Abstract
T-cell acute lymphoblastic leukemia (ALL) is a rare disease in adults with inferior survival outcomes compared with those seen in pediatric patients. Although potentially curable with ∼50% survival at 5 years, adult patients with relapsed disease have dismal outcomes with <10% of patients surviving long term. This review will discuss the diagnosis and management of adult patients with newly diagnosed T-cell ALL with an emphasis on the immunophenotypic and genetic analyses required to assign prognosis, risk stratify, and guide post-remission therapy. The evidence for the main components of complex T-cell ALL treatment regimens is described. The importance of monitoring minimal residual disease is emphasized, with a discussion of the different methods used. The results of hematopoietic cell transplantation are analyzed, and recommendations made about which patients should be considered for this intervention. The treatment of the adolescent and young adult group is delineated, and the role of using “pediatric-inspired” regimens in older adults considered. We also describe the current data and potential future options for the use of novel therapies, including nelarabine and γ-secretase inhibitors, in adult patients with T-cell ALL.
Introduction
T-cell lymphoblastic disease is an uncommon disorder in adults. Surveillance, Epidemiology, and End Results Program registry data shows an overall incidence of acute lymphoblastic leukemia (ALL) at 17.3 per million with T-cell ALL comprising ∼25% of all ALL in adults.1 The World Health Organization defines T-cell ALL and T-cell lymphoblastic lymphoma (LBL) as the same disease.2 T-cell ALL is used when there is extensive bone marrow involvement, whereas T-cell LBL is preferred when there is primarily a mass lesion with <20% to 25% blasts in the marrow. Management of T-LBL will not be discussed specifically in this review. T-ALL patients frequently present with a high tumor burden with hyperleukocytosis and large mediastinal masses. Although T-cell ALL is a highly aggressive disease, it is potentially curable in adults with superior 5-year overall survival (OS) rates compared with B-cell ALL (48% vs 41%).1,3,4
Diagnosis of T-lineage ALL
Diagnosis of T-cell ALL requires a combination of morphology, immunophenotyping, and cytogenetic analysis, the results of which have important prognostic implications for patient management.
The immunophenotype of T-cell ALL corresponds to the stage of intrathymic T-cell differentiation and provides the basis for the European Group for the Immunological Characterization of Leukaemias subclassification of T-cell ALL (Table 1).5 Lymphoblasts in T-cell ALL are TdT+ in addition to cytoplasmic CD3+, the only lineage specific marker for lymphoblastic T-cell disease. Other T-cell markers including CD1a, CD2, CD4, CD5, CD7, and CD8 are variably expressed according to the degree of T-cell differentiation. Several study groups have shown a relationship between prognosis and the degree of T-cell differentiation at diagnosis. The Gruppo Italiano Malattie Ematologiche dell’Adulto reported a 91% complete remission (CR) rate in patients with cortical/mature disease as opposed to 56% in those with pre-/pro- T disease.6 The Medical Research Council/Eastern Cooperative Oncology groups performed central immunophenotypic studies of 108 patients uniformly treated on the United Kingdom ALL (UKALL) XII/E2993 trial.3 CD1a-negativity was associated with an increased relapse rate and a lower survival (39% vs 64%; P = .01). Patients with cortical T-cell ALL in the same study had the most favorable prognosis with 5-year OS rates of 62%.4 Others have confirmed these findings,7,8 although Jain et al recently reported no difference in OS between T-cell immunologic subtypes in a total of 111 patients with T-cell ALL/LBL.9
T-cell CD antigen expression in pro-, pre-, cortical (thymic), and mature T-cell ALL cyCD3 indicates cytoplasmic CD3; sCD3, surface CD3
| . | cyCD3 . | CD7 . | CD5 . | CD2 . | CD1a . | sCD3 . | CD34 . |
|---|---|---|---|---|---|---|---|
| Pro-T | + | + | — | — | — | — | ± |
| Pre-T | + | + | + | + | — | — | ± |
| Cortical (thymic) | + | + | ± | ± | + | ± | — |
| Mature | + | + | ± | + | — | + | — |
| . | cyCD3 . | CD7 . | CD5 . | CD2 . | CD1a . | sCD3 . | CD34 . |
|---|---|---|---|---|---|---|---|
| Pro-T | + | + | — | — | — | — | ± |
| Pre-T | + | + | + | + | — | — | ± |
| Cortical (thymic) | + | + | ± | ± | + | ± | — |
| Mature | + | + | ± | + | — | + | — |
Early T-precursor ALL (ETP)
A novel subgroup of T-cell ALL, ETP-ALL, has recently been described and is characterized by a distinct gene expression profile and immunophenotype.10 ETP cells derive from a subset of immature thymocytes that retain the ability to differentiate into both T-cell and myeloid lineages, suggesting direct derivation from hematopoietic stem cells.11 ETP-ALL blasts are CD8− and CD1a−, CD5weak, and express one or more myeloid or stem cell marker.10 The gene expression profile of ETP-ALL has strong similarities to that of normal and myeloid leukemic stem cells and is distinct from non–ETP-ALL. An additional entity of “near” ETP, based on CD5 and myeloid antigen expression, has also been defined.12 ETP-ALL accounts for ∼15% of all T-cell ALL in children10,13,14 and ∼35% in adult T-cell disease,9,15,16 and is associated with high levels of minimal residual disease (MRD) post-induction chemotherapy14 and inferior long-term outcomes.7,9,10,13 However, data from the UKALL 2003 pediatric and young adult ALL trial suggested that these patients have an intermediate-risk outcome when treated on a contemporary protocol.17 Similar results have been reported by the Children’s Oncology Group.14 ETP-ALL has recently been shown to be dependent on aberrant JAK-STAT signaling,18 and data from the Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL) imply that the poor outcome is related to HOXA expression.19
Cytogenetics
Cytogenetic analysis in B-cell ALL has become an important part of the diagnosis and planning of treatment strategy in both pediatric and adult practice. Although there are an increasing number of molecular abnormalities associated with T-cell ALL, which may represent important targets for future novel therapies, few are currently required for routine diagnosis and treatment planning (Table 2).
The diagnostic work-up of adults with T-cell ALL: essential tests and tests that are currently research based
| Essential tests required for routine diagnosis and management of T-cell ALL . | Non-essential tests currently being investigated within clinical trials in T-cell ALL . |
|---|---|
| Immunophenotyping | |
| Determination of pre-T/pro-T, cortical, and mature T-cell phenotype | Determination of ETP subtype |
| Oncogenetic analysis | |
| Identification of patients with a complex karyotype | NOTCH1/FBXW7 mutations |
| PTEN/NK-RAS mutations | |
| JAK/STAT mutations | |
| NUP21-ABL1 fusion | |
| IL-7 receptor mutations | |
| Molecular assessment for identification of T-cell receptor rearrangement for MRD monitoring |
| Essential tests required for routine diagnosis and management of T-cell ALL . | Non-essential tests currently being investigated within clinical trials in T-cell ALL . |
|---|---|
| Immunophenotyping | |
| Determination of pre-T/pro-T, cortical, and mature T-cell phenotype | Determination of ETP subtype |
| Oncogenetic analysis | |
| Identification of patients with a complex karyotype | NOTCH1/FBXW7 mutations |
| PTEN/NK-RAS mutations | |
| JAK/STAT mutations | |
| NUP21-ABL1 fusion | |
| IL-7 receptor mutations | |
| Molecular assessment for identification of T-cell receptor rearrangement for MRD monitoring |
IL-7, interleukin 7.
The genetic lesions underlying T-cell ALL will be discussed in detail elsewhere in this series. Therefore, we will briefly discuss only the oncogenetic abnormalities that are either required for standard practice or are currently being evaluated within treatment algorithms in the context of clinical studies (Tables 2 and 3).
Selected current trials in T-cell ALL in adolescent and adult patients
| Trial group/trial . | Description of study . | Population . | Primary end point . | Trial # . |
|---|---|---|---|---|
| UKALL 14 (UK NCRI) | Phase 3 nelarabine in induction randomization | Adults aged 25-65 y with de novo ALL | EFS | NCT01085617 |
| UKALL 2011 (UK NCRI) | Phase 3 randomized comparison of dexamethasone and methotrexate schedules | Children and young adults up to 25 y with de novo ALL and LBL | Steroid-related toxicity; CNS and marrow relapse rate | ISRCTN64515327 |
| MD Anderson | Phase 2 nelarabine/HyperCVAD | Children and adults | CR | NCT00501826 |
| US Intergroup | Pediatric-like regimen | 21-40 y | Survival | NCT00558519 |
| DFCI | LY3039478 (notch inhibitor) | Relapsed/refractory disease; 2 y and older | Overall remission rate | NCT02518113 |
| GRAALL | Pediatric-like regimen | Adults (median age, 31 y) | EFS | NCT00327678 |
| NCI | Phase 3, bortezomib randomization | 18 y and older | EFS | NCT02112916 |
| GIMEMA | Phase 2, lineage-targeted methotrexate dosing | 18-65 y | EFS | NCT02067143 |
| Trial group/trial . | Description of study . | Population . | Primary end point . | Trial # . |
|---|---|---|---|---|
| UKALL 14 (UK NCRI) | Phase 3 nelarabine in induction randomization | Adults aged 25-65 y with de novo ALL | EFS | NCT01085617 |
| UKALL 2011 (UK NCRI) | Phase 3 randomized comparison of dexamethasone and methotrexate schedules | Children and young adults up to 25 y with de novo ALL and LBL | Steroid-related toxicity; CNS and marrow relapse rate | ISRCTN64515327 |
| MD Anderson | Phase 2 nelarabine/HyperCVAD | Children and adults | CR | NCT00501826 |
| US Intergroup | Pediatric-like regimen | 21-40 y | Survival | NCT00558519 |
| DFCI | LY3039478 (notch inhibitor) | Relapsed/refractory disease; 2 y and older | Overall remission rate | NCT02518113 |
| GRAALL | Pediatric-like regimen | Adults (median age, 31 y) | EFS | NCT00327678 |
| NCI | Phase 3, bortezomib randomization | 18 y and older | EFS | NCT02112916 |
| GIMEMA | Phase 2, lineage-targeted methotrexate dosing | 18-65 y | EFS | NCT02067143 |
DFCI, Dana-Farber Cancer Institute; GIMEMA, Gruppo Italiano Malattie Ematologiche dell’Adulto; NCI, National Cancer Institute; UK NCRI, United Kingdom National Cancer Research Institute.
Cytogenetic analysis of 303 of 356 patients with T-cell ALL treated on UKALL XII/E2993 was successful in 204 patients (67%) and demonstrated an abnormal karyotype in 72% of patients. The 8% of patients with a complex karyotype (≥5 abnormalities) had significantly inferior outcomes with OS 19% vs 51% at 5 years (P = .006) (Table 2). The 10 patients with 17p abnormalities had 20% survival (confidence interval, 0% to 45%) but this was not statistically different to the remaining T-cell ALL cases.4
At least 60% of adult patients with T-cell ALL will have a mutation of the NOTCH1/FBXW7 (N/F) pathway at diagnosis.20 The majority of studies that have examined the prognostic impact of N/F pathway mutations have concluded that they are associated with a good response to therapy and improved survival.21-28 However, not all studies report statistically significant results and there is clear heterogeneity in survival rates when comparing similar protocols. It is unclear whether these differences are driven by variable response to therapy, or simply because these mutations are secondary events and few studies have comprehensively factored in the effect of other genetic drivers. One exception is the study by Trinquad et al,29 who elegantly proposed an oncogenetic classifier for 212 adult patients with T-cell ALL based on the presence or absence of mutations in the N/F pathway, as well as the phosphatase and tensin homolog deleted on chromosome 10 (PTEN) and natural killer (NK)-RAS pathways. They described a good-risk group with significantly superior OS and cumulative incidence of relapse (CIR) rates (82% OS and 15% CIR) for patients with mutations in the N/F pathway with no associated mutations in PTEN or NK-RAS. All other patients, including those with mutated N/F genes plus mutations in PTEN or NK-RAS were classified as poor risk with OS 44% and CIR 54%. When combined with MRD assessment post-induction on a pediatric-inspired protocol, investigators identified a good-risk group (MRD <10−4 and good-risk genetic profile) with a 5-year OS of 91%.30 However, it should be noted that an inferior outcome was not reported for T-cell ALL patients with PTEN or RAS pathway mutations treated on UKALL 2003.27 These inconsistent results emphasize the need for a study, which comprehensively assesses all prognostic factors: demographic (age, sex), tumor burden (white cell count [WCC]), early response (MRD), as well as the full spectrum of genetic lesions. The latter must include both primary class defining abnormalities (eg, TAL1, LMO2, TLX1, TLX3, NKX2-1, and MEF2C and those driving HOXA expression), as well as key secondary lesions (eg, mutations and deletions of NOTCH1, FBWX7, NRAS, KRAS, PTEN, TP53, and CDKN2A/B, etc).
Treatment of T-cell ALL
Induction therapy
The goal of induction therapy in adult ALL is to achieve a deep remission with low treatment-related toxicity and rapid hematologic recovery to enable prompt progression to further therapy. Although induction regimens vary, most use anthracyclines (daunorubicin or doxorubicin), steroids (dexamethasone or prednisolone), and vincristine plus central nervous system (CNS)-directed prophylaxis with administration of intrathecal (IT) methotrexate. It is usual to include an intensification phase that contains cyclophosphamide and cytarabine.
Effective depletion of extracellular asparagine by administration of the enzyme l-asparaginase has long been an important component of the treatment of pediatric ALL, with T-cell ALL patients benefiting from intensified dosing schedules.31 In recent years, the use of pegylated Escherichia coli-derived (polyethylene glycol-asparaginase [PEG-ASP]) l-asparaginase has been shown to improve outcomes in children,32-36 offering a longer half-life and a lower risk of anti-asparaginase antibody formation. The majority of current adult induction regimens include l-asparaginase, although there is significant variability in how it is delivered. Cyclosphosphamide/vincristine/adriamycin/dexamethasone (HyperCVAD), most commonly used in the United States, contains no asparaginase and may be associated with inferior outcomes in patients with T-cell ALL in some series.37,38 In contrast, the “pediatric-inspired” regimens frequently used in adolescent and young adults (AYAs) contain up to 15 doses of PEG-ASP in the first 30 weeks of therapy.39 PEG-ASP used in combination with a multiagent regimen has been shown to effectively deplete asparagine in adult ALL,35,40 resulting in high CR rates and improved OS outcomes in patients with T-cell ALL, both compared with patients with B-cell ALL and patients treated with native enzyme.35 The German Multicentre Adult ALL (GMALL) study group report improved survival outcomes with intensified PEG-ASP scheduling in a large cohort of patients.41 Similarly, the Dana-Farber Cancer Institute reported excellent results with an intensified PEG-ASP regimen with 75% 3-year OS in T-cell ALL patients.42 Despite interest in intensifying regimens with additional doses of PEG-ASP in adults, there are clear data showing greater toxicities of asparaginase with increasing age, and the optimum dose for adults remains uncertain and may vary with age.43-46 There is a need to investigate the best methods of delivering l-asparaginase in adults with T-cell ALL within a large phase 3 trial.
Patients with T-cell ALL are more likely to have involvement of the CNS at presentation than patients with B-cell ALL (9.6% vs 4.4%; P = .001).47 Patients with CNS disease at diagnosis had inferior 5-year OS outcomes compared with patients without CNS involvement on the UKALL XII/E2993 trial (42% vs 29%) due to an increased risk of both systemic and CNS relapse.47 Pediatric trials have demonstrated improved event-free survival (EFS) outcomes for patients with T-cell ALL when high-dose IV methotrexate (4 doses of 5 g/m2) is added as an intensification phase.48 Although some groups have cited decreased CNS relapses as the primary reason for the improved outcomes,48 others have demonstrated improved systemic disease control with high-dose methotrexate (HDM) as the main benefit.49 Although there are limited data for HDM in the specific treatment of adult T-cell ALL, most ALL trial groups have adopted the use of HDM (doses range from 1-5 g/m2 in adult protocols) in addition to IT chemotherapy within their T-cell ALL protocols (Table 4).4,6,8,9,38,50-52 Combining HDM and PEG-ASP in adults with T-cell ALL is the subject of several ongoing trials (Table 3). The risk of CNS relapse in adult patients on modern regimens who have no evidence of CNS disease at presentation is ∼5%, with or without cranial radiotherapy.47,50,53
Prospective clinical trials in adults with T-cell lymphoblastic disease
| Reference . | Number with T-cell disease . | Disease type . | Treatment given . | CR and OS rates . |
|---|---|---|---|---|
| 6 | 90 | ALL | LAL0496 protocol with CNS RT | CR-NS, OS-NS |
| 52 | 87 | ALL | ALL 90, 93, and 97 protocols of JALSG; CNS RT for high WCC; alloHCT for almost half | CR 75.8% |
| OS 35% (5 y) | ||||
| 8 | 744 | ALL | GMALL 05/93, 06/99, and 07/2003; No alloHCT in CR1 | CR 86% |
| OS 47% (10 y) | ||||
| 50 | 76 | ALL | GRAALL 2003; CNS RT for all; alloHCT for high-risk patients | CR 99% |
| EFS 62% (42 mo) | ||||
| 4 | 356 | ALL | UKALL XII/E2993; alloHCT for those with sibling donor; RCT for the rest chemo vs autograft CNS RT for chemo only arm | CR 94% |
| OS 48% (5 y) | ||||
| 38 | 24 | ALL | HyperCVAD; alloHCT for high-risk patients (4%) | CR 89% |
| OS 47% (5 y) | ||||
| 51 | 40 | ALL/LBL | HyperCVAD plus nelarabine | CR 91% |
| OS 63% (3 y) | ||||
| 9 | 111 | ALL/LBL | HyperCVAD, HyperCVAD plus nelarabine, or augmented BFM | CR 88% |
| OS 52% (5 y) |
| Reference . | Number with T-cell disease . | Disease type . | Treatment given . | CR and OS rates . |
|---|---|---|---|---|
| 6 | 90 | ALL | LAL0496 protocol with CNS RT | CR-NS, OS-NS |
| 52 | 87 | ALL | ALL 90, 93, and 97 protocols of JALSG; CNS RT for high WCC; alloHCT for almost half | CR 75.8% |
| OS 35% (5 y) | ||||
| 8 | 744 | ALL | GMALL 05/93, 06/99, and 07/2003; No alloHCT in CR1 | CR 86% |
| OS 47% (10 y) | ||||
| 50 | 76 | ALL | GRAALL 2003; CNS RT for all; alloHCT for high-risk patients | CR 99% |
| EFS 62% (42 mo) | ||||
| 4 | 356 | ALL | UKALL XII/E2993; alloHCT for those with sibling donor; RCT for the rest chemo vs autograft CNS RT for chemo only arm | CR 94% |
| OS 48% (5 y) | ||||
| 38 | 24 | ALL | HyperCVAD; alloHCT for high-risk patients (4%) | CR 89% |
| OS 47% (5 y) | ||||
| 51 | 40 | ALL/LBL | HyperCVAD plus nelarabine | CR 91% |
| OS 63% (3 y) | ||||
| 9 | 111 | ALL/LBL | HyperCVAD, HyperCVAD plus nelarabine, or augmented BFM | CR 88% |
| OS 52% (5 y) |
BFM, Berlin Frankfurt Munster protocol; CNS RT, CNS radiotherapy; JALSG, Japan Adult Leukemia Study Group; NS, not significant; RCT, randomized controlled trial.
There is a paucity of data concerning the best consolidation therapy in adults for T-cell ALL and much current practice is based on pediatric trials. Most regimens use a delayed intensification block of therapy, repeating drugs used in induction, followed by a 2-year period of maintenance with 6-mercaptopurine and methotrexate, pulses of vincristine and steroids, plus additional CNS prophylaxis with IT chemotherapy (Table 4). Although maintenance is not required post-allogeneic hematopoietic cell transplantation (alloHCT), patients receiving a reduced intensity alloHCT (and less CNS-directed therapy) should have HDM prior to transplant and consideration of IT CNS prophylaxis post-HCT in order to minimize their risk of CNS relapse, although the value of this strategy is not proven.54
MRD
Monitoring response to treatment by MRD assessment should be a standard of care in all patients with ALL treated with curative intent. Standardized methods for molecular assessment of MRD identify rearrangements of the immunoglobulin heavy chain gene or T-cell receptor genes by using a large panel of consensus primers spanning the junctional regions of V, (D), and J gene segments. The random insertion and deletion of nucleotides into those segments generates clone-specific rearrangements, which can be sequenced to allow the generation of patient-specific real-time quantitative polymerase chain reaction assays for quantification in ∼90% of patients, with a quantitative range of 10−4. This method has an international standard and is quality-controlled within the European Study Group on MRD. Flow cytometry-based quantification of a “leukemia-associated immunophenotype” is also widely used. This technique has a lower sensitivity (10−3) and is less well standardized due to the wide range of approaches used, although the Euroflow consortium is working toward establishing an international standard.55 Next-generation sequencing can also be used to identify and quantify the same clone-specific re-arrangements and this forms the basis of a commercial Food and Drug Administration-approved test.
Many studies have confirmed early MRD response as a powerful predictor of long-term survival in adult patients with ALL.56-59 GMALL examined MRD at 9 time points in adults receiving GMALL 09/99 ALL therapy and showed that, using their treatment protocols, molecular MRD quantification after 2 courses of therapy (10 to 16 weeks from diagnosis) was the most predictive of outcomes with patients failing to achieve molecular remission (MRD >10−4) post-induction having inferior survival compared with patients who were MRD-negative (MRD <10−4) (42% vs 80%; P < .001).57 Seventy-two of the 196 patients (37%) within this study had T-cell disease.
The GRAALL analyzed the prognostic significance of MRD at 6 weeks from the initiation of induction therapy on the pediatric-inspired GRAALL 2003 and 2005 trials in 163 patients with T-cell ALL (total patients studied = 423). They defined a high-risk group as those with an MRD level ≥10−4 and unfavorable cytogenetics (no N/F mutation and/or N/K-RAS mutation and/or PTEN mutation).30 The 59% of patients in this high-risk group had markedly inferior OS compared with the good-risk group (62% vs 91% at 5 years).
Although it is reasonable to recommend that T-cell ALL patients with persistent MRD should be candidates for an alloHCT in first CR (CR1),60 there are currently no data to show that intervention with novel agents or allogeneic transplantation can overcome the relapse risk and this is currently the focus of many clinical trials (Table 3).
HCT
Allogeneic transplantation
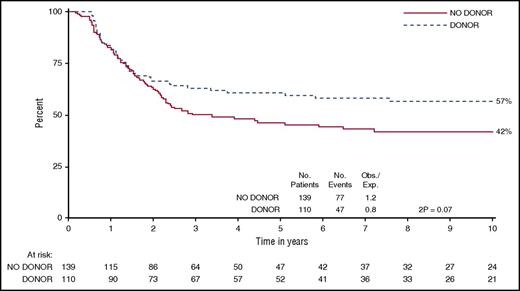
alloHCT is the most effective but also the most toxic anti-leukemic therapy for adults with ALL.61 Traditionally, myeloablative alloHCT, with a wide variety of conditioning regimens, has been used in the treatment of ALL. Etoposide and total body irradiation (13.2 Gy in 8 fractions) were used in UKALL XII/E2993. This trial assessed the role of a sibling donor alloHCT by a comparison of outcomes in those with vs those without a sibling donor (Figure 1).4 Two-hundred and fifty-three of 356 T-cell ALL patients were tissue typed; 110 had a sibling donor. OS at 5 years was 46% (38% to 55%) for the no donor group and 61% (51% to 70%) for the donor group (log rank P = .07, χ2 test of difference at 5 years; P = .02); this difference was maintained at 10 years (Figure 1). Having a sibling donor halved the chance of relapse (25% vs 51%; P < .0001) but modestly increased nonrelapse mortality (22% vs 12%; P = .06). The Passweg study showed that acute and/or chronic graft-versus-host disease reduced relapse 2.5-fold (Hazard ratio, 0.4) and that the graft-versus-leukemia effect was similar in patients with T- or B-cell disease.62 These results suggest that myeloablative alloHCT is an effective therapy in adults and can be considered for patients with high-risk T-cell disease (Table 5).4,9,10,17,29,30,44,60,63-65
Comparison of OS in patients with T-cell ALL who had a matched sibling donor vs those without a donor within the UKALL XII/E2993 trial.4
Comparison of OS in patients with T-cell ALL who had a matched sibling donor vs those without a donor within the UKALL XII/E2993 trial.4
Patients with T-cell ALL to be considered for alloHCT in first remission
| . | Indication* . | Reference . |
|---|---|---|
| 1 | Failure to achieve CR after induction | 60 |
| 2 | MRD >1 × 10−4 after 2 courses of therapy | 30, 60 |
| 3 | Presenting WCC >100 × 109/L† | 4 |
| 4 | Complex cytogenetics ≥5 abnormalities | 4, 30 |
| 5 | Early T-cell precursor ALL† | 9, 10, 17 |
| 6 | Unfavorable N/F/PTEN/RAS genetics | 29 |
| 7 | Age 40-65 y, especially if delays occur in treatment due to toxicity | 64 |
| . | Indication* . | Reference . |
|---|---|---|
| 1 | Failure to achieve CR after induction | 60 |
| 2 | MRD >1 × 10−4 after 2 courses of therapy | 30, 60 |
| 3 | Presenting WCC >100 × 109/L† | 4 |
| 4 | Complex cytogenetics ≥5 abnormalities | 4, 30 |
| 5 | Early T-cell precursor ALL† | 9, 10, 17 |
| 6 | Unfavorable N/F/PTEN/RAS genetics | 29 |
| 7 | Age 40-65 y, especially if delays occur in treatment due to toxicity | 64 |
TRM, treatment-related mortality.
Donor quality and the Hematopoietic Cell Transplantation Comorbidity index also affect the decision.65 alloHCT may reduce relapse risk but this should be balanced against the predicted TRM.
MRD negativity may override these unfavorable prognostic factors.
Total body irradiation-based conditioning, although effective in eradicating leukemic cells, causes considerable extramedullary toxicity.61 For this reason, the UK NCRI group has been prospectively studying reduced-intensity conditioning sibling and unrelated donor allografting in older patients (>40 years), in an attempt to reduce TRM but retain the graft-versus-leukemia effect. With 22 months median follow up, 120 patients (15% T cell, median age 51 years, 54% high-risk cytogenetics) had conditioning with fludarabine, melphalan, and alemtuzumab, and experienced 66% survival at 22 months with 18% TRM, suggesting that reduced-intensity conditioning allograft is a valid option for patients >40 years.64 The low TRM was partly due to a low incidence of grade 2-4 graft-versus-host disease but the 20% relapse rate so far may increase. The number of T-cell patients was insufficient for them to be analyzed separately. Patients with MRD >10−4 at alloHCT had a lower EFS (Hazard ratio 2.75; P = .047) compared with those that were MRD-negative ≤10−4). This patient subgroup may require additional pre-transplant therapy in an attempt to achieve MRD-negativity before transplant. TRM will also be affected by the source of stem cells; recent data shows that cord blood produces equivalent survival to unrelated donor HCT.66 There are no mature results of haploidentical HCT in adult ALL; this requires prospective study. Where possible, all patients with T-cell ALL should be treated within a prospective clinical trial. However, a list of potential indications for alloHCT in first CR1 is presented in Table 5.
Autologous stem cell transplantation
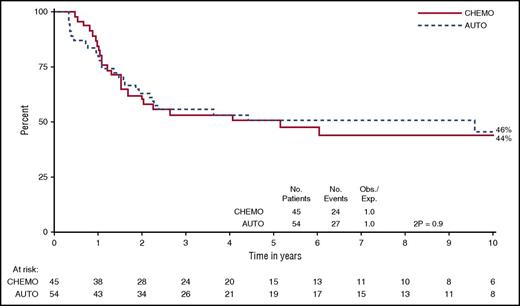
UKALL XII/E2993, the only randomized trial to date comparing autograft with standard chemotherapy, showed an 8% survival advantage for 2 years of maintenance chemotherapy over an autograft.61 Ninety-nine of the patients randomized to autograft vs chemotherapy in this study had T-cell ALL. Survival was the same in both arms (Figure 2). Although the study was not powered to analyze the T-cell subset separately, a test of heterogeneity did not suggest patients with T-cell disease should be treated differently to those with B-cell disease (P = .2).4 Further analysis suggested that patients with MRD-negativity (<10−4) prior to autograft have better outcomes than those with persistent MRD, but there is no evidence that autograft is superior to standard chemotherapy in any group of patients with ALL.67
Comparison of OS in patients with T-cell ALL treated with autologous stem cell transplantation (auto) or chemotherapy (chemo) within the UKALL XII/E2993 trial.4
Comparison of OS in patients with T-cell ALL treated with autologous stem cell transplantation (auto) or chemotherapy (chemo) within the UKALL XII/E2993 trial.4
In a Russian study, 28 of 77 (36%) patients with T-cell ALL had a BEAM (BCNU, etoposide, cytarabine, and melphalan) autograft in CR1. Although a selected group, 100% of autografted patients survived and none have relapsed with a 27-month median follow up.68
Treatment of T-cell ALL in AYAs
The incidence of T-cell ALL rises throughout childhood and adolescence (5% of all cases of ALL are <5 years and 27.6% >16 years; P ≤ .0001).69 It is widely accepted that AYA patients with ALL have improved outcomes when treated on pediatric protocols with intensified scheduling of l-asparaginase without the routine use of HCT.50,70-77 Hough et al69 reported OS outcomes of 72% at 5 years for AYA patients aged 16 to 25 years treated on a risk-adapted pediatric protocol where the strongest predictor for survival was immunoglobulin heavy chain gene or T-cell receptor-defined MRD status after induction. Patients with low-risk MRD (<0.01%) had a 93% EFS at 5 years compared with 64% EFS at 5 years for those with high-risk MRD (at least 0.01%). Over 75% of AYA patients with T-cell ALL had high risk or indeterminate MRD post-induction in this study.
Although there is broad agreement that young adults with ALL should be treated on intensive “pediatric-inspired” protocols, there is a lack of clarity on how we define an AYA. In the United Kingdom, anyone up to 25 years of age is considered a young adult, whereas other groups use a cutoff up to 40 years.78 In addition, the definition of what constitutes a “pediatric-inspired” protocol is not clear in the modern era where adult protocols are also based on an MRD risk-based stratification with intensified use of steroids, asparaginase, and other non-myelotoxic agents such as vincristine. Pediatric protocols rarely employ alloHCT in CR1 and yet we know that alloHCT is the most active anti-ALL therapy.61 A subgroup of AYA patients with T-cell ALL are likely to benefit from alloHCT in CR1, but we currently have no risk stratification models for use of alloHCT in frontline therapy in this group. In the United Kingdom, AYA patients with T-cell ALL treated on the NCRI UKALL 2011 trial (ISRCTN64515327) are risk-stratified on MRD alone. Those with very high-risk MRD (>10−1 at day 29 or 5 × 10−3 at week 14) are assigned nelarabine plus multiagent chemotherapy,79 followed by HCT. The current GRAALL study (NCT00327678) stratifies AYA ALL patients for alloHCT based on their oncogenetic classifier.30 The results of these trials are awaited with interest (Table 3). Finally, the toxicity of intensive “pediatric-inspired” protocols with increasing patient age remains a significant cause of morbidity and mortality,69 and needs careful evaluation within the context of large phase 3 studies.
Relapse
The overall outcome of relapsed ALL in adults is poor with <7% of patients surviving at 5 years.80 There is no agreed standard of care in adults with relapsed T-cell ALL, but standard chemotherapy regimens such as FLAG (fludarabine, cytarabine, and granulocyte colony-stimulating factor) ± idarubicin only result in 30% to 40% response rates with 6 months median OS in responders.81
Nelarabine
Nelarabine is the only new drug that is licensed specifically for use in relapsed/refractory T-cell ALL/LBL. Initial phase 1/2 pediatric trials reported response rates of 14% to 55% to single-agent nelarabine for patients with relapsed/refractory T-cell lymphoblastic disease, with neurotoxicity being the major toxicity affecting 18% of patients.82,83 Phase 2 trials in adults with relapsed/refractory T-cell ALL/LBL reported similarly encouraging response rates (41% to 46%) to single-agent nelarabine with 28% 1-year OS.84,85 Importantly, 80% of the 36% of patients achieving CR in the Gökbuget study were able to proceed to alloHCT, with 1-year OS for the whole cohort of 24% and 31% for patients reaching HCT. Although 16% of patients experienced some neurologic toxicity, grade 3-4 toxicities were only observed in 7% of patients (n = 9), and most were transient and reversible. The use of nelarabine in combination with other chemotherapeutic agents, particularly cyclophosphamide, has shown promising response rates in children with relapsed/refractory disease.79
These encouraging results in relapsed disease have created an interest in the use of nelarabine in the initial (upfront) therapy of T-cell ALL. A pilot study from the Children’s Oncology Group added nelarabine to an intensified Berlin Frankfurt Munster protocol for children and young adults, initially reserving this therapy for slow early responders.86 Equivalent outcomes for the slow early responders who received nelarabine (73% with 5-year EFS) and the rapid early responders who did not receive nelarabine (69% with 5-year EFS; P = .64) were reported (Table 5). More recently, Jain et al51 reported the outcomes for 40 adult patients with T-cell ALL/LBL when treated in induction with nelarabine plus HyperCVAD. This single-arm phase 2 study reported a CR rate of 91% with disease-free survival at 3 years of 63% (Table 5). Reported toxicities were acceptable, with all neurologic toxicities being fully reversible (22% grades 2-4).
Novel agents in T-cell ALL
γ-Secretase is required for NOTCH1 signaling through mutant receptors in T-cell ALL and may be an attractive target for therapeutic intervention for the 60% of patients with T- cell ALL with a mutation in the NOTCH-1 signaling pathway.87,88 γ-Secretase inhibitor (GSI) drugs are in early development. A phase 1 study with the GSI BMS-906024 showed activity in 8 of 25 relapsed patients.89 Other studies are now ongoing.90
The recently reported NUP214-ABL1 fusion gene, reported in 6% of adult T-cell ALL, results in a constitutively activated tyrosine kinase and confers an inferior prognosis.91 Tyrosine kinase inhibitors, including imatinib, have been reported to inhibit the resultant mutant tyrosine kinase in vitro, and offer an interesting and novel therapeutic option in the minority of T-cell ALL patients with this genetic abnormality.92
Ruxolitinib shows promise in in vitro studies in T-cell ALL blasts and may have activity in the 30% of T-cell ALL patients with increased JAK-STAT signaling.18 Approximately 40% of T-cell ALL expresses CD30, making brentuximab an interesting therapeutic option in these cases for relapsed, refractory disease.93
CD19 chimeric antigen receptor T-cell (CAR-T) therapy is achieving remarkable results in children and adults with relapsed/refractory pre-B ALL, but this therapeutic modality is not yet a reality for patients with T-cell ALL. There is shared expression of most targetable surface antigens between normal and malignant T cells leading to death of the CAR-T cells (fratricide mechanism) or profound immunodeficiency. However, Mamonkin et al reported targeting CD5 (which is expressed by normal and malignant T cells), and demonstrated activity against T-cell ALL in vivo and in murine xenograft models with only a limited degree of fratricide.94
Conclusion
About half of adults with T-cell ALL who are intensively treated are currently cured. Further improvements are anticipated with intensification of protocols in younger adults, frontline use of newer agents such as nelarabine, judicious use of HCT, and possibly the use of novel agents and CAR-T cells in poor responders. As always, a better understanding of disease biology will enable the use of specific targeted therapy.
Acknowledgments
The authors thank Adele Fielding for critically reviewing the manuscript and making a number of important suggestions; Anthony Moorman for several helpful comments on the section on cytogenetics and genetics; and Jenny Bird for manuscript accuracy and consistency.
Authorship
Contribution: D.I.M. and C.R. wrote, edited, and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David I. Marks, University Hospitals Bristol National Health Service Foundation Trust, Adult BMT Unit, Bristol BS2 8ED, United Kingdom; e-mail: david.marks@uhbristol.nhs.uk.