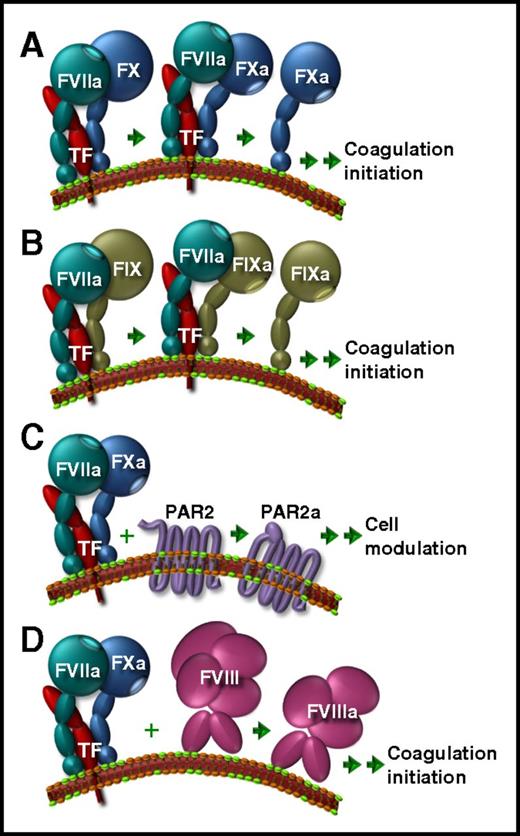
The functions of maestro TF. The TF/FVIIa complex initiates activation of FX to FXa (A) or FIX to FIXa (B) localized to an anionic phospholipid-containing membrane (green lipid polar head groups). (C) Nascent TF/FVII/FXa is an important cell modulator via cleavage of PAR2 to expose its tethered ligand in the stimulated form (PAR2a). (D) Newly defined in the current article is an additional TF/FVIIa/FXa function, which initiates FVIII activation. Double arrows indicate multistep processes.
The functions of maestro TF. The TF/FVIIa complex initiates activation of FX to FXa (A) or FIX to FIXa (B) localized to an anionic phospholipid-containing membrane (green lipid polar head groups). (C) Nascent TF/FVII/FXa is an important cell modulator via cleavage of PAR2 to expose its tethered ligand in the stimulated form (PAR2a). (D) Newly defined in the current article is an additional TF/FVIIa/FXa function, which initiates FVIII activation. Double arrows indicate multistep processes.
TF is a stimulus-induced protein cofactor. Found on the cell surface, it is best known as the trigger of localized blood clot formation and the keystone of the extrinsic branch of coagulation.2 Whether clotting is initiated or not is predominantly orchestrated by the availability of protein cofactors, like the initiator TF, but also includes the chorus of amplifying cofactors FVa and FVIIIa. This cofactor trio enhances the production of functional clotting proteases by hundreds of thousand fold. In this context, TF increases the activity of FVIIa for localized proteolytic activation of FX to FXa (see figure panel A) and FIX to FIXa (see figure panel B).3 Once FXa releases from the TF/FVIIa complex, it combines with the cofactor FVa for generation of thrombin, and FIXa combines with its respective cofactor FVIIIa to amplify the FXa production originated by TF/FVIIa. Thrombin is the principal biological effector within the coagulation pathway and escalates its own production by activating FV and FVIII to form FVa and FVIIIa, respectively. When sufficient thrombin accumulates to overcome the constitutive anticoagulant threshold in plasma, clot formation occurs.
Dissociation of the enzymatic product FXa from the binary cofactor/protease complex, TF/FVIIa, is relatively slow. As a constituent of the nascent ternary complex, TF/FVIIa/FXa, TF has an additional important cofactor role in cell stimulation via protease-activated receptor 2 (PAR2) (see figure panel C). This accounts for its links to a myriad of pathologies, including cancer,4 obesity,5 pain,6 and viral infections,7 in addition to vascular disease. The roles of TF taken together make it the grand conductor of the physiological hemostatic and inflammatory responses.
Because of the importance of the cofactors FVa and FVIIIa, a critical question in coagulation is how they are initially activated prior to the thrombin crescendo. The accepted mode of early FV activation is by TF/VIIa-produced FXa.8 However, it is still a puzzle whether early activation of FVIII is a raised baton that precedes sufficient thrombin buildup for feedback amplification. In the article by Kamikubo et al, several expert laboratories combine to solve the riddle by showing that the constituents comprising the nascent TF/FVIIa/FXa complex harmonize for effective FVIIIa production.
Most of the clotting enzymes are multifunctional, creating a technical challenge to unambiguously discern new functions. Therefore, mouse models, plasma experiments, purified protein experiments, and specific inhibitory agents were used by the authors to evaluate the role of TF/FVIIa/FXa in FVIII activation. In a mouse model of carotid artery occlusion induced by 7% ferric chloride, TF- and FXI-specific antibodies that individually inhibit the extrinsic and intrinsic (FVIII-dependent) branches of coagulation, respectively, functioned independently toward attenuating occlusion. Surprisingly, the response differed with 8% ferric chloride, which induced a more severe lesion. Here, the low antibody concentrations that were used in the preceding experiment only attenuated clot formation when they were combined. At higher doses of antibody, only the anti-FXI antibody was inhibitory, not the TF-specific antibody. Of importance to dissect the mechanism, the authors used similar combinations of antibody in a complementary femoral vein occlusion model and revealed that antibody-mediated clot formation was attenuated only in the presence of purified FVIII, but not FVIIIa. The combined in vivo data suggest a role for TF in FVIII activation.
To determine which constituents of the TF/FVIIa/FXa complex may be required for FVIII activation, experiments were conducted in plasma or by systematically reconstituting select purified proteins. Furthermore, Kamikubo et al employed a specific TF/FVIIa inhibitor (nematode anticoagulant peptide c2), an FXa inhibitor (tick anticoagulant peptide), and recombinant FVIIa whose protease active site was genetically inactive (iFVIIa). They also used 2 variants of FVIIa (T99Y and E1554A) that stabilized the nascent TF/FVIIa/FXa complex. These were used to further investigate the proposed mechanism by following several parameters including thrombin generation, FVIIIa activity, FVIII cleavage to FVIIIa, and clot volume in reconstituted FVII-deficient blood under flow conditions. Their conclusion was that the full TF/FVIIa/FXa ensemble was involved. Although FVIIa is required for conversion of FX to FXa to favor formation of the nascent complex, it is FXa that proteolytically activates FVIII as a player within TFa/FVIIa/FXa (see figure panel D), similar to PAR2 cleavage.9
Because thrombin feeds back to activate FVIII, the authors carefully excluded this complication by conducting experiments in the presence of specific thrombin inhibitors (hirugen, dansylarginine N-[3-ethyl-1,5-pentanediyl]amide) or genetically modified thrombin that has a nonfunctional catalytic site (S195A). The authors concluded that like many previous reports, thrombin indeed generates significant FVIIIa, but not all of it. The remainder is attributed to TF/FVIIa/FXa in their experiments.
The studies presented here show that TF arranges the coagulation concerto by not only initiating canonical FXa and FIXa production, but also activating FVIII to FVIIIa. Its newly described cadence is highly specific and does not contribute to activation of FV, although FVIII and FV share domain and amino acid motif homology. Thus, TF/FVIIa contributes to the initiation of coagulation by distinct mechanisms dictated by the temporal association of FXa with TF/FVIIa and may ameliorate bleeding risk for thrombin-targeted anticoagulants.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal