Abstract
T-cell acute lymphoblastic leukemia (T-ALL) is a highly proliferative hematologic malignancy that results from the transformation of immature T-cell progenitors. Aberrant cell growth and proliferation in T-ALL lymphoblasts are sustained by activation of strong oncogenic drivers promoting cell anabolism and cell cycle progression. Oncogenic NOTCH signaling, which is activated in more than 65% of T-ALL patients by activating mutations in the NOTCH1 gene, has emerged as a major regulator of leukemia cell growth and metabolism. T-ALL NOTCH1 mutations result in ligand-independent and sustained NOTCH1-receptor signaling, which translates into activation of a broad transcriptional program dominated by upregulation of genes involved in anabolic pathways. Among these, the MYC oncogene plays a major role in NOTCH1-induced transformation. As result, the oncogenic activity of NOTCH1 in T-ALL is strictly dependent on MYC upregulation, which makes the NOTCH1-MYC regulatory circuit an attractive therapeutic target for the treatment of T-ALL.
Introduction
T-cell acute lymphoblastic leukemia (T-ALL) is an early lymphoid neoplasia characterized by diffuse infiltration of the bone marrow by malignant lymphoblasts expressing immature T-cell surface markers.1 This aggressive hematologic malignancy typically shows a very active proliferative rate and high tumor burden, with high white blood cell counts and frequent large thymic masses and pleural effusions. This accelerated tumor growth may reflect in part the capacity of immature thymocytes to divide at high rates at critical steps during early T-cell development; that growth is fueled by the activation of oncogenic pathways closely linked to the mechanisms that drive cell growth and proliferation in early T-cell progenitors.
Early T-cell precursors enter the thymus at the cortical-medullary junction2 where they are exposed to the instructive signals of the thymic microenvironment that induce cell growth and proliferation primarily via the stimulus of interleukin-73,4 and stem cell factor.5 These uncommitted lymphoid precursors express the NOTCH1 receptor6 and are exposed to high levels of the NOTCH ligand Δ-like 4 expressed in the surface of thymic epithelial cells while they circulate along the thymic cortex.7 Activation of NOTCH1 signaling has an instructive role promoting cell lineage commitment toward a T-cell fate.8 In the absence of NOTCH1, early lymphoid progenitors fail to read the developmental cues of the thymic microenvironment and become B cells instead.6,9
Early uncommitted thymocytes, named double-negative (DN) cells because they lack surface expression of the CD4 and CD8 antigens, can be subdivided into four stages of differentiation based on the expression of CD44 and CD25 (DN1, CD44+CD25–; DN2, CD44+CD25+; DN3, CD44–CD25+; and DN4, CD44–CD25–) (recently reviewed in Shah and Zúñiga-Pflücker10 ). As cells progress into the DN3 stage of development, they complete rearrangement of the TCRB locus and express on their surface a constitutively active receptor named pre-TCR (composed of an invariable pre-T α chain) and the rearranged TCRB.11 Pre-TCR signaling coincides with the highest levels of NOTCH signaling and induces marked cell proliferation as thymocytes progress from the DN3 stage of development to become DN4 cells first and then intermediate single-positive (ISP) cells, a transition population that expresses CD4 in humans or CD8 in mice in the absence of surface CD3.11 Marked proliferation at the pre-TCR signaling phase is required to expand the pool of cells with a successful TCRB rearrangement entering the CD4+CD8+ double-positive (DP) stage of differentiation. This represents a key feature of T-cell development required for effective production of immune-competent T cells because subsequent stages of thymocyte development will ablate more than 95% of these precursors to ensure that they are competent for immune recognition but do not recognize self-antigens.12
NOTCH1 activation in these rapidly dividing already T-cell–committed progenitors is critical for supporting growth and cellular metabolism in close interaction with the PI3K signaling pathway.13 When thymocyte precursors differentiate into DP cells, they cease to proliferate and rearrange their TCRA loci. Surface expression of a complete TCR composed of TCRB and TCRA chains determines the capacity of T cells to interact with the major histocompatibility complex (MHC) and recognize antigens. Intrathymic T-cell development is completed with checkpoints for the capacity of DP T cells to recognize the MHC (positive selection) and then for the resulting single-positive CD4 and CD8 cells to interact with MHCs loaded with self-peptides without eliciting a strong autoimmune-prone response (negative selection). In all, only a small fraction of thymic precursors complete positive selection and survive negative selection to leave the thymus and populate the peripheral lymphoid organs. In this context, T-ALL originates as a result of the multistep accumulation of genetic alterations in oncogenes and tumor suppressors, which coordinately disrupt key developmental pathways responsible for the normal control of cell growth, proliferation, survival, and differentiation during thymocyte development.1,14
Oncogenic NOTCH1 activation in T-ALL
NOTCH1 is a class I transmembrane protein that directly transduces extracellular signals into gene expression changes that function as ligand-activated transcription factors (Figures 1 and 2).15,16 NOTCH1 signaling is initiated by interaction of the receptor with Δ-like and Jagged ligands expressed on the surface of neighboring cells. This ligand-receptor interaction triggers the cleavage of the extracellular domains of the receptor by the ADAM10 metalloprotease, which facilitates the subsequent proteolytic cleavage by the γ-secretase complex17-23 in the transmembrane region of the receptor. Upon release from the membrane, the cytoplasmic intracellular portion of NOTCH1 (ICN1) translocates into the nucleus. There, nuclear ICN1 associates with the RBPJ DNA-binding protein and activates gene expression of target genes via recruitment of MAML transcriptional co-activators.19,20,24,25 Of note, this multistep activation process is amenable to pharmacologic inhibition at multiple levels (Figure 2). Thus, NOTCH1 inhibitory antibodies can block ADAM10 processing26-28 and small molecule γ-secretase inhibitors (GSIs), which suppress the release of ICN1 from the membrane,29 can effectively abrogate the activation of NOTCH1 transcriptional programs.30
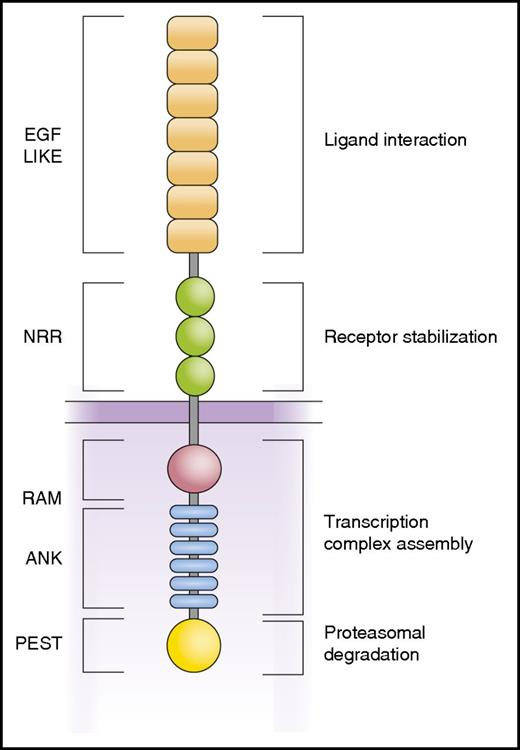
Schematic representation of the NOTCH1 receptor. NOTCH1 is a class I transmembrane protein with a modular structure. The N-terminal EGF repeats are involved in interaction with Jagged and Δ-like ligands. The NRR holds the receptor inactive in the absence of ligand by limiting the access of the ADAM10 protease. Intracellular domains include the RAM (RBP-Jκ–associated module) and ANK (ankyrin repeat) domains involved in the interaction with the RBPJ DNA binding protein and the recruitment of transcriptional coactivators and the C-terminal PEST domain responsible for termination of NOTCH1 signaling by FBXW7-mediated proteasomal degradation.
Schematic representation of the NOTCH1 receptor. NOTCH1 is a class I transmembrane protein with a modular structure. The N-terminal EGF repeats are involved in interaction with Jagged and Δ-like ligands. The NRR holds the receptor inactive in the absence of ligand by limiting the access of the ADAM10 protease. Intracellular domains include the RAM (RBP-Jκ–associated module) and ANK (ankyrin repeat) domains involved in the interaction with the RBPJ DNA binding protein and the recruitment of transcriptional coactivators and the C-terminal PEST domain responsible for termination of NOTCH1 signaling by FBXW7-mediated proteasomal degradation.
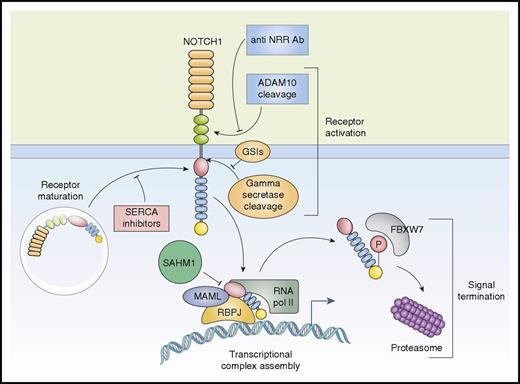
Activation and therapeutic targeting of the NOTCH1 signaling in T-ALL. The NOTCH1 receptor is synthesized as a precursor protein that undergoes maturation in the trans-golgi network before being expressed in the plasma membrane. Maturation and processing of NOTCH1-mutant proteins that destabilize the NRR regulatory region are sensitive to inhibition of SERCA calcium channels with thapsigargin. Activation of NOTCH1 is initiated by cleavage of the receptor by ADAM10 protease. Antibodies (Ab) that recognize and protect the NRR preclude metalloprotease processing of NOTCH1, thus abrogating receptor activation. After ADAM10 processing, NOTCH1 is cleaved in the transmembrane region by the γ-secretase complex, which can be inhibited by small-molecule GSIs. After release from the membrane, the intracellular active portion of the receptor translocates to the nucleus where it interacts with the RBPJ DNA-binding protein and recruits the MAML1 transcriptional coactivator. Assembly of the ICN1-RBPJ-MAML1 complex and activation of NOTCH1 target genes can be inhibited by SAHM1, a stapled peptide. P, phosphorylation; pol II, polymerase II.
Activation and therapeutic targeting of the NOTCH1 signaling in T-ALL. The NOTCH1 receptor is synthesized as a precursor protein that undergoes maturation in the trans-golgi network before being expressed in the plasma membrane. Maturation and processing of NOTCH1-mutant proteins that destabilize the NRR regulatory region are sensitive to inhibition of SERCA calcium channels with thapsigargin. Activation of NOTCH1 is initiated by cleavage of the receptor by ADAM10 protease. Antibodies (Ab) that recognize and protect the NRR preclude metalloprotease processing of NOTCH1, thus abrogating receptor activation. After ADAM10 processing, NOTCH1 is cleaved in the transmembrane region by the γ-secretase complex, which can be inhibited by small-molecule GSIs. After release from the membrane, the intracellular active portion of the receptor translocates to the nucleus where it interacts with the RBPJ DNA-binding protein and recruits the MAML1 transcriptional coactivator. Assembly of the ICN1-RBPJ-MAML1 complex and activation of NOTCH1 target genes can be inhibited by SAHM1, a stapled peptide. P, phosphorylation; pol II, polymerase II.
NOTCH1 was first implicated in the pathogenesis of T-ALL as the target oncogene of the t(7;9)(q34;q34.3) translocation.30 This rare chromosomal rearrangement present in about 1% of patients with T-ALL, places an N-terminal truncated form of NOTCH1 under the control of strong T-cell–specific enhancer in the vicinity of the TCRB locus resulting in high levels of expression of a truncated NOTCH1 receptor either as ICN1 or as a membrane-bound protein (ΔE-NOTCH1), which lacks the NOTCH1 extracellular domains and is constitutively processed into ICN1 by γ-secretase.31,32
NOTCH1 is also recurrently activated by viral integration in T-ALL tumors generated by retroviral mutagenesis in mice.33 Yet the central role of NOTCH1 as the driver of human T-ALL transformation became apparent only with the identification of activating NOTCH1 mutations present in more than 65% of patients with T-ALL.34 Most T-ALL–associated NOTCH1 mutations either disrupt the negative regulatory region (NRR), an intramolecular lock that protects the extracellular portion of the receptor from cleavage by ADAM10 in the absence of ligand,35,36 or result in truncation of the PEST domain located in the C-terminal portion of NOTCH1 and involved in the termination of NOTCH1 signaling by proteasomal degradation of ICN1.37-39 In addition, about 20% of patients with T-ALL harbor mutations in FBXW7,40-42 an F-box factor that recognizes phosphorylated motifs in the PEST domain of NOTCH1 and directs ICN1 for ubiquitination and subsequent proteasomal degradation.37-39 Thus, 2 convergent mechanisms—ligand-independent receptor activation and impaired signaling termination via ICN1 stabilization—can contribute to aberrant NOTCH1 activation in T-ALL (Figure 3).
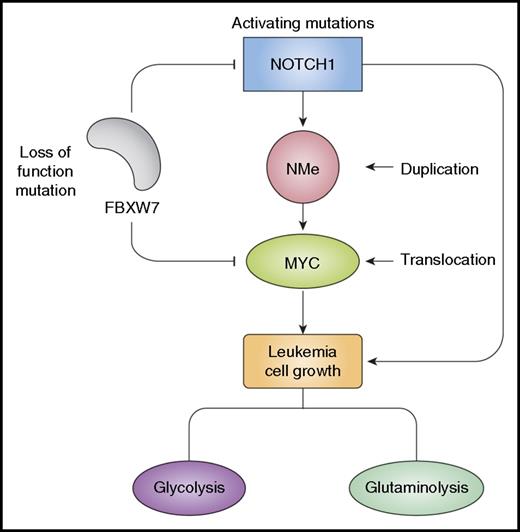
NOTCH1 and MYC regulate leukemia cell growth. Activation of NOTCH1 signaling in T-ALL is typically triggered by activating mutations in the NOTCH1 gene and loss of function mutations in FBXW7. NOTCH1 directly activates MYC expression via NMe, a long-range NOTCH-dependent T-cell–specific enhancer duplicated in about 5% of T-ALLs. Chromosomal translocations involving MYC can also be found in 1% of T-ALLs, and FBXW7 mutations contribute to activating MYC expression by stabilizing the MYC protein. NOTCH1 and MYC regulate common transcriptional targets in a feed-forward loop circuitry that promotes leukemia cell growth, proliferation, and self-renewal.
NOTCH1 and MYC regulate leukemia cell growth. Activation of NOTCH1 signaling in T-ALL is typically triggered by activating mutations in the NOTCH1 gene and loss of function mutations in FBXW7. NOTCH1 directly activates MYC expression via NMe, a long-range NOTCH-dependent T-cell–specific enhancer duplicated in about 5% of T-ALLs. Chromosomal translocations involving MYC can also be found in 1% of T-ALLs, and FBXW7 mutations contribute to activating MYC expression by stabilizing the MYC protein. NOTCH1 and MYC regulate common transcriptional targets in a feed-forward loop circuitry that promotes leukemia cell growth, proliferation, and self-renewal.
Notably, around one fifth of patients with T-ALL show co-occurrence of both mechanisms because of dual mutations disrupting the NRR and PEST regions of NOTCH1 or the presence of a NOTCH1 NRR-disrupting mutation together with an FBXW7 mutation.34 Of note, the oncogenic activity of NOTCH1 is highly dose dependent and is strongly enhanced by the synergistic effect of these 2 mutation-driven activation mechanisms which, when combined, result in very high levels of NOTCH1 signaling.43 The frequency of NOTCH1 mutations varies between different clinico-biologic molecular groups of T-ALLs.34,44,45 Early T-cell precursor T-ALLs have a lower frequency of NOTCH1-activating mutations,46 and among cortical T-ALLs, the frequency of NOTCH1 mutations is highest in early cortical leukemias associated with TLX1 and TLX3 activation and lower in late cortical tumors characterized by activation of the TAL1 transcription factor oncogene.34,44,45 These differences in the distribution of NOTCH1 mutations may be related to the biology of the normal thymic counterparts for each of these groups; NOTCH1 signaling and dependence increases along thymocyte development peaking at the transition between DN and DP thymocytes and then being turned off as cells mature along the DP stage of thymocyte development.47,48
Mechanistically, NOTCH1 mutations in T-ALL hijack the physiologic role of NOTCH signaling in promoting T-cell lineage commitment, cell growth, and proliferation during thymocyte development.49 Gene expression profiling analyses of T-ALL cell lines that harbor activating mutations in NOTCH1 after blocking of NOTCH signaling with GSIs coupled with the genome-wide mapping of NOTCH1 binding sites placed NOTCH1 atop a broad transcriptional network primarily dominated by genes involved in the upregulation of anabolic pathways, including amino acid and nucleotide metabolism, ribosome biogenesis, and protein biosynthesis.30 Transcriptional downregulation of this metabolic signature upon NOTCH1 inhibition is coupled with decreased cell growth with G1 cell cycle arrest and decreased cell size.32,34 Moreover, detailed phenotypic and metabolomic analyses of NOTCH1-dependent tumors has shown that abrogation of oncogenic NOTCH1 signaling induces a metabolic crisis, which, in addition to transcriptional downregulation of anabolic genes, includes transcriptional upregulation of catabolic pathways (proteasome, ubiquitination, autophagy), decreased glycolytic and glutaminolitic flux, and increased autophagy.50 Of note, autophagy can help sustain leukemia cell survival in the context of NOTCH1 inhibition by providing an alternative source of precursors for cell metabolism because genetic suppression of autophagy markedly enhances the antileukemic effects of GSI treatment.50 Moreover, NOTCH1-dependent T-ALL cells seem to be particularly reliant on glutaminolysis for cell growth, and genetic and pharmacologic inhibition of glutaminase has strongly synergistic antitumor effects in combination with NOTCH1 inhibition.50 Beyond the activation of this anabolic signature, HES1 (a transcriptional repressor directly controlled by NOTCH1) plays important roles in thymocyte development and is required for NOTCH1-induced tumor initiation and maintenance.26,51,52 Additional important targets of NOTCH1 of biologic relevance in thymocyte development and leukemia include NOTCH1 itself,53 IL7RA,54 pre-TCRA,55 IGF1R,56 and the LUNAR long noncoding RNA.57
It is important to note that NOTCH1 mutations work in concert with many other genetic alterations in T-cell transformation. The TAL1 oncogene is aberrantly expressed in T-ALL as a result of chromosomal translocations and focal deletions or mutations in T-ALL, most often in concert with the LMO1 and LMO2 oncoproteins. Analysis of transgenic models of TAL1-LMO1–induced T-ALL that focused on the effect of this oncogenic transcriptional complex at the earliest stages of T-cell transformation revealed insightful information on its interplay with NOTCH1 signaling.58,59 Activation of TAL1 in developing thymocytes correlated with increased NOTCH1 expression and induced upregulation of NOTCH1 target genes.58 Transgenic expression of TAL1-LMO1 reprogrammed DN3 thymocytes into self-renewing preleukemic cells, which was suppressed by inhibition of NOTCH1 signaling.59 Moreover, expression of hyperactive Notch1 in this model enhanced the self-renewal and transplantability of TAL1-LMO1 thymic progenitors, expanding the self-renewing pool from DN3 cells to all DN populations and ISP cells.58
MYC in T-ALL
The MYC oncogene encodes a basic helix-loop-helix leucine zipper transcription regulator that is broadly involved in the control of gene expression and in the regulation of genes involved in cell cycle regulation, cell metabolism, ribosome biogenesis, and DNA replication.60-63 MYC transcriptionally controls the expression of factors involved in the biosynthesis of macromolecules required to support cell growth and proliferation, including genes that regulate the cell cycle, ribosome biogenesis, and protein translation.64-66 In addition, MYC regulates glycolysis,67,68 glutaminolysis,69 mitochondrial biogenesis,70 oxidative phosphorylation,68 and energy production to support cell growth. Studies of genome-wide transcription profiling support a broader role for MYC in transcription regulation functioning as a general transcriptional cofactor in association with the RNA polymerase II complex.71,72 It has been proposed that MYC may also function as a transcription-independent regulator of the initiation of replication by binding to minichromosome maintenance protein complexes at replication origins.73
Originally identified as the overexpressed product of the t(8;14)(q24;q32) translocation in Burkitt lymphoma,74 high levels of MYC expression are found in the majority of human tumors, and MYC activation is widely involved in malignant transformation,75,76 with a direct pathogenic role in tumor initiation, progression, and maintenance.77 Several mechanisms directly contribute to MYC overexpression in cancer, including chromosomal translocation, gene amplification, and somatic mutations that lead to increased protein stability.75,76 In addition, a diverse set of oncogenic signaling pathways, including WNT and MAP kinase signaling, can induce transcriptional and posttranscriptional upregulation of MYC.78-80
In the hematopoietic system, MYC is required for the correct balance between self-renewal and differentiation of hematopoietic stem cells81-85 and plays important roles in lymphoid cell development and maturation.86-91 During thymocyte development, Myc is dynamically regulated, shows increased levels of expression in the transition between DN3 and DN4 cells, and is downregulated as thymocytes mature into the DP stage.92 Mechanistically, activation of Myc is coupled with pre-TCR signaling, which induces rapid elevation of Myc protein levels.86,87 Loss of Myc in the thymus consistently causes markedly reduced thymocyte numbers as a result of suppressed cell proliferation and growth downstream of pre-TCR signaling.86,87
Strong oncogenic activity of MYC in T-ALL has been solidly demonstrated in mouse93,94 and zebrafish95 leukemia models. High levels of MYC are broadly present in T-ALL,96 and MYC expression is strictly required for T-ALL cell growth, proliferation, and leukemia-initiating cell activity.30,97-99 Early on, MYC was implicated in the pathogenesis of T-ALL as the target of the t(8;14)(q24;q11) translocation, a rare but recurrent chromosomal translocation that induces MYC overexpression by placing the MYC gene under the control of strong T-cell–specific enhancer elements in the vicinity of the TCRA/TCRD.100-103 In addition, NOTCH1 signaling upregulates MYC expression.30,104,105 The MYC protein has a very short half-life and, as is the case with intracellular NOTCH1, regulation of MYC protein levels by proteasomal degradation is also mediated by FBXW7.106-108 Consequently, T-ALLs with FBXW7 mutations show not only prolonged and augmented NOTCH1 signaling but also MYC stabilization and increased MYC protein levels (Figure 3).40,41 About 20% of patients with T-ALL show loss of PTEN,109 a critical negative regulator of the PI3K-AKT signaling pathway,110 and PTEN loss has also been linked with MYC stabilization and increased MYC protein levels.96 Mechanistically, PTEN suppresses activation of AKT1, which phosphorylates and inactivates GSK3B,111 a serine-threonine kinase implicated in the phosphorylation-mediated degradation of the MYC oncoprotein.112,113
NOTCH1-MYC transcriptional regulatory axis
The convergent role of NOTCH1 and MYC as drivers of cell growth in immature T cells and in the context of T-ALL is wired in a feed-forward loop transcriptional circuitry114 in which NOTCH1 directly activates MYC expression, and NOTCH1 and MYC control an overlapping repertoire of cell growth target genes (Figure 3).30,115,116 Early studies showed that NOTCH1 inactivation induced transcriptional downregulation of MYC expression in T-ALL, and NOTCH1 binding was allocated to regulatory elements in the MYC promoter.30,104,105 In addition, global mapping of NOTCH1 and MYC target genes showed broad binding of MYC to NOTCH1-regulated promoter sequences.115 Moreover, forced MYC expression can rescue inhibition of cell growth induced by GSI treatment in some NOTCH1-dependent human and mouse T-ALL cell lines.30,104 In this context, it is worth noting that MYC translocated leukemias are characteristically wild type for NOTCH1 and FBXW7.103 Animal models of T-ALL with recurrent Myc activation through chromosomal translocations, such as those triggered by loss of Pten117 or activation of β-catenin,118 do not show Notch1 activation, which supports a functional redundancy between NOTCH1 and MYC in T-ALL. Yet direct demonstration of the regulation of MYC by NOTCH1 remained elusive because active forms of NOTCH1 failed to induce any significant increase in MYC promoter activity in reporter assays, which suggests a potential role for long-range regulatory elements or indirect mechanisms in the control of MYC downstream of NOTCH1.
The MYC oncogene is subject to dynamic transcriptional regulation orchestrated by a network of proximal and distal regulatory elements implicated in the regulation of MYC expression in response to different growth-promoting signals and with diverse tissue specificity. The relevance of MYC regulation in tumorigenesis is highlighted by genome-wide association studies that link genetic variants located distally 5′ from the MYC locus with risk susceptibility to several different cancers, including breast, bladder, prostate, and colon.119-122 Of note, these cancer-risk alleles are located in enhancer elements123,124 that associate with the MYC promoter via long-range chromatin loops.125-127 Among these risk variants, the rs6983267 single nucleotide polymorphism is located in an enhancer required for Wnt-driven intestinal tumor formation128 and affects the binding of the TCF7L2 transcription factor implicated in Wnt signaling and colon cancer tumorigenesis.123,126,127
Mapping of recurrent genetic alterations that overlap extragenic regulatory elements has identified focal amplifications located ∼450 and ∼800 kb 3′ from MYC in lung adenocarcinoma and uterine corpus endometrial carcinoma, respectively.129 Notably, these areas of focal amplification encompass tissue-specific enhancers that interact with the MYC promoter via chromatin looping.129 Similarly, a cluster of enhancers located 1.7 Mb downstream from Myc interacts with the Myc promoter via a long-range chromatin loop in myeloid leukemia cells.130 This regulatory region is the target of focal amplifications present in about 3% of human acute myeloid leukemias,130-132 thus supporting a role for increased enhancer activity in the deregulation of MYC expression and the pathogenesis of this disease.
Mapping of enhancers loaded with high levels of the BRD4 epigenetic regulator in diffuse large B-cell lymphoma cells, a hallmark of dynamic transcriptional regulation, have identified additional candidate regulatory sequences in the vicinity of the MYC locus.133 In T-ALL, the specific link between NOTCH1 activation and MYC expression was ultimately elucidated with the identification a T-cell–specific NOTCH1-controlled distal enhancer located +1,427 kb telomeric to the MYC locus (Figure 4).97,134 This highly evolutionarily conserved regulatory element named “NMe” (for NOTCH MYC enhancer) is strongly occupied by NOTCH1 in T-ALL cells.97,134,135 NMe directly interacts with proximal regulatory sequences in the MYC promoter forming a long-range chromatin loop and confers orientation-independent NOTCH1 regulation in association with the MYC promoter in reporter assays.97,134 Notably, analysis of chromatin marks revealed that NMe could be selectively active only in T cells.97 In agreement, NMe knockout mice were viable and fertile and developed normally with no defects other than a marked thymic atrophy resulting from severe depletion of DP thymocytes.97
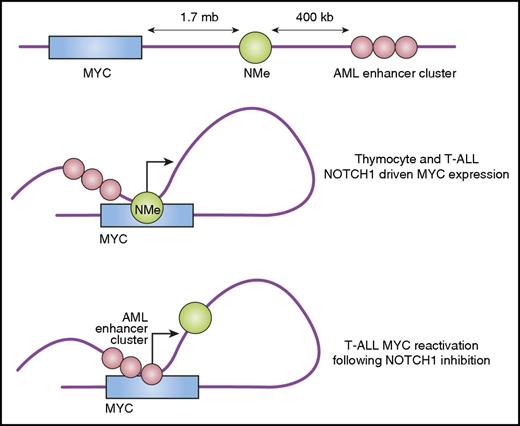
Long-range enhancers control the expression of MYC in T-ALL. During T-cell development and in the context of T-ALL, NOTCH1 activates MYC expression via interaction of the NMe long-range enhancer with regulatory elements in the MYC promoter. In some T-ALL cell lines, sustained NOTCH1 inhibition with GSIs selects a persistent population in which MYC is reactivated independently of NOTCH via recruitment of a further distal group of enhancers normally active in the myeloid compartment (acute myeloid leukemia or AML enhancer cluster).
Long-range enhancers control the expression of MYC in T-ALL. During T-cell development and in the context of T-ALL, NOTCH1 activates MYC expression via interaction of the NMe long-range enhancer with regulatory elements in the MYC promoter. In some T-ALL cell lines, sustained NOTCH1 inhibition with GSIs selects a persistent population in which MYC is reactivated independently of NOTCH via recruitment of a further distal group of enhancers normally active in the myeloid compartment (acute myeloid leukemia or AML enhancer cluster).
Loss of NMe markedly reduced Myc expression levels selectively in DN3, DN4, and ISP T-cell progenitors, which resulted in a thymocyte developmental defect that perfectly recapitulates that of T-cell–specific Myc knockout mice.86,97 Analysis of leukemia development in NMe knockout mice highlighted the relevance of this enhancer and of the NOTCH1-Myc regulatory axis in T-cell transformation.116 Thus, bone marrow progenitors from NMe–deficient mice failed to generate leukemia upon expression of oncogenic NOTCH1.97 Moreover, loss of 1 copy of NMe was sufficient to decrease leukemia penetrance in this setting, indicating that the leukemogenic activity of NOTCH1-Myc is highly dose dependent.97 Similarly, deletion of NMe in fully established NOTCH1-induced leukemias resulted in markedly reduced Myc expression, abrogation of Myc-controlled transcriptional programs, decreased proliferation, and impaired tumor progression with marked suppression of leukemia-initiating cell activity.97 In addition, and because it was the case in tumor initiation experiments, loss of 1 copy of NMe in established tumors partially impaired leukemia progression.97 The relevance of the oncogenic role of NMe–mediated NOTCH1-induced MYC regulation is further underscored by the identification of recurrent somatic focal duplications of the NMe enhancer in about 5% of T-ALL patients.97
Therapeutic opportunities
The high prevalence of NOTCH1-activating mutations and the central role of the NOTCH1-MYC signaling axis as the driver of cell proliferation and growth in T-ALL provides a rationale for the development of targeted strategies toward inhibition of NOTCH signaling in this disease.136 As mentioned before, small-molecule GSIs can effectively suppress NOTCH signaling by blocking intramembrane proteolytic processing of NOTCH1 by the γ-secretase complex.137 Notably, cleavage by the γ-secretase complex is required for the activity of both NRR and PEST domain mutant forms of NOTCH1. Treatment of T-ALL cell lines harboring NRR, PEST, or combined NRR-PEST mutations consistently results in loss of intracellular activated NOTCH1 and downregulation of NOTCH1 target genes. But it is worth noting that PEST- and FBXW7-mutant cells have increased intracellular NOTCH1 half-life and that the clearance of active NOTCH1 after GSI treatment may be slower in these cells. A more gradual decrease of NOTCH1 signaling in this setting could influence the response to GSI therapy. But one should also consider that cells with higher levels of active NOTCH1, as is the case in tumors with NRR-PEST and NRR-FBXW7 mutations, may have higher addiction to NOTCH1 signaling, which could render them more sensitive to the effects of NOTCH1 inhibition with a GSI. However, these hypotheses still require direct experimental testing.
GSI treatment of T-ALL cell lines with activating mutations in NOTCH1 induces rapid clearance of activated NOTCH1 protein and downregulation of NOTCH1 target genes, including MYC.30,32,34,104,138 Moreover, mouse NOTCH1-induced leukemias can be effectively treated with GSIs,50,139 and in a subset of human T-ALL tumor lines, suppression of oncogenic NOTCH1-induced G1 cell cycle arrest and decreased cell size.32,34 In addition, inhibition of NOTCH1 signaling has been associated with decreased leukemia-initiating cell activity.48,56,140,141 Yet GSIs have shown only limited therapeutic activity in the clinic to date.136,141-144
Multiple factors may condition the response to anti-NOTCH1 therapies in T-ALL. It should be noted that although NOTCH1 mutations as biomarkers of response can function as tumor-initiating lesions present in all leukemia populations,145,146 they can also be acquired during disease progression and found as subclonal genetic alterations,147,148 in which case anti-NOTCH1 therapies may impair only a fraction of the tumor cells. In addition, only a fraction of T-ALL cell lines with activating mutations in NOTCH1 respond to GSI treatment despite effective clearance of activated intracellular NOTCH1 and downregulation of NOTCH1 target genes.109 Of note, GSI-resistant T-ALL lines show mutational loss of PTEN and consequent constitutive activation of PI3K-AKT signaling.109 Objectively, loss of Pten decreased the dependence of NOTCH1 signaling for growth, proliferation, and survival and results in loss of the therapeutic window of GSIs and progression under therapy in animal studies.50 However, it should be noted that loss of Pten does not completely revert all the transcriptional and phenotypic effects of NOTCH1 inhibition.50,149 Mechanistically, loss of Pten can induce resistance to GSI treatment in mouse models of T-ALL by promoting increased glycolysis and metabolic reprogramming of leukemia cell growth.50,109 Many GSI-resistant human T-ALL cell lines have co-occurring mutations in FBXW7, which may further contribute to GSI resistance by ameliorating GSI-induced MYC downregulation.40,41 Thus, although MYC is transcriptionally downregulated in PTEN-null T-ALL cell lines and in mouse models of NOTCH1-induced T-ALL with loss of Pten, it is worth noting that most human GSI-resistant T-ALL cell lines also have FBXW7 mutations,40,41 which extend the half-life of the MYC protein. Thus, although MYC protein levels eventually decrease as NOTCH1 is turned off, changes in the kinetics of MYC inactivation could contribute to modulation of the response to GSI treatment.
Prolonged in vitro exposure to GSI in T-ALL cell lines induces the selection of a GSI-persistent population with NOTCH1-independent cell growth that is capable of reverting to a GSI-sensitive state if it is cultured in the absence of drug (Figure 4).150 Notably, these GSI-persistent cells show upregulation of MYC expression, which can be epigenetically abrogated with BRD4 inhibitors.150 A similar phenomenon—restoration of leukemia cell growth and NOTCH1-independent upregulation of MYC expression—has been associated with leukemia progression in a T-ALL patient with an initial response to GSI therapy.151 Interestingly, in both cases, MYC reactivation was coupled with the activation of a group of BRD4-dependent enhancers located 1.7 Mb telomeric to the MYC gene (400 kb downstream of NMe) that normally drive MYC expression in myeloid cells.130,134
In all, these observations support the notion that the antileukemic effects of NOTCH1 inhibition can be bypassed by different, sometimes convergent, genetic and epigenetic mechanisms capable of supporting NOTCH-independent leukemia cell growth. In this context, combination therapies that target NOTCH1 downstream and parallel effector pathways, including the combination of GSIs with CDK inhibitors,152 drugs targeting nuclear factor κB,40 glutaminase inhibitors,50 and inhibitors blocking the PI3K-AKT-mTOR pathway109,138,139 have been shown to increase the antileukemic effects of GSIs. In addition, inhibition of NOTCH signaling with a GSI can sensitize glucocorticoid-resistant T-ALL cell lines to glucocorticoid-induced apoptosis.153 Importantly, glucocorticoids can also transcriptionally downregulate MYC expression in T-ALL,153,154 which provides an added rationale for the combination of steroid therapy and GSIs. However, systemic inhibition of NOTCH signaling with GSIs, which abrogate the activity of all 4 NOTCH receptors (NOTCH1-4) is not devoid of toxicity. Genetic inhibition of NOTCH signaling in animal models via deletion of the Rbpjk gene in the gut155 or using double Notch1/Notch2 conditional knockout mice156 induces intestinal secretory metaplasia, a phenotype that is recapitulated upon pharmacologic inhibition of the Notch pathway with GSIs.155,157 Of note, and of potential importance for the development of GSIs as anti-NOTCH1 therapies in the clinic, glucocorticoid treatment can protect experimental animals from gastrointestinal toxicity induced by systemic inhibition of NOTCH signaling.153,158-160
Additional strategies toward NOTCH1 inhibition include blocking NOTCH1 signaling with inhibitory antibodies, interfering with the processing of NOTCH1-mutant proteins, and blocking the activity of the NOTCH1 transcriptional complex in the nucleus. Anti-NOTCH1 antibodies binding to the NRR region of the receptor can block the NOTCH1 signaling that interferes with ADAM10 processing.26-28,161,162 These inhibitory antibodies bind to and stabilize the NRR, which further protects NOTCH1 from metalloprotease cleavage. Of note, most NRR mutations do not disrupt binding and inhibition by anti-NRR antibodies, and a diverse set of NRR-mutant forms of NOTCH1 have been shown to be amenable to antibody-mediated inhibition. Yet NOTCH1 receptors that bear rare mutations in which amino acid insertions generate a duplicated or constitutively sensitive metalloprotease cleavage site located outside the NRR region34,163 seem to be refractory to inhibition with anti-NRR antibodies.27 Anti-NOTCH1 antibodies have shown antileukemic effects in vitro and in vivo against T-ALL cell lines, and in primary-derived T-ALL, xenografts can suppress leukemia-initiating cell activity and potentiate the antitumor effects of glucocorticoids.26-28 A particularly attractive feature of this approach is that Notch1 blocking antibodies spare the activity of Notch2 in the intestine, thus precluding the development of overt gastrointestinal toxicity.164
In addition, SAHM1, a stabilized α-helical peptide that interferes directly with the recruitment of MAML1 into the NOTCH-RBPJ transactivation complex, has been shown to induce rapid and robust NOTCH inhibition with strong antileukemic effects against both human T-ALL cell lines and a mouse model of NOTCH1-induced T-ALL.165 Yet, none of these strategies selectively abrogates the activity of mutant NOTCH1. It is possible that heterogeneity in the composition and function of the γ-secretase complex could be exploited to develop tissue-specific inhibitors that selectively inactivate NOTCH activation in T-cells. In addition, the NOTCH1 receptor protein undergoes a complex process of maturation and processing in endoplasmic vesicles before locating in the plasma membrane,166 a process that seems more efficient in the case of wild-type NOTCH1 than in NRR-mutant NOTCH1 receptor proteins,167 and is dependent on the activity of sarcoplasmic/endoplasmic reticulum calcium ATPase (SERCA) channels.168 As a result, SERCA inhibition with thapsigargin impairs the expression of mature NOTCH1 protein with preferential suppression of NRR-mutated NOTCH1 receptors.168
Moreover, the NOTCH1-MYC signaling axis could also be targeted via direct suppression of MYC, a strategy that could effectively abrogate leukemia cell growth even in the context of parallel or convergent genetic alterations that lead to MYC activation. Transcriptionally, expression of the MYC oncogene is tightly regulated by a complex network of tissue-specific enhancers and long-range enhancers126,130,169 organized into super-enhancers, broad areas of active open chromatin characterized by the presence of H3K27 acetylation that favor very dynamic transcriptional regulation.170,171 The activity of these super-enhancers is dependent on BRD4, an epigenetic regulator that binds to the H3K27 acetylation mark.172 In this context, JQ1, a small-molecule BRD4 inhibitor that abrogates MYC expression in multiple settings,173 induces strong therapeutic responses in mouse models of NOTCH1-induced T-ALL and against human T-ALL xenografts.98,99,174 Similarly, MYC protein translation is also dynamically regulated, and T-ALL cells seem particularly dependent on protein translation for cell growth proliferation and survival.175 Silvestrol, an inhibitor of eIF4A (a helicase required for CAP-mediated translation that preferentially abrogates the expression of proteins with highly structured 5′ untranslated regions with CGG quadruplexes), induces decreased expression of T-ALL oncogenic factors including MYC and NOTCH1 and shows strong antileukemic effects against T-ALL cell lines and in leukemia xenografts.175 Finally, MYC activity is dependent on its interaction with the MAX transcriptional co-regulator.176 Thus, small molecules that interfere with the assembly of MYC-MAX transcriptional complexes177,178 may offer additional opportunities to abrogate the oncogenic transcriptional circuitries controlled by MYC.
Conclusion
Dissection of the signaling, transcriptional, epigenetic, and metabolic circuitries downstream of oncogenic NOTCH1 has brought to the fore a key major role for MYC as a driver of the growth of T-ALL leukemia cells. The feed-forward loop transcriptional network coupling NOTCH1 and MYC expression provides a mechanism for PI3K and additional signaling pathways that regulate MYC expression to amplify the effects of NOTCH1 in cell growth during thymocyte development. In the context of T-ALL, activation of parallel and downstream oncogenic effectors that activate cell growth can attenuate the antileukemic effects of anti-NOTCH1 therapies. Drug combinations that target NOTCH1 and these alternative growth-supporting mechanisms offer improved opportunities for the development of highly active targeted therapies for the treatment of T-ALL.
Acknowledgments
The authors thank Daniel Herranz for his insightful comments and editing of the manuscript.
This work was supported by a postdoctoral fellowship (M.S.-M.) from the Rally Foundation.
Authorship
Contribution: M.S.-M. and A.F. designed and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Adolfo Ferrando, Columbia University Medical Center, Irving Cancer Research Center 402A, 1130 St. Nicholas Ave, New York, NY 10032; e-mail: af2196@columbia.edu.