Abstract
Angioimmunoblastic T-cell lymphoma (AITL) is an uncommon subtype of mature peripheral T-cell lymphoma (PTCL). The history of AITL is much longer and deeper than the literature would suggest given the many names that have preceded it. Advanced-stage disease is common with uncharacteristic laboratory and autoimmune findings that often slow or mask the diagnosis. Significant strides in the immunohistochemical and molecular signature of AITL have brought increased ability to diagnose this uncommon type of PTCL. The 2016 World Health Organization classification of lymphoid neoplasms recently acknowledged the complexity of this diagnosis with the addition of other AITL-like subsets. AITL now resides under the umbrella of nodal T-cell lymphomas with follicular T helper phenotype. Induction strategies continue to focus on increasing complete remission rates that allow more transplant-eligible patients to proceed toward consolidative high-dose therapy and autologous stem cell rescue with improving long-term survival. There are several clinical trials in which recently approved drugs with known activity in AITL are paired with induction regimens with the hope of demonstrating long-term progression-free survival over cyclophosphamide, doxorubicin, vincristine, and prednisone. The treatment of relapsed or refractory AITL remains an unmet need. The spectrum of AITL from diagnosis to treatment is reviewed subsequently in a fashion that may one day lead to personalized treatment approaches in a many-faced disease.
Introduction: “many names but all serve”
Angioimmunoblastic T-cell lymphoma (AITL) is now a well-established subtype of mature peripheral T-cell lymphoma (PTCL).1 Many names precede the currently recognized designation by the 2008 World Health Organization classification of lymphoid neoplasms and has been expanded in the 2016 revision.2 Chronologically acknowledged within the literature are angioimmunoblastic lymphadenopathy with dysproteinemia, immunoblastic lymphadenopathy, and lymphogranulomatosis X. All are predecessor synonyms to AITL and often forgotten in systemic reviews.3-5
There are many faces to AITL including the historic descriptive nomenclature referencing AITL as benign immune activation of the B cell despite the fatal natural history. Advances that allowed assessment in T- and B-cell clonality in the 1980s uncovered the malignant T-cell nature thus referred to as AITL.6-9 Herein, we briefly review the characteristics of AITL and discuss the evolving treatment landscape.
Epidemiology
Spanning more than 4 decades of analysis, the incidence of AITL appears to have changed little since the 1970s. AITL represents only 1% to 2% of all cases non-Hodgkin lymphoma (NHL), but nearly 1 in 5 cases of PTCL diagnosed per annum.3,10 AITL afflicts advanced-age individuals with a median age of diagnosis of 65 years of age without a notable gender predisposition.11,12 AITL carries an inverse geographic tropism than other PTCL subtypes as it is more common in Europe (28.7%) than in Asia (17.9%).10 Although this may be related to the increase in natural killer/T-cell lymphoma seen in Asia rather than a true difference. Similarly, to natural killer/T-cell lymphoma, AITL has been showed to have an intricate relationship to Epstein-Barr virus (EBV). Paradoxically, in AITL the B cells demonstrate active viral infection, whereas the malignant T cells are spared. Furthermore, there appears to be no specific ethnic predisposition or geographic pockets spread by trade good routes, historic slave routes, and the Bering Strait land bridge as proposed in human T-cell lymphotropic virus type 1–associated acute T-cell leukemia/lymphoma).13
Clinical and pathologic findings: “the house AITL”
AITL may have the highest ratio of case reports to incidence of diagnosis when compared with all NHL subtypes given the multitude of peculiar presentations. The majority of diagnoses are rendered after weeks to months of patients reporting nonlocalizing symptoms, transient physical exam findings, and a broad range of serologic or radiographic findings.14
Ironically, B-symptoms (fevers, unintentional weight loss, and/or drenching night sweats) and lymphadenopathy remain the most common complaints and physical findings traversing again the 40-year span of this process.3,10 The lymphadenopathy seen on computer tomography (CT) is often meager with nodal disease with low bulk (1.5 to 3 cm) and variable standard uptake values on positron emission tomography.15-17 Nearly 70% of patients will have bone marrow involvement, and early stage AITL is very uncommon (10%).18,19 Hepatosplenomegaly is also seen in a modest proportion at diagnosis. The antecedent findings of rash can be seen in 20% to 50% of AITL patients, but the skin manifestation can range from urticarial lesions to nodular tumors.10,19 Many rashes appear following exposure to antimicrobial administration, and rarely is overt cutaneous AITL seen.20,21 Involvement of other extranodal sites appears to be less common, but disease-based variables appear stable over time (Table 1).22
Clinical and laboratory features of AITL
| Publications reviewed . | 1975 to 1999* . | 2007 to 2016* . |
|---|---|---|
| ATIL cases reviewed | 77 | 556 |
| Males, % | 42-48 | 56-74 |
| Median age range | 62-68 | 62-65 |
| General clinical features, % | ||
| B-symptoms | 29-85 | 55-77 |
| Performance status >1 | 57 | 37-50 |
| LDH elevation | 25-74 | 60-86 |
| Advanced stage (III/IV) | 94 | 81-92 |
| Low-risk IPI (0-1) | No reported | 11-21 |
| Areas of involvement, % | ||
| Multiple nodal stations | 97-100 | 76-99 |
| Bone marrow | 61 | 28-70 |
| Skin rash | 38-48 | 31-45 |
| Laboratory tests, % | ||
| Anemia | 40-83 | 33-65 |
| Positive DAT (Coomb’s) | 43-57 | 13-75 |
| Thrombocytopenia | 9 | 20-31 |
| Hypergammaglobulinemia | 50-77 | 50-84 |
| Hypereosinophilia | 29-39 | 32-34 |
| Publications reviewed . | 1975 to 1999* . | 2007 to 2016* . |
|---|---|---|
| ATIL cases reviewed | 77 | 556 |
| Males, % | 42-48 | 56-74 |
| Median age range | 62-68 | 62-65 |
| General clinical features, % | ||
| B-symptoms | 29-85 | 55-77 |
| Performance status >1 | 57 | 37-50 |
| LDH elevation | 25-74 | 60-86 |
| Advanced stage (III/IV) | 94 | 81-92 |
| Low-risk IPI (0-1) | No reported | 11-21 |
| Areas of involvement, % | ||
| Multiple nodal stations | 97-100 | 76-99 |
| Bone marrow | 61 | 28-70 |
| Skin rash | 38-48 | 31-45 |
| Laboratory tests, % | ||
| Anemia | 40-83 | 33-65 |
| Positive DAT (Coomb’s) | 43-57 | 13-75 |
| Thrombocytopenia | 9 | 20-31 |
| Hypergammaglobulinemia | 50-77 | 50-84 |
| Hypereosinophilia | 29-39 | 32-34 |
DAT, direct anti-globulin test; IPI, International Prognostic Index; LDH, lactate dehydrogenase.
Article referenced in reference section; not all parameters were recorded for all patients.
AITL can masquerade as an immune activator with an elevated sedimentation rate and lead to positive autoimmune tests including rheumatoid factor, anti–smooth muscle, and also by manifesting with circulating immune complexes or cold agglutinins.23 Concurrent dysproteinemia on serum protein electrophoresis is often a polyclonal gammopathy. A monoclonal gammopathy is less common but has been reported in 10% of cases with the possibility of clonal plasmacytosis.12,24 Warm autoimmune (DAT) hemolytic anemias can be seen as an initial presentation but are less common. Lastly, eosinophilia with or without infection etiology has been described.12 Circulating disease seen on peripheral blood smear is uncommon but can be seen on peripheral blood flow cytometry.25,26
Cell of origin
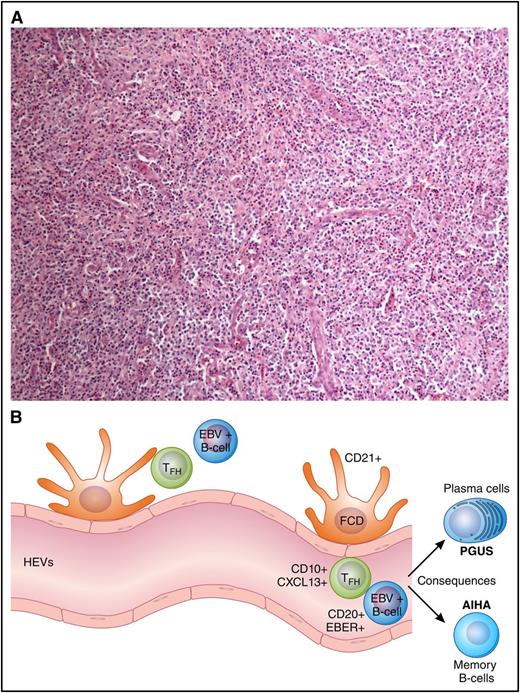
The cell of origin for AITL is the follicular T helper (TFH) cell, an effector T-cell subset. This was concretely proven by molecular analysis noting the genetic similarity of TFH to AITL cells.27 In normal immunology, the presence of TFH cells is a crucial checkpoint for B-cell activation and differentiation within a germinal center.28 Notably, after antigen stimulation, germinal centers arise and create a crescendo of B-cell activity. TFH cells at outer zones of secondary lymphoid tissue help chaperone centroblasts’ evolution to the more mature centrocyte. This interaction elicits a strong signal for differentiation to either plasma cells or memory B cells. Although immunologic tolerance within the TFH is important for prevention of autoimmune disorders, complete dysregulation the TFH cell can possibly lead to germinal center anarchy and subsequent development of AITL (Figure 1).
Architecture, immunophenotype, and consequences of AITL. (A) Lymph node effacement devoid of follicles with characteristic CXCL13+ T cells. (B) An illustration of the cellular components of the microenvironment and immunophenotype in AITL including HEVs, EBV+ B cells, and TFH. The B-cell activation leads to consequences commonly of AIHA and hypergammaglobulinemia (PGUS).
Architecture, immunophenotype, and consequences of AITL. (A) Lymph node effacement devoid of follicles with characteristic CXCL13+ T cells. (B) An illustration of the cellular components of the microenvironment and immunophenotype in AITL including HEVs, EBV+ B cells, and TFH. The B-cell activation leads to consequences commonly of AIHA and hypergammaglobulinemia (PGUS).
Morphology and immunophenotype
Similar to the Reed-Sternberg cells of Hodgkin lymphoma, the malignant TFH cells in AITL represent a minority of the cellular components of a malignant lymph node. The architecture of the lymph node is commonly effaced and devoid of follicles.29 Higher power demonstrates a full house of the immune repertoire including ample immunoblasts, B cells, plasma cells, eosinophils, histiocytes, and epithelioid cells. Notable other features include an irregular proliferation of follicular dendritic cells (FDCs) and proliferative high endothelial venules (HEVs). The malignant TFH cells appear to reside in close proximity to HEVs.30 The majority of large B cells can be shown by in situ hybridization to have active EBV infection, whereas while the malignant TFH cells will not.31
The rudimentary immunophenotype of a TFH is CD3, CD4, and CD10 positive.29,31,32 The T-cell receptor is α-β with often aberrant loss of CD5 and/or CD7.33,34 CD30 expression is seen in ∼20% of cases and only recently has become therapeutically relevant.35 The near uniform expression of cytoplasmic CXCL13 has improved the diagnostic confidence.36 CXCL13 appears to be a more specific compared with CD10 given the variable expression reported.37 Furthermore, TFH expression of program death receptor 1 (PD-1), ICOS, BCL-6, and CD200 continue to support an expression pattern that distinguishes AITL from a spectrum of benign lymphoproliferative disorders to other PTCL subtypes that have a TFH cell of origin.32,38-40 To help associate the TFH with HEVs and FDCs, other stains may be performed that highlight the FDCs, specifically CD21.41
Cytogenetic and molecular testing
Karyotypic abnormalities in AITL are seen in 9 out of 10 cases, but whether they are specific to the clonal T cell remains controversial.22,42 The most common abnormalities are trisomy of chromosome 3 and 5.43 Loss of TP53 is an uncommon event, but clonal complexity is a poor prognostic marker.44 To date, no characteristic abnormalities have been found that have led to a therapeutic option.
An expanding literature exists for the clonal assessment of AITL with CD4+ T-cell clonality being proved in >80% of cases in 8 out of 11 reports.22 A clonal B-cell population has been found in as high as 41% of cases with concurrent clonal AITL.45 Molecular profiling of AITL has become more robust in recent years, and gene expression patterns can now discern AITL from other PTCL subtypes.46 The molecular signature of AITL has a substantial contribution from FDCs, B cells, and other stromal components.47 This microenvironment signature may be prognostic in AITL. Gene expression profiling continues to identify many aberrations including TET2 (47% to 73%), DNMT3A (33%), and IDH2-R172 (20% to 40%) unlocking interesting differences when compared with B-cell lymphomas and some similarities to myeloid malignancies.48-50 However, these mutation are unlikely sufficient to drive lymphomagenesis. Recently, gain-of-function mutations in the T-cell receptor in AITL patients have been described.51-53 RHOA, a guanosine triphosphatase involved in cytoskeleton reorganization, was found to be mutated (G17V) in ∼60% of AITL cases analyzed. RHOA mutation is commonly seen in TET2 mutations proposing a multihit process toward genesis of AITL and may occur at several stages of T-cell development.53-55
Prognosis: “AITL has been called many things, but kind is seldom one of them”
The natural history of AITL is likely the most variable among the PTCL subtypes. Despite the variability, the overall prognosis for AITL remains poor with a 5-year median survival of 32%.11 The IPI, a prognostic index namely for aggressive B-cell NHL, has demonstrated a 5-year overall survival (OS) advantage of 56% for IPI 0/1 vs 25% for IPI 4/5. An attempt to better define a prognostic index has been attempted specifically for AITL patients. The prognosis in AITL included age >60, Eastern Cooperative Oncology Group (ECOG) performance status >2, extranodal sites of disease >1, presence of B symptoms, and a platelet count <150 × 109/L as defined entities.10 Additively, the variables differentiated a low-risk group (0-1 risk factors) with a 5-year OS rate of 44% and high-risk group (2-5 risk factors) associated with 5-year OS 24%. The clinical application of any prognostic index remains an area of clinical interest with risk-adapted treatment approaches a goal worth striving toward.
Induction: game of regimens
The uniqueness of AITL does not just lie in its biology but also continues into its treatment. Commonly responses to induction therapy whether single-agent oral therapies or intensive combination chemotherapies are often dramatic but fraught with primary progression or short duration of remission.56 There remains no gold standard chemotherapy for the treatment of newly diagnosed AITL. Risk-adapted strategies have been proposed based on the prognosis in AITL and are yet to be tested in clinical trials but appear to be a reasonable starting point.57 Up-front studies with strategies solely for the inclusion and treatment of AITL are limited because of the incidence of AITL. Often the best data are descriptive reports of outcomes within a group and limited subgroup comparison against other PTCL subtypes. This leads to many limitations in data interpretation. Nevertheless, in many ways a measuring stick for research in PTCL subtypes continues to be whether CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) alone remains the gold standard or a new one will emerge.
CHOP chemotherapy disappointingly continues to be the customary up-front treatment despite retrospective evidence of possible lack of efficacy for anthracycline-based regimens.11 To acknowledge the baseline of CHOP in PTCL, CHOP carries a complete response (CR) rate of 39% in the up-front setting and specifically in AITL a 53% CR rate.58 The impact of more intensive regimens has been investigated in combination with other aggressive NHLs. The addition of etoposide to CHOP (CHOEP) in PTCL continues to demonstrate promise as an induction regimen with high overall response rates (ORRs) at 82% and a CR rate of 51%.59 The ORR/CR for the AITL cohort of 30 patients was not reported. With noted limitations for cross-study comparisons, it appears CHOEP may provide the ability to allow for deeper remissions prior to high-dose therapy and autologous stem cell rescue (HDT-ASCR) given that the most common reason for not proceeding to HDT-ASCR after CHOP was disease progression rather than toxicity.60
The approval of 3 single agents in relapsed/refractory (rel/ref) PTCL has led to further studies in combination with CHOP. The regimen of romidepsin plus CHOP at varying doses of romidepsin has been reported in a phase 1/2 study in PTCL.61 In the 35 patients evaluable for response, the CR rate was 51% with a median progression-free survival (PFS) of 21 months. The phase 1 study of belinostat-CHOP in untreated PTCL patients reported a maximum tolerated dose of 1000 mg days 1 to 5 in combination with CHOP.62 The CR rate in the 21 patients evaluable was 67%. Lastly, despite pralatrexate being approved first in the rel/ref setting, the regimen of pralatrexate plus CHOP (Fol-CHOP; #NCT02594267) in untreated patients continues to accrue in a phase 1 trial.
The regimen of ACVBP (doxorubicin, cyclophosphamide, vindesine, bleomycin, and prednisone) not unlike what was seen in diffuse large B-cell lymphoma was superior to CHOP in a retrospective study that included PTCL.63 This regimen is unlikely to make an impact in North America because of the lack of availability of vindesine, and it should be noted that the regimen carries a fairly intensive consolidative package after the induction phase. Bortezomib, a proteasome inhibitor, has been combined with both CHOP and ACVBP in phase 2 clinical trials in PTCL. In the phase 2 study with bortezomib-CHOP that enrolled 46 patients with all PTCL subtypes, the ORR was 76% with CR 65%.64 AITL represented 17% of the population with an improved 3-year overall survival (OS) advantage when compared with other non-AITL populations. Surprisingly, the bortezomib-ACVBP study did not note higher ORR and CR rate to historical ACVBP.65
The use of dose-adjusted etoposide, prednisone, oncovin, cyclophosphamide, doxorubicin has been proposed in the National Comprehensive Cancer Networks guidelines based on institutional preference rather than significant peer-reviewed data.66
There have been studies on alternatives to up-front CHOP tested in clinical trials using the hypothesis that a nonanthracycline regimen may indeed be better in PTCL. An example would be the PEGS (cisplatinum, etoposide, gemcitabine, methylprednisolone) regimen, which carried a disappointing ORR of 39% in the 33 patients eligible for response. The ORR was similar at 33% in the AITL patients treated. The median PFS was 7 months.67 The most recent trial by the T-cell consortium also attempted to exploit this hypothesis by using an alternating regimen of CEOP (cyclophosphamide, etoposide, vincristine, prednisone) and pralatrexate.68 In total, 33 patients (8 AITL) were treated with a CR rate of 52% and a 2-year PFS of 39%. The CR rate was 25% for the AITL patients. This regimen is not being further explored; however, as discussed previously, the pralatrexate-CHOP study continues to accrue.
Understandably, given the unique microenvironment in AITL, some regimens have tried to attack the vascular irregularities and B-cell proliferation that can be seen in the diagnostic tissues likely as a result of vascular endothelial growth factor and EBV reactivation, respectively. Bevacizumab, a vascular endothelial growth factor antagonist, has been combined with CHOP with bevacizumab maintenance in a phase 2 study.69 Thirty-nine patients were evaluable with 9 completing the entire planned regimen. The CR rate was 49% with a 1-year PFS of 44%. This regimen was found to have a significant cardiac toxicity and was not further explored.70 Rituximab, an anti-CD20 monoclonal antibody, was employed in combination with CHOP in a phase 2 study.71 Twenty-five patients underwent rituximab-CHOP therapy for 6 cycles. The ORR was 80% with 44% CR or CR unconfirmed. The 2-year OS rate was 62%. It is unknown how many patients proceeded to consolidative therapy after completion of therapy.
The second monoclonal antibody alemtuzumab, a monoclonal antibody against CD52, has also been combined with CHOP (A-CHOP). Alemtuzumab, unlike rituximab, has the unique characteristic of being able to target both T and B cells that express CD52. However, it is well known that the efficacy must be balanced with opportunistic viral infection like cytomegalovirus (CMV) or EBV-related lymphoma, which has limited its used in many other NHLs. Three phase 2 studies in newly diagnosed PTCL including AITL have been conducted. The first study was done in Europe with 25 patients that included 6 AITL patients.72 All 6 patients experienced a response. Infectious nonhematologic complications were the most common complications. In the second trial with A-CHOP 16 out of 20 patients experienced a response with a CR rate of 65%.73 Two of the 3 patients (66%) with AITL responded. The durability/tolerability remains in question as the 1-year OS was only 44%, again hampered by infectious complication leading to study closure, a common issue in PTCL therapy. Lastly, a phase 2 study that allowed up to 8 cycles of A-CHOP enrolled 20 patients.74 The ORR was 90% with 13% CR/CR unconfirmed. The 2-year event-free survival was low at 27%. CMV prophylaxis was given in the regimen, but 2 patients experienced EBV-associated lymphoma. A-CHOP appears to have potential in raising the CR comparatively to CHOP but may be best used in patients that are qualitatively (immunoglobulin M/immunoglobulin G) and quantitatively (polymerase chain reaction) negative for CMV at diagnosis and be considered for HDT-ASCR to improve on durability.
Several phase 3 randomized clinical trials are planned or continued to accrue in PTCL with the inclusion of AITL. The romidepsin-CHOP (#NCT01796002), belinostat-CHOP (planned), and brentuximab vedotin-CHOP (#NCT01777152) are all planned, quickly accruing or already accrued with presentation of data forthcoming. To our knowledge, none of the trials allow for consideration of HDT-ASCR for consolidation. This will allow for the comparative primary end points that may lead to approval of each drug. The combination of histone deacetylase (HDAC) inhibitors plus CHOP will be exciting for patients participation with AITL given the notable improved ORR with AITL compared with other PTCL subtypes in their respective phase 2 registration trials.75 However, to our knowledge none of the randomized trials were powered to assess for specific outcomes in the AITL cohorts enrolled.
As demonstrated previously, many have tried to pair approved agents for relapsed or refractory PTCL with CHOP or other chemotherapies, but other groups have tried to build on the CHOEP backbone. Lenalidomide, a well-established immunomodulatory agent in B-cell NHL and multiple myeloma, also has single-agent activity in PTCL in small phase 2 studies.76 A currently accruing multi-institutional phase 1/2 study (#NCT02561273) led by the T-cell Consortium includes lenalidomide with CHOEP in newly diagnosed PTCL including AITL. Unique to this trial design is the ability to allow for up-front consolidative autologous transplant or proceeding with lenalidomide maintenance. Lenalidomide is given for 10 days per cycle. An accruing European study specifically for newly diagnosed AITL also has employed lenalidomide for 14 days per cycle but with CHOP (#NCT01553786).
Consolidation: “wildfire”
Philosophy remains entrenched in the management of AITL. Treatment discussions at the time of diagnosis seem to be related to whether a patient is considered to be “transplant eligible” or “transplant ineligible” but acknowledge that in many situations “transplant maybe” may be the most common given the significant disease-related subjective symptoms and objective complications that are present at the time of diagnosis.77 Furthermore, acknowledging the difficulty in assessing an “intent-to-transplant” population in PTCL limits the complete utility of HDT-ASCR. Nevertheless, HDT-ASCR has become an increasingly used modality in those who are chemosensitive by either CT or positron emission tomography/CT depending on radiographic availability. A large retrospective analysis from the European Group for Blood and Marrow Transplantation reported an improved response rate of 76% and a 5-year OS of 44% in PTCL.78 HDT-ASCR has been analyzed solely in AITL with 56% of individuals remaining without evidence of relapse at 4 years.79 Patients that are chemosensitive continue to have a survival advantage compared with those that were not in at least a partial remission at the time of transplant. Recent prospective outcomes of HDT-ASCR after CHOEP-based induction regimens have been reported by intent to treat/transplant; 72% proceeded to HDT-ASCR overall.59 Those with AITL had a 5-year PFS of 49% and OS 52%. These results in AITL patients treated with what could be considered “maximal chemotherapy exposure” with intensive CHOEP induction and consolidative HDT-ASCDR still signal an ongoing opportunity for possible posttransplant maintenance approaches given the relatively low 5-year PFS and OS. An example that is trying to improve on these values would be a currently accruing lenalidomide post–HDT-ASCR maintenance trial (#NCT01035463).
Rel/ref AITL: dragon’s breath or Valerian steal
Rel/ref AITL remains a common and difficult situation. The optimal approach to management is unclear, and in rel/ref PTCL patients who have been excluded from HDT-ASCR after induction, the median OS was 5.5 months.80 Divergent treatment paradigms have evolved: short-course combinations vs single-agent continuous therapy. Short-course combinations include historical treatments borrowed from relapsed aggressive B-cell lymphomas including classic inpatient regimens like ICE (ifosphamide, carboplatin, etoposide), DHAP (dexamethasone, cytarabine, cisplatinum), and ESHAP (etoposide, methylprednisolone, cisplatinum, cytarabine), as well as outpatient regimens like Gem-P (gemcitabine, cisplatinum, methylprednisolone), GDP (gemcitabine, cisplatinum, dexamethasone), and bendamustine.81-87 These regimens have been reported in PTCL, but outcomes in AITL specifically have infrequently been identified or extensively analyzed. Combination chemotherapy regimens given inpatient have been shown to have higher response rates, for instance an ORR of 70% with ifosphamide, carboplatin, etoposide, but are limited because of cumulative hematologic toxicities relegating the use to 3 to 4 cycles and short PFS.88 Similarly, an outpatient experience with bendamustine was reported in 60 patients with rel/ref PTCL with a high ORR of 50%, but most received <4 cycles.87 In those that responded, the median duration of response was short at 3.5 months, and the median OS was 6.2 months. Nevertheless, in a patient who deferred transplant after induction and remained “transplant eligible,” these combination approaches may provide a window of response to allow for HDT-ASCR in second remission. This approach has been retrospectively studied in rel/ref PTCL, which included AITL and appears to be a reasonable clinical option in chemosensitive patients.89
Continuous therapy paradigms continue to gather steam in PTCL and more specifically AITL where single-agent continuous therapy approaches have been attempted dating back to steroid-only approaches in the 1970s. Continuous therapy approaches allowed treatment until progression or intolerance with a premium on maintenance of quality of life. The ORR may be lower, but without cumulative toxicities, the potential benefits of this approach are noted in the median durations of response, and if progression is seen, maintenance of the performance status will likely afford another line of therapy.
The continuous therapy approach has resulted in the US Food and Drug Administration (FDA) approval of 3 single agents for the treatment of rel/ref AITL (Table 2). Pralatrexate was the first drug FDA approved for use in rel/ref PTCL.90 The phase 2 study pralatrexate in relapsed or refractory PTCL study included 111 patients with rel/ref PTCL; however, only 13 patients were included. The ORR was 29% with an 11% CR rate. An 8% ORR was seen in AITL patients. The second drug FDA approved for rel/ref PTCL was romidepsin.75,91 In 2 phase 2 studies, romidepsin had an ORR of 38% in patients with either cutaneous or peripheral T-cell lymphomas and 25% in those with PTCL alone. Romidepsin had a 30% ORR in AITL in the second trial. The median duration of response in these patients was 17 months. The latest drug to be approved in rel/ref PTCL was a second HDAC inhibitor belinostat.92 The phase 2 study included 120 evaluable patients with 22 patients with AITL. The ORR was 25.8%, with 10.8% achieving a CR in the entire cohort, whereas AITL had an ORR of 45%. CD30 expression is seen to a lesser extent in AITL when compared with anaplastic large cell lymphoma. Brentuximab vedotin has been explored in a study that allowed AITL. In that series, in the AITL accrued the ORR was 54% with 5 of the 7 responding patients obtaining a CR despite significantly less significant CD30 expression by immunohistochemistry.93 Cyclosporine, a known immunosuppressant commonly used in solid organ transplantation, was tested in 12 AITL patients with an ORR of 75%, which provided support for further exploration in a phase 2 study that was discontinued because of low accrual.94 Lenalidomide, another immunomodulation agent, has been explored in rel/ref PTCL in a phase 2 study. In the interim report, 24 patients were enrolled with 7 AITL patents. The ORR was 29% with 2 patients experiencing a partial remission.76
Overall response rates reported for FDA-approved agents for AITL vs PTCL-NOS
| Subtype . | Pralatrexate . | Romidepsin . | Belinostat . |
|---|---|---|---|
| PTCL-NOS, % | 32 | 29 | 23 |
| AITL, % | 8 | 30 | 46 |
| Subtype . | Pralatrexate . | Romidepsin . | Belinostat . |
|---|---|---|---|
| PTCL-NOS, % | 32 | 29 | 23 |
| AITL, % | 8 | 30 | 46 |
PTCL-NOS, peripheral T-cell lymphoma, not otherwise specified.
Both romidepsin and belinostat have FDA-approved labels inclusive for rel/ref AITL and have now moved into the frontline setting as described previously. Unfortunately, both drugs are unlikely to be able to differentiate themselves as a standard up-front regimen in AITL in combination with CHOP because of the inability to enrich for AITL with the pressures applied in registration trials in uncommon diseases. Pralatrexate, as noted previously, had an ORR of 8% in patients with AITL. Acknowledging limited AITL cases in the pivotal trial, pralatrexate is likely better placed after progression from an HDAC inhibitor or on a combination clinical trial. Unexpected signals have been seen in clinical trials in AITL as described with a high ORR seen with belinostat and brentuximab vedotin. Although belinostat can capture some of the AITL market share, the role of brentuximab vedotin, lenalidomide, and cyclosporine may not ever be further explored unless diagnostic biomarkers can help us predict who will not respond to certain classes of known novel agents and furthermore expand the combination potential as new signals demonstrate the possibility of use of JAK-2 inhibitors, hypomethylating agents, and lastly IDH2 inhibitors all now entering into clinical trials.
Conclusion: end is certain … time is not
There is no doubt that AITL has many faces despite a common lymphoma presentation. The rapid advances in immunophenotypic and molecular diagnostics will only help secure the confidence of the diagnosis likely leading to earlier treatment in less symptomatic patients. This coupled with rapid introduction of novel agents to standard chemotherapy backbones may reduce primary refractory disease with a resultant rise in the tail of the OS curve of AITL. In the end, the overwhelming concern remains that with the veil over AITL the nonlymphoma physician specialist must know how to look before one can hope to see.
Authorship
Contribution: J.M.V. and M.A.L. wrote and edited the manuscript.
Conflict-of-interest disclosure: J.M.V. has institutional research grants with Acerta Pharma, Allos Therapeutics/Spectrum, Bristol-Myers Squibb Company, Celgene Corporation, Incyte Corporation, Janssen Biotech, Kite Pharma, Merck Sharp & Dohme Corporation, Onyx Seattle Genetics, Inc., and US Biotest. M.A.L. has institutional research grants with Amgen, Inc., Bristol-Myers Squibb Company, Celgene Corporation, Constellation Pharmaceuticals, Janssen Scientific Affairs, LLC, JUNO Therapuetics, Janssen R&D LLC, Memorial Sloan Kettering Cancer Center, Merck Sharp & Dohme Corporation, Pharmacyclics, and TG Therapeutics, Inc. M.A.L. received honorarium from Epizyme.
Correspondence: Julie M. Vose, Chief, Division of Hematology/Oncology, University of Nebraska Medical Center, 987680 Nebraska Medical Center, Omaha, NE 68198-7680; e-mail: jmvose@unmc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal