with anti-FVIII MoAb C5 (0.5 µg/mL)24 or anti-FV AHV-5146 (1 µg/mL). FVIIIa and FVa were quantified by infrared detection with the Odyssey infrared imager (Li-COR, Lincoln, NE), calibrated with known FVIIIa and FVa quantities.
Coagulation activation in reactions with purified components
Reactions in 50 mM Tris-buffered saline, pH 7.4, with 0.1% bovine serum albumin included 0.7 nM FVIII, 3 nM FV, 135 nM FX, and 1 µM prothrombin without or with 4 μM dansylarginine N-(3-ethyl-1,5-pentanediyl)amide (DAPA). TFPIα and other inhibitors were added as indicated. Reactions were initiated by rTF (50 or 400 pM) with FVIIa (200 or 500 pM), FIXa (2 or 10 nM), or both added with 2.5 mM CaCl2 and incubated at 37°C for the indicated times. After quenching the reaction with 10 mM EDTA, generated FXa was measured with S-2765 (180 µM). FVIIIa procoagulant activity was measured as FIXa-dependent FXa generation; FVIIIa procoagulant activity generated by the nascent TF-FVIIa-FXa complex was calculated by subtracting the amount of FXa produced in reactions initiated by FVIIa and FIXa individually from that produced in reactions initiated by FVIIa/FIXa combined. When indicated, 200 nM lepirudin was used to inactivate possible thrombin contamination.
Ferric chloride-induced thrombosis in mice
Animal procedures complied with the Guide for Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of The Scripps Research Institute. Vascular injury was induced in C57BL/6J mice by 1 0.8-μL drop of 7% (0.26 M) or 8% (0.30 M) FeCl3⋅6H2O applied on the carotid artery for 3 min; or a 0.4-μL 4% (0.15 M) drop for 1 min on the femoral vein, followed by rinsing. Antibodies were administered by bolus injection into the catheterized jugular vein. FVIII and FVIIIa were administered by a bolus injection (1.4 pmol), followed by maintenance with continuous infusion at the rate of 0.47 pmol/min for 15 min. Time to first occlusion after injury and flow index were quantified as has been described.22 Additional details for experimental procedures are described in the supplemental Methods.
Study approval
Studies involving human subjects were approved by appointed institutional review boards. Human volunteers gave informed consent to participate in the studies before blood collection, and experiments were performed in accordance with the Declaration of Helsinki.
Statistics
Group variances were evaluated with Levene’s median and Bartlett’s tests; differences were evaluated with 1-way analysis of variance (ANOVA) or the Kruskal-Wallis test, followed by Tukey’s and Dunn’s tests, respectively, for multiple comparisons. Data were transformed as y = log10y when necessary to obtain homoscedasticity. Software packages used were GraphPad Prism (version 7; GraphPad Software, La Jolla, CA) and XLSTAT (Addinsoft, Paris, France).
Results
The TF pathway activates FVIII in vivo
As was previously shown, contact phase FXII20,25,26 and TF22,27 contribute to experimental thrombosis in the ferric chloride-induced carotid artery occlusion model, but how this happens remains unclear. We found that MoAbs blocking the TF function22 or FXI activation by FXIIa25 independently reduced occlusion after a vessel wall lesion caused by 7% (0.26 M) FeCl3 (Figure 2A). After a lesion by 8% (0.3 M) FeCl3 the same MoAb concentrations were ineffective, but a higher dose of the anti-FXI MoAb, not of the anti-TF, still prevented thrombosis (Figure 2A). Thus, even after the more severe lesion, thrombogenesis required FIXa generation by FXIIa-FXIa.20,25 Remarkably, under the latter conditions, combining individually inactive low doses of anti-TF and anti-FXI MoAbs reduced arterial occlusion significantly (Figure 2A), confirming a role for the TF pathway in this intrinsic coagulation-dependent model of thrombus formation. To explain such findings, we hypothesized that TF might contribute to activate FVIII, the essential cofactor for the intrinsic tenase protease, FIXa. As visualized in the femoral vein, FVIIIa, but not FVIII, prevented inhibition of FeCl3-induced fibrin deposition by the low-dose anti-TF/anti-FXI MoAb combination but not by high-dose anti-FXI alone (Figure 2B-C). These results ruled out the hypothesis that FIXa generated by TF-FVIIa or alternative pathways used exogenously provided FVIIIa to trigger thrombosis, supporting the idea that TF-FVIIa contributes to FVIII activation in vivo.
TF pathway and FVIII activation in vivo. (A) Carotid artery occlusion after injury by 7% (0.26 M) or 8% (0.3 M) FeCl3⋅6H2O in C57BL/6J mice treated with anti-TF 21E10, anti-FXI 14E11 MoAbs, or both, as indicated (n = 5-20 in different groups); control mice (panel C) were injected with buffer or isotype-matched nonimmune mouse IgG. (B) Femoral vein occlusion after injury by 4% (0.15 M) FeCl3⋅6H2O in mice (n = 3-7) receiving FVIII or FVIIIa (1.4 pmol bolus followed by 0.47 pmol/min for 15 min) before injury and MoAb treatment. Results in panel A (top) and panel B (dot plots; median and interquartile range) were analyzed with Kruskal-Wallis/Dunn tests; results in panel A (bottom) (dot plot; mean and 95% confidence interval) were analyzed with ANOVA/Tukey tests. (C) Fibrin formation in the femoral vein. Control mice were injected with phosphate-buffered saline (top left). FVIII injection does not prevent the antithrombotic effect of 9 µg/g anti-TF/65 ng/g anti-FXI MoAbs combined (top right). FVIIIa injection bypasses inhibition by this antibody combination (bottom left). FVIIIa cannot bypass inhibition by a full dose (125 ng/g) of anti-FXI MoAb alone (bottom right). (D) Representative TG (n = 3) induced by 0.15 pM rTF, 20 pM FIXa, or both in citrated human PRP (180 ⋅ 103 platelets/μL) recalcified with 18 mM CaCl2 at 37°C and containing 30 μg/mL CTI to block FXIIa and 40 µg/mL rabbit anti-TFPI IgG (left) or nonimmune IgG (right). (E) Representative TG (n = 2) induced by rTF, FIXa, or both as above in recalcified FIX-deficient PPP containing 50 μg/mL CTI and 180 ⋅ 103 normal washed platelets per microliter. *P < .05. **P < .01. ***P < .001.
TF pathway and FVIII activation in vivo. (A) Carotid artery occlusion after injury by 7% (0.26 M) or 8% (0.3 M) FeCl3⋅6H2O in C57BL/6J mice treated with anti-TF 21E10, anti-FXI 14E11 MoAbs, or both, as indicated (n = 5-20 in different groups); control mice (panel C) were injected with buffer or isotype-matched nonimmune mouse IgG. (B) Femoral vein occlusion after injury by 4% (0.15 M) FeCl3⋅6H2O in mice (n = 3-7) receiving FVIII or FVIIIa (1.4 pmol bolus followed by 0.47 pmol/min for 15 min) before injury and MoAb treatment. Results in panel A (top) and panel B (dot plots; median and interquartile range) were analyzed with Kruskal-Wallis/Dunn tests; results in panel A (bottom) (dot plot; mean and 95% confidence interval) were analyzed with ANOVA/Tukey tests. (C) Fibrin formation in the femoral vein. Control mice were injected with phosphate-buffered saline (top left). FVIII injection does not prevent the antithrombotic effect of 9 µg/g anti-TF/65 ng/g anti-FXI MoAbs combined (top right). FVIIIa injection bypasses inhibition by this antibody combination (bottom left). FVIIIa cannot bypass inhibition by a full dose (125 ng/g) of anti-FXI MoAb alone (bottom right). (D) Representative TG (n = 3) induced by 0.15 pM rTF, 20 pM FIXa, or both in citrated human PRP (180 ⋅ 103 platelets/μL) recalcified with 18 mM CaCl2 at 37°C and containing 30 μg/mL CTI to block FXIIa and 40 µg/mL rabbit anti-TFPI IgG (left) or nonimmune IgG (right). (E) Representative TG (n = 2) induced by rTF, FIXa, or both as above in recalcified FIX-deficient PPP containing 50 μg/mL CTI and 180 ⋅ 103 normal washed platelets per microliter. *P < .05. **P < .01. ***P < .001.
To challenge this concept, we measured TG in PRP. In reactions containing an inhibitory polyclonal antibody blocking TFPI and CTI preventing FXI activation by FXIIa,28 we defined the concentrations of FIXa or relipidated rTF yielding comparable TG (Figure 2D, left). At the same concentration but without TFPI blockade (Figure 2D, right), rTF produced little thrombin and late in the reaction, but enhanced TG with FIXa added concurrently. This amplification of FIXa-triggered TG required FVIII at <10% plasma concentration (supplemental Figure 1) but not FIX (Figure 2E), excluding additional TF-dependent FIXa generation.16,17 Because FVIII concentrations sufficient to prevent severe spontaneous bleeding in FVIII-deficient patients29 could support synergistic TG amplification, the conditions of this assay appear to be relevant for assessing hemostatic competence in PRP.
The TF-FVIIa-FXa complex activates FVIII
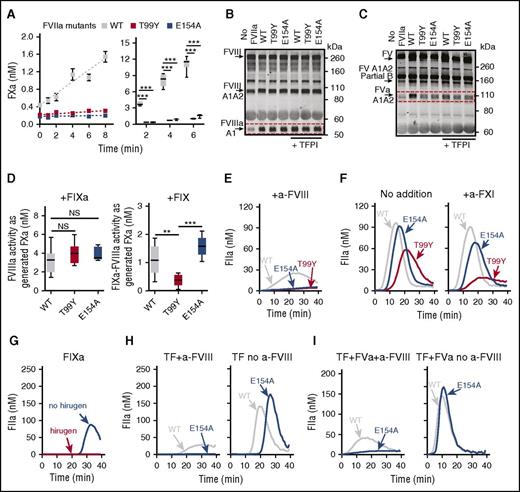
We explored the mechanism of TF-induced priming of intrinsic coagulation using a sensitive 2-stage TG assay. In FVII-deficient reconstituted PRP, 0.15 pM rTF with FVIIa wild-type (WT) produced little thrombin (<20 pM in 11 min), similar to that generated by the active site mutant FVIIa S195A (iFVIIa)30 or FIXa alone (Figure 3A). However, combining FIXa with rTF and FVIIa WT, but not inactive iFVIIa, amplified TG ∼5-20 times in 5-11 min (Figure 3A). Thus, the additive quantities of thrombin produced in this assay by TF-FVIIa and FIXa separately were far less than those yielded by the 2 combined, demonstrating a synergistic interaction linking TF-initiated and intrinsic coagulation upstream of thrombin generation.
The TF initiation complex activates FVIII. (A) TG was induced by 20 pM FIXa; or 400 pM FVIIa WT or inactive iFVIIa; or FIXa combined with FVIIa WT or iFVIIa added into FVII-deficient reconstituted PRP (180 ⋅ 103 platelets/μl) containing 30 μg/mL CTI, 0.15 pM rTF, and 18 mM CaCl2, followed by incubation for 5 (n = 2-5), 8 (n = 2-5), or 11 (n = 3) min at 37°C. (B) FVIII (1.4 nM) activation by 400 pM rTF and 500 pM FVIIa with or without 135 nM FX in reactions containing 40 nM TFPIα and 200 nM lepirudin incubated for 30 s at 37°C. After blocking residual FXa and TF-FVIIa with 70 nM of TAP and 20 µg/mL each of MoAbs 5G9 and 12C7, respectively, FVIIIa clotting activity (n = 2-4) was measured by adding the reaction mixture into FVIII-deficient plasma with 10 nM FIXa, 20 µM phospholipids, and 8 mM CaCl2. (C) TG as in panel A but induced by a preformed complex of 10 nM rTF, 10 nM iFVIIa, 5 nM FXa, and 40 nM TFPIα or NAPc2 added at the indicated TF/FXa concentrations without (left) or with (right) 10 pM FIXa. Incubation for 8 min at 37°C (n = 3-5). Results in panels A-C are shown as 25th-75th percentile bars (minimum-to-maximum [min-to-max] whiskers, line at the median) or, when n ≤ 3, min-to-max floating bars (line at the mean); analysis by ANOVA/Tukey tests (after y = log10y transformation in panel C). (D) Representative immunoblots (n = 2) of FVIIIa and FVa generation after 30 or 60 s in reactions with the indicated combinations of 200 pM FXa, 500 pM iFVIIa, 40 nM NAPc2, and 1 µM TAP added to 400 pM rTF, 700 pM FVIII, 3 nM FV, and 200 nM lepirudin. (E) Representative immunoblots showing the effect of TAP (1 µM) on FVIII activation by TF-FVIIa (400 and 500 pM, respectively) and 135 nM FX (top). Reactions, containing 700 pM FVIII, 3 nM FV, and 200 nM lepirudin also, were incubated for 30 s at 37°C. Quantification of experiments as shown in the top panel (n = 4), presented as 25th-75th percentile bars and analyzed by 2-tailed t test (bottom). (F) Dose response of FVa effect on 1 µM prothrombin conversion to thrombin in reactions containing 10 pM FXa, 50 pM rTF, and 700 pM FVIII incubated for 180 s at 37°C; mean ± 95% confidence interval (CI) (n = 4). (G) Dose response of FVa effect on FVIII activation by 10 pM FXa in reactions with 50 pM rTF, 700 pM FVIII, and 200 nM lepirudin incubated for 180 s at 37°C. FVIIIa activity (mean ± 95% CI, n = 4) was measured as generated FXa in the presence of 2 nM FIXa and expressed as percentage of that measured in the absence of FVa. (H) Representative immunoblots (n = 3) of FVIIIa generation in reactions as in panel G (left). Quantification of generated FVIIIa (n = 3) expressed as in panel G and shown by min-to-max floating bars (right); analysis by 1-sample t test. *P < .05. **P < .01. ***P < .001.
The TF initiation complex activates FVIII. (A) TG was induced by 20 pM FIXa; or 400 pM FVIIa WT or inactive iFVIIa; or FIXa combined with FVIIa WT or iFVIIa added into FVII-deficient reconstituted PRP (180 ⋅ 103 platelets/μl) containing 30 μg/mL CTI, 0.15 pM rTF, and 18 mM CaCl2, followed by incubation for 5 (n = 2-5), 8 (n = 2-5), or 11 (n = 3) min at 37°C. (B) FVIII (1.4 nM) activation by 400 pM rTF and 500 pM FVIIa with or without 135 nM FX in reactions containing 40 nM TFPIα and 200 nM lepirudin incubated for 30 s at 37°C. After blocking residual FXa and TF-FVIIa with 70 nM of TAP and 20 µg/mL each of MoAbs 5G9 and 12C7, respectively, FVIIIa clotting activity (n = 2-4) was measured by adding the reaction mixture into FVIII-deficient plasma with 10 nM FIXa, 20 µM phospholipids, and 8 mM CaCl2. (C) TG as in panel A but induced by a preformed complex of 10 nM rTF, 10 nM iFVIIa, 5 nM FXa, and 40 nM TFPIα or NAPc2 added at the indicated TF/FXa concentrations without (left) or with (right) 10 pM FIXa. Incubation for 8 min at 37°C (n = 3-5). Results in panels A-C are shown as 25th-75th percentile bars (minimum-to-maximum [min-to-max] whiskers, line at the median) or, when n ≤ 3, min-to-max floating bars (line at the mean); analysis by ANOVA/Tukey tests (after y = log10y transformation in panel C). (D) Representative immunoblots (n = 2) of FVIIIa and FVa generation after 30 or 60 s in reactions with the indicated combinations of 200 pM FXa, 500 pM iFVIIa, 40 nM NAPc2, and 1 µM TAP added to 400 pM rTF, 700 pM FVIII, 3 nM FV, and 200 nM lepirudin. (E) Representative immunoblots showing the effect of TAP (1 µM) on FVIII activation by TF-FVIIa (400 and 500 pM, respectively) and 135 nM FX (top). Reactions, containing 700 pM FVIII, 3 nM FV, and 200 nM lepirudin also, were incubated for 30 s at 37°C. Quantification of experiments as shown in the top panel (n = 4), presented as 25th-75th percentile bars and analyzed by 2-tailed t test (bottom). (F) Dose response of FVa effect on 1 µM prothrombin conversion to thrombin in reactions containing 10 pM FXa, 50 pM rTF, and 700 pM FVIII incubated for 180 s at 37°C; mean ± 95% confidence interval (CI) (n = 4). (G) Dose response of FVa effect on FVIII activation by 10 pM FXa in reactions with 50 pM rTF, 700 pM FVIII, and 200 nM lepirudin incubated for 180 s at 37°C. FVIIIa activity (mean ± 95% CI, n = 4) was measured as generated FXa in the presence of 2 nM FIXa and expressed as percentage of that measured in the absence of FVa. (H) Representative immunoblots (n = 3) of FVIIIa generation in reactions as in panel G (left). Quantification of generated FVIIIa (n = 3) expressed as in panel G and shown by min-to-max floating bars (right); analysis by 1-sample t test. *P < .05. **P < .01. ***P < .001.
To identify the reaction that, apart from thrombin, could yield FVIIIa for FIXa-dependent coagulation, we considered TF-FVIIa8,9 and FXa5,-7 as potential FVIII activators. First, we determined that TF-FVIIa without FX (or TF-FX without FVIIa) generated no or minimal FVIIIa activity but that TF-FVIIa with FX (generating FXa) produced substantial amounts of FVIIIa with all reactants at physiologically relevant concentrations (Figure 3B). To distinguish between functions of FXa released from or still associated with TF-FVIIa, we formed a stable TF-iFVIIa-FXa complex with the nematode anticoagulant protein (NAP) c2.31,32 Use of iFVIIa excluded FVIIa catalytic activity, whereas FXa is known to retain catalytic function in the formed NAPc2 complex.32 This complex failed to induce TG in FVII-deficient reconstituted PRP but markedly enhanced FIXa-induced TG (Figure 3C), suggesting that FXa associated with TF-FVIIa can activate FVIII. As was expected, a similar complex formed with TFPIα, which inhibits FXa, was inactive alone and did not support FIXa-dependent TG in PRP (Figure 3C). Surprisingly, the NAPc2-stabilized TF-iFVIIa-FXa complex could activate purified FVIII but not the homologous cofactor FV (Figure 3D). Specific inhibition with tick anticoagulant peptide (TAP) confirmed that FXa in the stabilized TF-iFVIIa-FXa complex activated FVIII (Figure 3D), implying that nascent FXa still associated with TF-FVIIa can exert the same function (Figure 3E).
We next evaluated whether free FXa, which triggers coagulation by forming the prothrombinase complex, could be a physiologic FVIII activator akin to its function in generating the prothrombinase cofactor, FVa.4 On the same procoagulant membrane used in the experiments above, increasing concentrations of FVa markedly stimulated prothrombin conversion by a low concentration of FXa (Figure 3F). In contrast, generation of FVIIIa activity (Figure 3G) or proteolytic cleavage of FVIII (Figure 3H) by the same FXa concentration was progressively inhibited by adding FVa in the same concentration range, indicating that FXa bound to FVa cannot efficiently activate FVIII. Thus, nascent FXa associated with TF-FVIIa preferentially activates FVIII, triggering the intrinsic coagulation pathway. Subsequent FXa transfer from TF-FVIIa into the prothrombinase complex with FVa marks the transition to direct TF-induced coagulation.
The TF-FVIIa-FXa complex activates FVIII independently of thrombin
To assess the relative roles of the nascent TF-FVIIa-FXa complex and thrombin in generating FVIIIa, we studied procofactor activation on rTF-bearing phospholipid vesicles mixed with purified FX, prothrombin, FVIII, and FV. Activation of FVIII after FVIIa addition was partially inhibited by blocking thrombin with DAPA or by replacing normal prothrombin with the inactive S195A mutant,33 but ∼15% FVIIIa was still detectable under both conditions (Figure 4A). Importantly, although thrombin could generate more FVIIIa, as was expected from efficient FVIII cleavage in the solution phase, the amount of VIII activated by the TF-initiated reaction in the absence of active thrombin was sufficient for full function of membrane assembled FVIIIa-FIXa intrinsic tenase complex (Figure 4B; supplemental Figure 2A). In agreement with the results in PRP containing endogenous coagulation inhibitors (see Figure 2D), TFPIα that markedly suppressed direct FXa generation by TF-FVIIa (supplemental Figure 2B) had limited effect on the formation of functional FVIIIa-FIXa complex in TF-initiated reactions (Figure 4C). The latter was also resistant to inhibition by TFPIα with the cofactor protein S34,35 ; partial inhibition by protein S alone likely resulted from competition for limited procoagulant surfaces (supplemental Figure 2C). Consistent with the observed thrombin-independent FVIII activation by nascent FXa in PRP, FVIII activation by TF-FVIIa-FXa was not influenced by von Willebrand factor (VWF) binding FVIII (supplemental Figure 2D).
Thrombin-independent FVIII activation by nascent TF-FVIIa-FXa. (A) Representative immunoblots showing FVIIIa A1 activation fragment generation induced by 200 pM FVIIa and 50 pM rTF (0.37 μM phospholipids) in reactions containing 700 pM FVIII, 3 nM FV, 135 nM FX, 1 µM prothrombin (FII) (WT without or with 4 μM DAPA [n = 4] or inactive S195A mutant [n = 3]), and 2.5 mM CaCl2 incubated at 37°C for 120 s (top). Quantification of FVIIIa-A1 calibrated with known quantities of the fragment (bottom). (B) FVIIIa activity calculated from FXa generation in reactions as in panel A but with 10 nM FIXa added and incubated for 180 s at 37°C (n = 4-12). FXa generation dependent on FVIIIa-FIXa activity was calculated by subtracting FXa generated by FVIIa and FIXa added individually from that by FVIIa/FIXa added together. (C) FVIIIa activity generated and calculated in reaction as in panels A and B but with the addition of 10 nM TFPIα. Results in panel A (bottom) and panels B and C (shown as 25th-75th percentile bars, min-to-max whiskers, line at the median; or min-to-max floating bars with line at the mean when n ≤ 3) were analyzed by ANOVA/Tukey tests. (D) Effect of different hirugen concentrations on TG initiated by 10 pM FIXa in normal PRP (180 ⋅ 103 platelets/μL) containing 30 μg/mL CTI and 20 µg/mL anti-FXIa blocking MoAb O1A6 (n = 3). (E) Representative thrombograms initiated in normal PRP, containing CTI and anti-FXIa MoAb as in D, by 0.15 pM rTF, 10 pM FIXa, or both without (left) or with (right) 2 µM hirugen (n = 3). ***P < .001. NS, not significant.
Thrombin-independent FVIII activation by nascent TF-FVIIa-FXa. (A) Representative immunoblots showing FVIIIa A1 activation fragment generation induced by 200 pM FVIIa and 50 pM rTF (0.37 μM phospholipids) in reactions containing 700 pM FVIII, 3 nM FV, 135 nM FX, 1 µM prothrombin (FII) (WT without or with 4 μM DAPA [n = 4] or inactive S195A mutant [n = 3]), and 2.5 mM CaCl2 incubated at 37°C for 120 s (top). Quantification of FVIIIa-A1 calibrated with known quantities of the fragment (bottom). (B) FVIIIa activity calculated from FXa generation in reactions as in panel A but with 10 nM FIXa added and incubated for 180 s at 37°C (n = 4-12). FXa generation dependent on FVIIIa-FIXa activity was calculated by subtracting FXa generated by FVIIa and FIXa added individually from that by FVIIa/FIXa added together. (C) FVIIIa activity generated and calculated in reaction as in panels A and B but with the addition of 10 nM TFPIα. Results in panel A (bottom) and panels B and C (shown as 25th-75th percentile bars, min-to-max whiskers, line at the median; or min-to-max floating bars with line at the mean when n ≤ 3) were analyzed by ANOVA/Tukey tests. (D) Effect of different hirugen concentrations on TG initiated by 10 pM FIXa in normal PRP (180 ⋅ 103 platelets/μL) containing 30 μg/mL CTI and 20 µg/mL anti-FXIa blocking MoAb O1A6 (n = 3). (E) Representative thrombograms initiated in normal PRP, containing CTI and anti-FXIa MoAb as in D, by 0.15 pM rTF, 10 pM FIXa, or both without (left) or with (right) 2 µM hirugen (n = 3). ***P < .001. NS, not significant.
To demonstrate directly that rTF supports FVIII activation in a physiological plasma milieu independently of thrombin feedback reactions, we used hirugen (63-O-sulfo-tyr-hirudin[55-65]) to block thrombin exosite I required for cofactor activation.36,37 Hirugen dose-dependently inhibited FVIII activation by thrombin (supplemental Figure 3A) but not FXa generated by TF-FVIIa-FIXa (supplemental Figure 3B). In PRP with CTI and anti-FXI MoAb to block feedback TG amplification through increased FIXa generation by FXIa,38 2 μM of hirugen blocked FIXa-dependent TG (Figure 4D), demonstrating that this reaction required thrombin feedback activation of FVIII. In contrast, the same hirugen concentration failed to inhibit TG by FIXa combined with rTF, even though no TG could be detected when FIXa and rTF were added separately (Figure 4E). Thus, the TF pathway activates FVIII in plasma when thrombin feedback loops are inhibited. Experiments with mouse microvesicles39 generated from WT or human TF knock-in40 macrophages showed that thrombin-independent FVIIIa generation occurred also on a natural procoagulant surface with human or mouse TF-FVIIa (supplemental Figure 3C).
Intrinsic coagulation pathway activation by the nascent TF-FVIIa-FXa complex contributes to thrombin generation independently of direct extrinsic pathway function
To elucidate further how FXa generation by TF-FVIIa distinctly contributes to intrinsic pathway activation as opposed to direct extrinsic pathway TG, we studied 2 FVIIa mutants, T99Y and E154A, that cleave FX but display very low substrate turnover because of impaired FXa release.30,41 In a phospholipid-free assay or with phospholipid-reconstituted rTF, the FVIIa mutants produced an initial burst but, in contrast to FVIIa WT, could not sustain FXa generation (Figure 5A). Remarkably, TF complexes with both mutants were comparable to FVIIa WT in supporting FXa-dependent FVIII activation (supplemental Figure 4A), and importantly, TFPIα at supraphysiological concentrations (10 nM) did not appreciably influence this pathway of FVIIIa generation (Figure 5B). In marked contrast to FVIII, the FVIIa mutants failed to induce FV activation, and TFPIα inhibited FVa generation induced by FVIIa WT (Figure 5C).
FVIIa mutants with impaired FXa product turnover support FVIII activation by nascent FXa when thrombin feedback is blocked. (A) Time course (mean ± SEM) of 1 µM FX activation by 2 µM phospholipid-free soluble rTF with 10 nM FVIIa WT (n = 4-7), T99Y (n = 2-3), or E154A mutants (n = 3-4); incubation at 37°C (left). FXa generation in reactions with 50 pM phospholipid-reconstituted rTF, 200 pM FVIIa WT or mutants, 135 nM FX, and 700 pM FVIII incubated for 2 (n = 2-3), 4 (n = 4-5), or 6 (n = 6-9) min (right). (B) Representative immunoblots (n = 2) of FVIII activation by 50 pM rTF, 200 pM FVIIa WT or mutants, 135 nM FX, 700 pM FVIII, 3 nM FV, and 200 nM lepirudin, without or with 10 nM TFPIα incubated for 180 s. (C) Representative immunoblots (n = 2) of FV activation in reactions as in panel B incubated 420 s. (D) FVIIIa activity (25th-75th percentile bars, min-to-max whiskers, line at the median) generated as in panel B but without TFPIα, measured as FXa produced by 10 nM FIXa (n = 9 for FVIIa WT and T99Y; n = 5 for E154A) by ANOVA/Tukey tests (left). FVIIIa-FIXa activity generated with 90 nM FIX replacing FIXa; incubation 360 s (n = 5-6) (right). (E) Representative TG (n = 3) initiated by 2.5 pM rTF and 400 pM FVIIa WT or mutants in FVII-deficient reconstituted PRP containing 30 µg/mL CTI, 8 µg/mL anti-FVIII MoAb 8D4-blocking FVIIIa cofactor activity. (F) Representative TG (n = 3) as in panel E but without anti-FVIII MoAb and without (left) or with (right) 20 µg/mL anti-FXI MoAb O1A6-blocking FXIa activity in FIX activation. (G) Representative TG (n = 3) initiated by 20 pM FIXa in FVII-deficient reconstituted PRP containing 4 µM hirugen, 30 µg/mL CTI, 20 µg/mL anti-FXI MoAb. (H) Representative TG as in panel G but initiated by 2.5 pM rTF and 400 pM FVIIa WT or E154A with (left; n = 3) or without (right; n = 5) 8 µg/mL anti-FVIII MoAb. (I) Representative TG as in panel H but with added 3 nM FVa with (left; n = 4) or without (right; n = 5) anti-FVIII MoAb. **P < .01. ***P < .001.
FVIIa mutants with impaired FXa product turnover support FVIII activation by nascent FXa when thrombin feedback is blocked. (A) Time course (mean ± SEM) of 1 µM FX activation by 2 µM phospholipid-free soluble rTF with 10 nM FVIIa WT (n = 4-7), T99Y (n = 2-3), or E154A mutants (n = 3-4); incubation at 37°C (left). FXa generation in reactions with 50 pM phospholipid-reconstituted rTF, 200 pM FVIIa WT or mutants, 135 nM FX, and 700 pM FVIII incubated for 2 (n = 2-3), 4 (n = 4-5), or 6 (n = 6-9) min (right). (B) Representative immunoblots (n = 2) of FVIII activation by 50 pM rTF, 200 pM FVIIa WT or mutants, 135 nM FX, 700 pM FVIII, 3 nM FV, and 200 nM lepirudin, without or with 10 nM TFPIα incubated for 180 s. (C) Representative immunoblots (n = 2) of FV activation in reactions as in panel B incubated 420 s. (D) FVIIIa activity (25th-75th percentile bars, min-to-max whiskers, line at the median) generated as in panel B but without TFPIα, measured as FXa produced by 10 nM FIXa (n = 9 for FVIIa WT and T99Y; n = 5 for E154A) by ANOVA/Tukey tests (left). FVIIIa-FIXa activity generated with 90 nM FIX replacing FIXa; incubation 360 s (n = 5-6) (right). (E) Representative TG (n = 3) initiated by 2.5 pM rTF and 400 pM FVIIa WT or mutants in FVII-deficient reconstituted PRP containing 30 µg/mL CTI, 8 µg/mL anti-FVIII MoAb 8D4-blocking FVIIIa cofactor activity. (F) Representative TG (n = 3) as in panel E but without anti-FVIII MoAb and without (left) or with (right) 20 µg/mL anti-FXI MoAb O1A6-blocking FXIa activity in FIX activation. (G) Representative TG (n = 3) initiated by 20 pM FIXa in FVII-deficient reconstituted PRP containing 4 µM hirugen, 30 µg/mL CTI, 20 µg/mL anti-FXI MoAb. (H) Representative TG as in panel G but initiated by 2.5 pM rTF and 400 pM FVIIa WT or E154A with (left; n = 3) or without (right; n = 5) 8 µg/mL anti-FVIII MoAb. (I) Representative TG as in panel H but with added 3 nM FVa with (left; n = 4) or without (right; n = 5) anti-FVIII MoAb. **P < .01. ***P < .001.
Both FVIIa mutants with impaired FXa turnover formed a functional FVIIIa-FIXa intrinsic tenase complex when FIXa was available, but only the exosite mutant E154A produced FVIIIa-IXa activity when zymogen FIX was present instead (Figure 5D), in agreement with the fact that FVIIa T99Y,30 unlike E154A, cannot activate FIX (supplemental Figure 4B). Thus, complementing the ability to generate FIXa,15,16 direct activation of the antihemophilic FVIII cofactor by nascent FXa product of TF-FVIIa enables intrinsic pathway coagulation before TFPIα inhibitory control. We tested these conclusions in FVII-deficient reconstituted PRP in which, besides endogenous plasma coagulation inhibitors, platelets are an additional source of TFPIα.11 Under these conditions, FVIIa WT, but not E154A or T99Y mutants, induced TG in the presence of a neutralizing anti-FVIII MoAb (Figure 5E), confirming that the mutants could not directly generate thrombin in a plasma milieu. Without FVIII inhibition, TG by FVIIa E154A was only slightly slower than that by WT, whereas TG by FVIIa T99Y was clearly decreased. FXIa inhibition reduced markedly TG by FVIIa T99Y but less by WT or E154A (Figure 5F; supplemental Figure 4C), in agreement with the latter generating FIXa as well as FVIIIa.
To prove directly that thrombin-independent FVIII activation occurred in these reactions, we first verified that the thrombin exosite blocker, hirugen, abolished FIXa-initiated TG in FVII-deficient plasma (Figure 5G). In the presence of the same hirugen concentration, TG by mutant FVIIa E154A, not by FVIIa WT, was entirely FVIII dependent, and without FVIII inhibition, TG induced by FVIIa WT and E154A was of similar magnitude, but TG by the latter was clearly delayed (Figure 5H). To explain the delay, we reasoned that impaired direct FXa generation by the mutant FVIIa could reduce FVa cofactor generation for initial prothrombinase assembly. Indeed, adding FVa normalized the delay in FVIIIa-dependent TG by FVIIa E154A (Figure 5I). Accordingly, adding FVa to normal PRP accelerated TF-initiated TG but not more than did blocking TFPI function (supplemental Figure 4D). These data indicate that prothrombinase activity is regulated by TFPI control of FXa generation13,14 that contributes to FV activation4 and reinforce the concept that FVIII activation during TF-initiated coagulation generates FVIIIa-FIXa intrinsic tenase activity independently of thrombin feedback reactions and escaping TFPI control.
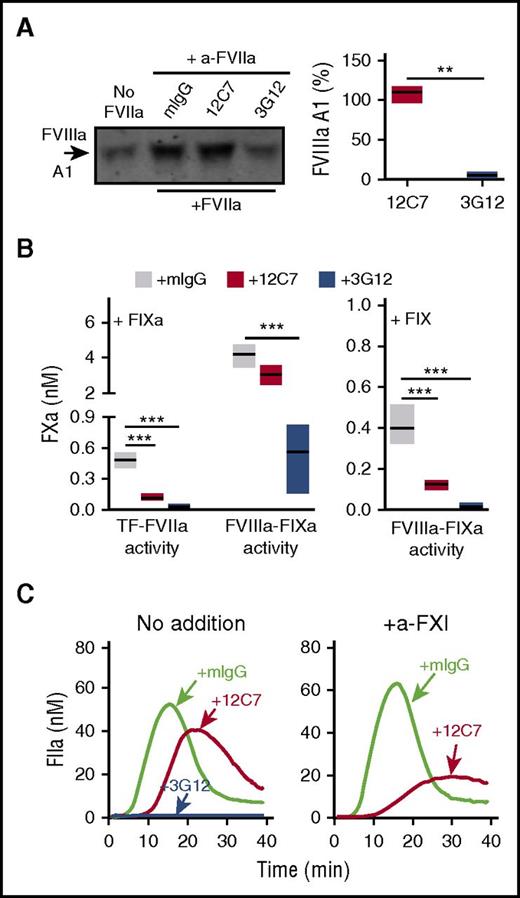
We then screened a library of MoAbs to FVIIa to identify a proof of principle inhibitor that could recapitulate the shift in functional properties (loss of efficient FXa and FIXa generation but not of FVIII activation) seen with the FVIIa mutant T99Y. In contrast to the fully inhibitory MoAb 3G12, antibody 12C7 had no effect on FVIII activation (Figure 6A). Moreover, it had no significant effect on the generation of intrinsic tenase activity when FIXa was present, but it markedly inhibited when FIX was supplied instead (Figure 6B). MoAb 12C7 attenuated TG in PRP but, as seen with FVIIa T99Y, rendered TG FXIa dependent (Figure 6C). Thus, results with mutant FVIIa molecules and inhibitory antibodies concordantly show that the TF-FVIIa complex can initiate intrinsic and extrinsic coagulation pathways in distinct reactions.
Anti-FVIIa MoAb 12C7 mimics FVIIa T99Y functional properties. (A) Representative immunoblot showing the effect of anti-FVIIa MoAbs 3G12 and 12C7 (20 µg/mL) on TF-dependent FVIIIa generation in reactions including 50 pM rTF, 200 pM FVIIa, 135 nM FX, 3.5 nM FVIII, 3 nM FV, and 200 nM lepirudin incubated for 120 s at 37°C (left). Quantification of the data on the left (n = 3; min-to-max floating bars, line at the mean) (right); differences were evaluated by Welch-corrected 2-tailed t test. (B) Anti-FVIIa MoAb 12C7, but not 3G12, preserves FVIIIa-dependent FXa generation by 10 nM FIXa (left; n = 3), but not 90 nM FIX (right; n = 3-5) in reactions containing 50 pM rTF, 200 pM FVIIa, 700 pM FVIII, 3 nM FV, 135 nM FX, 10 nM TFPIα, 200 nM lepirudin, and 2.5 mM CaCl2 incubated for 180 or 360 s, respectively, at 37°C. Anti-FVIIa MoAbs or control mouse IgG were added at 20 µg/mL. Results (min-to-max floating bars, line at the mean) were analyzed by ANOVA/Tukey tests. (C) Representative thrombograms (n = 2) showing the effect of anti-FVIIa MoAbs 3G12 and 12C7 (20 µg/mL) on 1.2 pM rTF-induced TG in normal PRP with CTI (30 µg/mL) and without (left) or with (right) addition of anti-FXI MoAb O1A6 (20 µg/mL) blocking FIX activation by FXIa. **P < .01. ***P < .001.
Anti-FVIIa MoAb 12C7 mimics FVIIa T99Y functional properties. (A) Representative immunoblot showing the effect of anti-FVIIa MoAbs 3G12 and 12C7 (20 µg/mL) on TF-dependent FVIIIa generation in reactions including 50 pM rTF, 200 pM FVIIa, 135 nM FX, 3.5 nM FVIII, 3 nM FV, and 200 nM lepirudin incubated for 120 s at 37°C (left). Quantification of the data on the left (n = 3; min-to-max floating bars, line at the mean) (right); differences were evaluated by Welch-corrected 2-tailed t test. (B) Anti-FVIIa MoAb 12C7, but not 3G12, preserves FVIIIa-dependent FXa generation by 10 nM FIXa (left; n = 3), but not 90 nM FIX (right; n = 3-5) in reactions containing 50 pM rTF, 200 pM FVIIa, 700 pM FVIII, 3 nM FV, 135 nM FX, 10 nM TFPIα, 200 nM lepirudin, and 2.5 mM CaCl2 incubated for 180 or 360 s, respectively, at 37°C. Anti-FVIIa MoAbs or control mouse IgG were added at 20 µg/mL. Results (min-to-max floating bars, line at the mean) were analyzed by ANOVA/Tukey tests. (C) Representative thrombograms (n = 2) showing the effect of anti-FVIIa MoAbs 3G12 and 12C7 (20 µg/mL) on 1.2 pM rTF-induced TG in normal PRP with CTI (30 µg/mL) and without (left) or with (right) addition of anti-FXI MoAb O1A6 (20 µg/mL) blocking FIX activation by FXIa. **P < .01. ***P < .001.
Intrinsic pathway activation by TF leads to fibrin formation in flowing blood
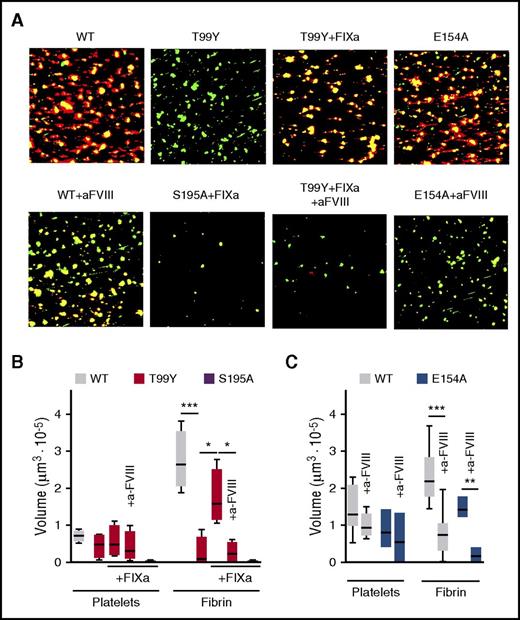
To evaluate whether TF-FVIIa initiates thrombus formation in flowing blood ex vivo when direct initial thrombin generation is limited, we surface-immobilized rTF at a low concentration sufficient for FVIII-dependent fibrin formation at a wall shear rate of 300 s−1. Under these conditions established with WT FVIIa, FVIIa T99Y were less thrombogenic in spite of supporting more platelet adhesion than were inactive FVIIa S195A (Figure 7A-B). Addition of 10 pM FIXa to blood containing FVIIa T99Y, but not containing inactive FVIIa S195A, restored FVIII-dependent fibrin formation (Figure 7A-B). In contrast, mutant FVIIa E154A, which is as defective as T99Y in direct TG (Figure 5E), supported FVIIIa-dependent thrombus formation similar to FVIIa WT when added without FIXa to FVII-deficient reconstituted blood (Figure 7A,C). This confirmed that the nascent FXa product of TF-FVIIa can directly generate FVIIIa functioning in the intrinsic tenase complex with the potential to enhance hemostasis in low-TF environments with limited direct TF-dependent thrombin generation activating feedback loops.
FVIIIa-dependent thrombus formation induced by the TF-FVIIa initiation complex in flowing blood. (A) Blood reconstituted with washed type 0 blood cells added to the original count into FVII-deficient citrated PPP with 200 pM WT or mutant FVIIa and without or with the inhibitory anti-FVIII MoAb C5 (25 μg/mL) was recalcified to 1.29 mM Ca2+ and perfused for 3.5 min at 300 s−1 wall shear rate. Where indicated, FIXa (20 pM) was added to blood. Representative confocal images are shown with superimposed green (platelet aggregates and leukocytes) and red (fibrin) fluorescence channels. Image side = 312 μm. (B) Quantification of the volume of platelet aggregates and deposited fibrin after adding FVIIa WT, T99Y, or S195A without or with anti-FVIII MoAb (n = 4-6 for the different conditions). (C) As in panel B, but after adding FVIIa WT or E154A (n = 3-8 for the different conditions). Results in panels B and C (shown as 25th-75th percentile bars, min-to-max whiskers, line at the median; or min-to-max floating bars, line at the mean when n ≤ 3) were evaluated by the ANOVA/Tukey tests. *P < .05. **P < .01. ***P < .001.
FVIIIa-dependent thrombus formation induced by the TF-FVIIa initiation complex in flowing blood. (A) Blood reconstituted with washed type 0 blood cells added to the original count into FVII-deficient citrated PPP with 200 pM WT or mutant FVIIa and without or with the inhibitory anti-FVIII MoAb C5 (25 μg/mL) was recalcified to 1.29 mM Ca2+ and perfused for 3.5 min at 300 s−1 wall shear rate. Where indicated, FIXa (20 pM) was added to blood. Representative confocal images are shown with superimposed green (platelet aggregates and leukocytes) and red (fibrin) fluorescence channels. Image side = 312 μm. (B) Quantification of the volume of platelet aggregates and deposited fibrin after adding FVIIa WT, T99Y, or S195A without or with anti-FVIII MoAb (n = 4-6 for the different conditions). (C) As in panel B, but after adding FVIIa WT or E154A (n = 3-8 for the different conditions). Results in panels B and C (shown as 25th-75th percentile bars, min-to-max whiskers, line at the median; or min-to-max floating bars, line at the mean when n ≤ 3) were evaluated by the ANOVA/Tukey tests. *P < .05. **P < .01. ***P < .001.
Discussion
Our findings delineate a novel function of the extrinsic TF-FVIIa complex, namely, providing selective feed-forward activation of the FVIII antihemophilic cofactor independently of thrombin feedback loops (Figure 1B). This specific reaction of nascent FXa escapes control by physiologic coagulation inhibitors in PRP or TFPIα in purified systems. Together with generation of the FIXa antihemophilic protease by TF-FVIIa,15,16 direct FVIII activation by TF-FVIIa-nascent FXa completes a pathway to FVIIIa-FIXa intrinsic tenase activity fully integrated within TF-initiated coagulation preceding direct activation of the common coagulation pathway.
Nascent TF-FVIIa-FXa generates FVIIIa, facilitating the formation of intrinsic tenase but without providing FVa for prothrombinase activity. Generating the latter requires FXa undocking from TF-FVIIa, thus exposing free FXa to inhibitory control. Therefore, the newly identified TF-FVIIa-FXa function allows for accumulation of active prohemostatic antihemophilic FVIIIa cofactor without increasing prothrombotic FVa. This may be of relevance for targeted FXa anticoagulants that, with comparable antithrombotic potency, have a lesser impact on hemostasis than do vitamin K antagonists.42,-44 Of note, such a mechanism is independent of thrombin feedback reactions and FXI activity and may thus support hemostasis during treatment with thrombin inhibitors or recently validated strategies targeting FXI.45
Selectivity for cofactor activation indicates distinct functional properties of FXa in complex with or released from TF-FVIIa. Although coagulation cofactor-enzyme complexes are typically geared toward efficient substrate turnover for rapid thrombin generation, throughout evolution the TF initiation complex appears to have preserved mechanisms favoring its stability. FX interacts with TF-FVIIa through an extended interface that is minimally affected by zymogen to enzyme transition.46,-48 In this interface, FVIIa residue E154, conserved across species, transmits conformational changes from the substrate-occupied active site of the protease41 and may thereby regulate subsequent product release. Elimination of this conformational switch was sufficient to segregate macromolecular substrate FX activation and product FXa turnover by TF-FVIIa. Thus, mutant FVIIa E154A helped inform on direct FVIII activation by nascent FXa associated with TF-FVIIa as well as the contribution of this novel pathway to thrombin generation and thrombogenesis in platelet-rich plasma and whole blood under flow.
Stability of the TF coagulation initiation complex likely represents the evolutionary advantage of preserving key signaling roles of TF-FVIIa-FXa that link coagulation activation and innate immunity.32 In line with efficient FVIII activation, FVIIa T99Y is fully functional in mediating TF-FVIIa-FXa activation of protease activated receptor (PAR) 2.30,32 Moreover, as seen for FVIIIa generation, resistance to functional inhibition by TFPIα is also an important feature of PAR signaling induced by TF-FVIIa-FXa in endothelial cells.49 This signaling complex is additionally stabilized by recruitment of the FXa-binding partner, endothelial protein C receptor (EPCR), in mouse and man.50,51 A key innate immune signaling role for the TF-FVIIa-FXa-EPCR complex recently emerged in dendritic cells,52 in which it is essential for toll-like receptor 4 induction of interferon-regulated genes. Negative regulation of this pathway by the alternative EPCR ligand, activated protein C, utilizes the canonical anticoagulant cofactor functions of FV and protein S.53 Thus, these and other nontraditional functions of the coagulation system54 likely use the same mechanistic features that simultaneously serve diverse roles in immunity, hemostasis, and injury repair.
Our findings provide the biochemical bases for defining distinct roles of TF in supporting hemostasis or contributing to thrombosis. One can envision application of mutants of FVIIa or the described antibody reagents to define the partial functions of TF in triggering thrombogenesis directly or provide activation of the intrinsic pathway of relevance for hemostasis. Ultimately, this should lead to the possibility of selectively assessing the effects of recent and future new anticoagulants on the dual roles of TF in hemostasis and thrombosis. The novel concepts on coagulation presented here may also have implications for the development and evaluation of hemostatic agents, providing protection from severe bleeding complications while avoiding adverse thrombotic complications in patients with underlying vascular pathologies.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Hiroshi Deguchi and Thomas Siegemund for technical advice on TG assays.
This work was supported by grants from the National Institutes of Health, National Heart, Lung, and Blood Institute (HL-117722 [Z.M.R.], HL-31950 [W.R. and Z.M.R.], and UM1 HL120877 [W.R.]), the MERU Foundation, Italy, and the Roon Research Center for Arteriosclerosis and Thrombosis at The Scripps Research Institute (Z.M.R.); and the Humboldt Foundation, Germany (W.R.).
Authorship
Contribution: Y.K. planned research, performed and interpreted coagulation studies, and contributed to writing the manuscript; G.L.M., A.Z., P.M., and J.N.O. performed mouse thrombosis and ex vivo thrombogenesis studies; A.S.R. prepared and characterized macrophage-derived microparticles; A.J.G., S.K., A.G., H.Ø., and L.C.P. provided essential reagents and reviewed the manuscript; and W.R. and Z.M.R. conceived and planned the study, interpreted results, and wrote the manuscript.
Conflict-of-interest disclosure: Z.M.R. is founder, President, and CEO of MERU-VasImmune, Inc., and has equity interest in Sedicidodici, s.r.l., 2 companies that are developing coagulation assays based on technology related to work presented in this article. Y.K. is a part-time employee of MERU-VasImmune. Oregon Health & Science University and A.G. may have a financial interest in the results of this study. H.Ø. and L.C.P. are employees of Novo Nordisk. The remaining authors declare no competing financial interests.
Correspondence: Zaverio M. Ruggeri, Department of Molecular Medicine, The Scripps Research Institute, MEM-175, 10550 N. Torrey Pines Rd, La Jolla, CA 92037; e-mail: ruggeri@scripps.edu; and Wolfram Ruf, Department of Immunology and Microbiology, The Scripps Research Institute, 10550 N. Torrey Pines Rd, La Jolla, CA 92037; e-mail: ruf@scripps.edu.


![Figure 3. The TF initiation complex activates FVIII. (A) TG was induced by 20 pM FIXa; or 400 pM FVIIa WT or inactive iFVIIa; or FIXa combined with FVIIa WT or iFVIIa added into FVII-deficient reconstituted PRP (180 ⋅ 103 platelets/μl) containing 30 μg/mL CTI, 0.15 pM rTF, and 18 mM CaCl2, followed by incubation for 5 (n = 2-5), 8 (n = 2-5), or 11 (n = 3) min at 37°C. (B) FVIII (1.4 nM) activation by 400 pM rTF and 500 pM FVIIa with or without 135 nM FX in reactions containing 40 nM TFPIα and 200 nM lepirudin incubated for 30 s at 37°C. After blocking residual FXa and TF-FVIIa with 70 nM of TAP and 20 µg/mL each of MoAbs 5G9 and 12C7, respectively, FVIIIa clotting activity (n = 2-4) was measured by adding the reaction mixture into FVIII-deficient plasma with 10 nM FIXa, 20 µM phospholipids, and 8 mM CaCl2. (C) TG as in panel A but induced by a preformed complex of 10 nM rTF, 10 nM iFVIIa, 5 nM FXa, and 40 nM TFPIα or NAPc2 added at the indicated TF/FXa concentrations without (left) or with (right) 10 pM FIXa. Incubation for 8 min at 37°C (n = 3-5). Results in panels A-C are shown as 25th-75th percentile bars (minimum-to-maximum [min-to-max] whiskers, line at the median) or, when n ≤ 3, min-to-max floating bars (line at the mean); analysis by ANOVA/Tukey tests (after y = log10 y transformation in panel C). (D) Representative immunoblots (n = 2) of FVIIIa and FVa generation after 30 or 60 s in reactions with the indicated combinations of 200 pM FXa, 500 pM iFVIIa, 40 nM NAPc2, and 1 µM TAP added to 400 pM rTF, 700 pM FVIII, 3 nM FV, and 200 nM lepirudin. (E) Representative immunoblots showing the effect of TAP (1 µM) on FVIII activation by TF-FVIIa (400 and 500 pM, respectively) and 135 nM FX (top). Reactions, containing 700 pM FVIII, 3 nM FV, and 200 nM lepirudin also, were incubated for 30 s at 37°C. Quantification of experiments as shown in the top panel (n = 4), presented as 25th-75th percentile bars and analyzed by 2-tailed t test (bottom). (F) Dose response of FVa effect on 1 µM prothrombin conversion to thrombin in reactions containing 10 pM FXa, 50 pM rTF, and 700 pM FVIII incubated for 180 s at 37°C; mean ± 95% confidence interval (CI) (n = 4). (G) Dose response of FVa effect on FVIII activation by 10 pM FXa in reactions with 50 pM rTF, 700 pM FVIII, and 200 nM lepirudin incubated for 180 s at 37°C. FVIIIa activity (mean ± 95% CI, n = 4) was measured as generated FXa in the presence of 2 nM FIXa and expressed as percentage of that measured in the absence of FVa. (H) Representative immunoblots (n = 3) of FVIIIa generation in reactions as in panel G (left). Quantification of generated FVIIIa (n = 3) expressed as in panel G and shown by min-to-max floating bars (right); analysis by 1-sample t test. *P < .05. **P < .01. ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/130/14/10.1182_blood-2017-02-767079/4/m_blood767079f3.jpeg?Expires=1769760055&Signature=nzuAIoqlZ4WpVFXy0W3zPh5QKo640Q~Vkp3cJ3wBTLdN0aYc5K8k9tUzbsj0wAc9JQN2UrsqWvx0Y-BP-yBGvxCXUXJT0wJEX8fn0rvw0xq0bmTzQeEE6xKEShblo0MSpxXX6Kkzcyie2ZXk2m1Eux4EJ~Trdrf2320PeYXK4J0XKn0X~30ALGWK5g6ROFxwL2PtBqFXOPHTCwbjfoov0K~Uj0UDcMSDYbQJktIapDBnRl3wL5-II8Uw7V5O40R80kQKFKf0yL7AxiBDv8YFxGC8cN7wO02l1WWir~J6xjG6l00YYtYgfu0SPdaK1Kg9DC5Ej-uZhsO3kVfvgc4Upw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Thrombin-independent FVIII activation by nascent TF-FVIIa-FXa. (A) Representative immunoblots showing FVIIIa A1 activation fragment generation induced by 200 pM FVIIa and 50 pM rTF (0.37 μM phospholipids) in reactions containing 700 pM FVIII, 3 nM FV, 135 nM FX, 1 µM prothrombin (FII) (WT without or with 4 μM DAPA [n = 4] or inactive S195A mutant [n = 3]), and 2.5 mM CaCl2 incubated at 37°C for 120 s (top). Quantification of FVIIIa-A1 calibrated with known quantities of the fragment (bottom). (B) FVIIIa activity calculated from FXa generation in reactions as in panel A but with 10 nM FIXa added and incubated for 180 s at 37°C (n = 4-12). FXa generation dependent on FVIIIa-FIXa activity was calculated by subtracting FXa generated by FVIIa and FIXa added individually from that by FVIIa/FIXa added together. (C) FVIIIa activity generated and calculated in reaction as in panels A and B but with the addition of 10 nM TFPIα. Results in panel A (bottom) and panels B and C (shown as 25th-75th percentile bars, min-to-max whiskers, line at the median; or min-to-max floating bars with line at the mean when n ≤ 3) were analyzed by ANOVA/Tukey tests. (D) Effect of different hirugen concentrations on TG initiated by 10 pM FIXa in normal PRP (180 ⋅ 103 platelets/μL) containing 30 μg/mL CTI and 20 µg/mL anti-FXIa blocking MoAb O1A6 (n = 3). (E) Representative thrombograms initiated in normal PRP, containing CTI and anti-FXIa MoAb as in D, by 0.15 pM rTF, 10 pM FIXa, or both without (left) or with (right) 2 µM hirugen (n = 3). ***P < .001. NS, not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/130/14/10.1182_blood-2017-02-767079/4/m_blood767079f4.jpeg?Expires=1769760055&Signature=4rAcjK-xqiAaeznLnJXZcE7CsRWDTOiyWVWKxcAUGMtvyQ7ugQ4nFKKjRTpKe15~NXUZKQ0uekvri8tCEVa0Z8bDNWC1yOuTQTMWKYEKg~BfM3Xp0M8tkvRXkaKtgpj4oNslManHjG8mS~kj6T1oYxYR2GsMeWE9AMgPhLGtWn-tfFfy1wd558BzAj7Iaxc34zJln4yrOw511AvEbI7pCkn61b0YLcmuZ5QG4Zqw6WzFUv4akS7Uqeq11~IzzRPg1BaIobuYesvA0GDMUbuUwmBrQo96T5ysgqO8UC55g~UIUxwbhpPz6comQ0TnpygqogYCybsFsQb2yKbxzeucpA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal