Key Points
BV has activity for SR-aGVHD.
The MTD of BV was 0.8 mg/kg every 2 weeks for 4 doses.
Abstract
Therapy for steroid-refractory acute graft-versus-host disease (SR-aGVHD) remains suboptimal. Preclinical data demonstrate increased CD30 expression on activated CD8+ T cells during aGVHD. Brentuximab vedotin (BV) is an antibody-drug conjugate targeting CD30. We conducted a multicenter phase 1 trial in 34 patients to establish the maximum tolerated dose (MTD) of BV for SR-aGVHD treatment. A 3+3 cohort design was conducted initially with BV given weekly × 3 doses followed by maintenance dosing (initial dose 0.6 mg/kg IV weekly). Six patients were treated with the initial weekly dosing scheme; 2 of these patients died of neutropenic sepsis complications. The trial was subsequently revised to escalating cohorts of 5 patients treated every 2 weeks × 4 doses with a 4-week dose-limiting toxicity (DLT) period. Twenty-eight patients were treated with every-2-week dosing (n = 10 at 0.6 mg/kg; n = 18 at 0.8 mg/kg). MTD was defined at 0.8 mg/kg with 1 DLT observed (sepsis). At day 28, the overall response rate was 38.2% with 5 complete responses (CRs; 14.7%) and 8 very-good-partial responses (23.5%). An additional 7 patients achieved CR by day 56. With 12 months’ follow-up on all patients, overall survival was 41% (95% confidence interval [CI], 25%-57%) at 6 months and 38% (95% CI, 22%-54%) at 12 months. CD30 expression on central memory CD8+, central memory CD4+, and regulatory T-lymphocyte subsets at enrollment was not associated with clinical response. BV is tolerable and has activity in SR-aGVHD and merits further investigation. This trial was registered at www.clinicaltrials.gov as #NCT01940796.
Introduction
Steroid-refractory acute graft-versus-host disease (SR-aGVHD) remains a major complication after allogeneic hematopoietic cell transplantation (HCT) and is associated with poor long-term survival.1-4 Many agents have been investigated and used for the treatment of SR-aGVHD, however, there is no clear standard and toxicities often include significant infection as a result of cumulative immunosuppression. Novel therapies that can target aGVHD without leading to further infection are sorely needed.
CD30 is a cell membrane molecule in the family of tumor necrosis receptors that is expressed on activated lymphocytes.5 Our preclinical study demonstrated increased expression of CD30 on activated CD8+ T cells at the time of aGVHD.6 We therefore hypothesized that selective targeting of CD8+/CD30+ T cells could be effective in treating aGVHD. Brentuximab vedotin (BV) is a CD30-directed antibody-drug conjugate consisting of the human CD30 antibody cAC10 and the microtubule-disrupting agent, monomethyl auristatin E (MMAE). BV is approved for the treatment of relapsed classical Hodgkin lymphoma7 and systemic anaplastic large-cell lymphoma8 and has also been preliminarily shown to be safe and effective for treatment of relapsed disease after allogeneic HCT.9 Here, we report the results of a multicenter phase 1 study investigating BV for the treatment of SR-aGVHD.
Methods
This study was approved by the local institutional review board at each of 4 centers. Informed consent was obtained from all patients. Multicenter coordination and monitoring was performed by the Massachusetts General Hospital Cancer Center Multicenter Coordinating Group. The diagnosis of aGVHD was made by treating providers and supported with tissue biopsies in the majority of cases. SR-aGVHD was defined as (1) progressive GVHD after at least 3 days of systemic corticosteroids (≥1 mg/kg per day of prednisone equivalent), (2) no improvement in GVHD after at least 7 days on ≥1 mg/kg per day of prednisone equivalent or insufficient improvement which warranted the addition of another agent, or (3) flare of GVHD symptoms during taper of corticosteroids. Exclusion criteria included the presence of acute/chronic GVHD overlap syndrome, significant organ dysfunction, and use of >1 systemic agent beyond corticosteroids for treatment of aGVHD. Patients were thus eligible if they failed steroids plus an additional agent used in sequence or started together as initial therapy. Agents used for GVHD prophylaxis were allowed to be continued and increased to target trough levels. Use of topical creams, oral nonabsorbable steroids and ursodiol were all permitted. For patients who met eligibility for recurrent GVHD upon tapering of steroids, steroids were allowed to be increased to a maximum of their initial baseline starting dose.
Patients were evaluable for toxicity and efficacy end point analyses if they received at least 1 dose of BV. Dose-limiting toxicity (DLT) was defined as: (1) any nonhematologic drug-related event that was Common Terminology Criteria for Adverse Events Version 4.0 (CTCAE v4.0) grade 3 or higher and either did not resolve to grade 0-1 or baseline within 7 days or resolved but then recurred after resuming BV; (2) peripheral neuropathy ≥ grade 2; or (3) ≥grade 3 infusion reaction that did not resolve to grade 1 or baseline within 24 hours. If patients discontinued treatment prior to the end of the DLT period for reasons unrelated to toxicity (ie, progression of GVHD), additional patients were enrolled. Response was assessed at study day 28 or at “end of treatment” for patients who discontinued treatment prior to day 28. aGVHD was graded by previously published consensus criteria.10 GVHD responses were scored as complete response (CR) and very-good-partial response (VGPR) per consensus criteria.11 All other changes were not included in the definition of overall response.
Two dose schedules of BV were evaluated. Initially, the study included weekly dosing for 3 weeks followed by maintenance dosing every 3 weeks for an 4 additional doses. A standard 3+3 dose-escalation cohort (0.6 mg/kg, 0.9 mg/kg, and 1.2 mg/kg) was planned to define the maximum tolerated dose (MTD). After treating the first 6 patients, the study was revised to dosing every 2 weeks for 4 doses only for safety purposes, and to enroll cohorts of 5 patients at each dose level (0.6 mg/kg, 0.8 mg/kg, 1.0 mg/kg, and 1.2 mg/kg). The primary end point was to define the MTD of BV in patients with SR-aGVHD. Secondary end points included identification of toxicities associated with BV, overall response rate at day 28, and overall survival at 6 months and 12 months.
Peripheral blood mononuclear cells were collected and cryopreserved at enrollment. Surface CD30 expression on central memory CD8 (CD8+CD45RO+CD62L+), CD4 (CD4+CD45RO+CD62L+), and regulatory T-cell (CD4+CD25+CD127−) subsets was evaluated by standard immunophenotyping methods as described previously.6 Differences in values between responders (VGPR or CR by day +56) and nonresponders (less than VGPR) were compared by the Wilcoxon rank sum test.
Results
Patient characteristics and toxicities
Thirty-five patients were enrolled and 34 patients were treated. One patient was initially enrolled, but improved prior to initiating treatment, and thus was taken off trial. Clinical and HCT characteristics of treated patients are presented in Table 1. Twenty-five patients met eligibility for SR-aGVHD because of progression or not improving on initial steroid therapy, whereas 9 patients qualified when they experienced a recurrence of symptoms upon steroid tapering. Three patients developed SR-aGVHD after recent donor leukocyte infusion. All but 3 patients were on active calcineurin inhibitor therapy at the time of enrollment. Organ involvement and grading of aGVHD at enrollment are presented in Table 2.
Clinical characteristics of participants
| Clinical characteristics . | No. . |
|---|---|
| N | 34 |
| Age, median (range), y | 56 (22-73) |
| Sex, M/F | 21/13 |
| Diagnosis, no. | |
| AML | 7 |
| ALL | 7 |
| MDS | 7 |
| NHL | 5 |
| MPN | 3 |
| Multiple myeloma | 2 |
| Other* | 3 |
| Donor | |
| Matched unrelated | 18 |
| Matched related | 7 |
| Mismatched unrelated | 5 |
| Umbilical cord blood | 4 |
| Stem cell source | |
| Peripheral blood stem cells | 29 |
| Bone marrow | 1 |
| Umbilical cord blood | 4 |
| Conditioning regimen | |
| Myeloablative | 11 |
| Reduced intensity | 23 |
| GVHD prophylaxis | |
| Tacrolimus/MTX | 17 |
| Tacrolimus/sirolimus ± MTX | 3 |
| Tacrolimus/MTX/other† | 7 |
| Tacrolimus or cyclosporine/MMF | 5 |
| Ex vivo T-cell depletion | 2 |
| aGVHD grade at enrollment | |
| II | 10 |
| III | 22 |
| IV | 8 |
| Time from aGVHD onset to day 1 of BV, median (range) | 31 d (6-185) |
| Time from day 0 of HCT (or DLI) to day 1 of BV, median (range) | 88 d (28-371) |
| Category of SR-aGVHD | |
| Progressing or not improving on initial steroids | 25 |
| Flare upon steroid tapering | 9 |
| BV dose level | |
| 0.6 mg/kg IV weekly × 3 | 3 |
| 0.9 mg/kg IV weekly × 3 | 3 |
| 0.6 mg/kg IV every other week × 4 | 10 |
| 0.8 mg/kg IV every other week × 4 | 18 |
| Clinical characteristics . | No. . |
|---|---|
| N | 34 |
| Age, median (range), y | 56 (22-73) |
| Sex, M/F | 21/13 |
| Diagnosis, no. | |
| AML | 7 |
| ALL | 7 |
| MDS | 7 |
| NHL | 5 |
| MPN | 3 |
| Multiple myeloma | 2 |
| Other* | 3 |
| Donor | |
| Matched unrelated | 18 |
| Matched related | 7 |
| Mismatched unrelated | 5 |
| Umbilical cord blood | 4 |
| Stem cell source | |
| Peripheral blood stem cells | 29 |
| Bone marrow | 1 |
| Umbilical cord blood | 4 |
| Conditioning regimen | |
| Myeloablative | 11 |
| Reduced intensity | 23 |
| GVHD prophylaxis | |
| Tacrolimus/MTX | 17 |
| Tacrolimus/sirolimus ± MTX | 3 |
| Tacrolimus/MTX/other† | 7 |
| Tacrolimus or cyclosporine/MMF | 5 |
| Ex vivo T-cell depletion | 2 |
| aGVHD grade at enrollment | |
| II | 10 |
| III | 22 |
| IV | 8 |
| Time from aGVHD onset to day 1 of BV, median (range) | 31 d (6-185) |
| Time from day 0 of HCT (or DLI) to day 1 of BV, median (range) | 88 d (28-371) |
| Category of SR-aGVHD | |
| Progressing or not improving on initial steroids | 25 |
| Flare upon steroid tapering | 9 |
| BV dose level | |
| 0.6 mg/kg IV weekly × 3 | 3 |
| 0.9 mg/kg IV weekly × 3 | 3 |
| 0.6 mg/kg IV every other week × 4 | 10 |
| 0.8 mg/kg IV every other week × 4 | 18 |
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; DLI, donor leukocyte infusion; F, female; M, male; MDS, myelodysplastic syndrome; MMF, mycophenolate mofetil; MPN, myeloproliferative neoplasm; MTX, methotrexate; NHL, non-Hodgkin lymphoma.
Other diagnoses: aplastic anemia, blastic plasmacytoid dendritic cell neoplasm, chronic myelomonocytic leukemia.
Other GVHD prophylaxis: 3, ATG; 1, ATG or placebo; 1, bortezomib; 1, Milatuzumab; 1, RGI-2001.
GVHD response to BV by dose level, organ involvement, and severity
| . | No. (%) . | ||
|---|---|---|---|
| CR . | VGPR . | OR . | |
| Drug dose | |||
| 0.6 mg/kg weekly × 3 | 1/3 (33) | 1/3 (33) | 2/3 (67) |
| 0.9 mg/kg weekly × 3 | 0/3 (0) | 0/3 (0) | 0/3 (0) |
| 0.6 mg/kg every other week × 4 | 2/10 (20) | 1/10 (10) | 3/10 (30) |
| 0.8 mg/kg every other week × 4 | 2/18 (11) | 6/18 (33) | 8/18 (44) |
| Overall | 5/34 (15) | 8/34 (24) | 13/34 (38) |
| Organs involved | |||
| Skin | 2/18 (11) | 3/18 (17) | 5/18 (28) |
| Intestine | 3/24 (12) | 7/24 (29) | 10/24 (42) |
| Liver | 2/11 (18) | 3/11 (27) | 5/11 (45) |
| GVHD grade at enrollment | |||
| II | 3/10 (30) | 1/10 (10) | 4/10 (40) |
| III | 2/22 (9) | 7/22 (32) | 9/22 (41) |
| IV | 0/2 (0) | 0/2 (0) | 0/2 (0) |
| . | No. (%) . | ||
|---|---|---|---|
| CR . | VGPR . | OR . | |
| Drug dose | |||
| 0.6 mg/kg weekly × 3 | 1/3 (33) | 1/3 (33) | 2/3 (67) |
| 0.9 mg/kg weekly × 3 | 0/3 (0) | 0/3 (0) | 0/3 (0) |
| 0.6 mg/kg every other week × 4 | 2/10 (20) | 1/10 (10) | 3/10 (30) |
| 0.8 mg/kg every other week × 4 | 2/18 (11) | 6/18 (33) | 8/18 (44) |
| Overall | 5/34 (15) | 8/34 (24) | 13/34 (38) |
| Organs involved | |||
| Skin | 2/18 (11) | 3/18 (17) | 5/18 (28) |
| Intestine | 3/24 (12) | 7/24 (29) | 10/24 (42) |
| Liver | 2/11 (18) | 3/11 (27) | 5/11 (45) |
| GVHD grade at enrollment | |||
| II | 3/10 (30) | 1/10 (10) | 4/10 (40) |
| III | 2/22 (9) | 7/22 (32) | 9/22 (41) |
| IV | 0/2 (0) | 0/2 (0) | 0/2 (0) |
OR, overall response.
Six patients were enrolled on the initial weekly dosing schedule, and 2 experienced profound neutropenia. Although 1 patient had rapid count recovery, both patients died of complications of neutropenia. Analysis of free MMAE levels from the first 6 patients showed inadequate MMAE clearance (data not shown) likely contributing to the observed neutropenia, and the study was subsequently revised. Twenty-eight patients were treated on the revised treatment schedule. One DLT was observed at the 0.8 mg/kg dose (sepsis and multiorgan failure). The DLT was thus defined at 0.8 mg/kg IV dosed every 2 weeks for 4 doses. Other toxicities that were potentially attributable to BV are listed in Table 3. None of these were deemed to be of significant clinical consequence, resolved with supportive care, and did not affect further administration of BV. Notably, no peripheral neuropathy was observed.
Toxicities observed on BV therapy
| Dose . | Grade 2 . | Grade 3 . | Grade 4 . | Grade 5 . |
|---|---|---|---|---|
| 0.6 mg/kg weekly | Anemia | Anemia | Thrombocytopenia | |
| Neutropenia | Neutropenia | |||
| Nausea | Abdominal pain | |||
| Elevated creatinine | Weakness (2) | |||
| Fatigue | ||||
| 0.9 mg/kg weekly | Edema | Anemia (2) | Neutropenia | Sepsis |
| Thrombocytopenia | Thrombocytopenia | |||
| Nausea | ||||
| 0.6 mg/kg every other week | Anemia | Neutropenia | Thrombocytopenia | |
| Neutropenia | Thrombocytopenia (3) | |||
| Pneumonia | ||||
| 0.8 mg/kg every other week | Neutropenia | Neutropenia | Neutropenia | |
| Thrombocytopenia (2) | Headache | Thrombocytopenia | ||
| Fatigue (2) | Hypoxia | Sepsis | ||
| Abdominal distention (2) | Ileus | |||
| Hypertension | Elevated bilirubin | |||
| Myalgias (2) | ||||
| Arthralgias |
| Dose . | Grade 2 . | Grade 3 . | Grade 4 . | Grade 5 . |
|---|---|---|---|---|
| 0.6 mg/kg weekly | Anemia | Anemia | Thrombocytopenia | |
| Neutropenia | Neutropenia | |||
| Nausea | Abdominal pain | |||
| Elevated creatinine | Weakness (2) | |||
| Fatigue | ||||
| 0.9 mg/kg weekly | Edema | Anemia (2) | Neutropenia | Sepsis |
| Thrombocytopenia | Thrombocytopenia | |||
| Nausea | ||||
| 0.6 mg/kg every other week | Anemia | Neutropenia | Thrombocytopenia | |
| Neutropenia | Thrombocytopenia (3) | |||
| Pneumonia | ||||
| 0.8 mg/kg every other week | Neutropenia | Neutropenia | Neutropenia | |
| Thrombocytopenia (2) | Headache | Thrombocytopenia | ||
| Fatigue (2) | Hypoxia | Sepsis | ||
| Abdominal distention (2) | Ileus | |||
| Hypertension | Elevated bilirubin | |||
| Myalgias (2) | ||||
| Arthralgias |
Numbers in parentheses refer to the number of patients experiencing each toxicity. Grading of toxicities was done by CTCAE v4.0
GVHD response and outcomes
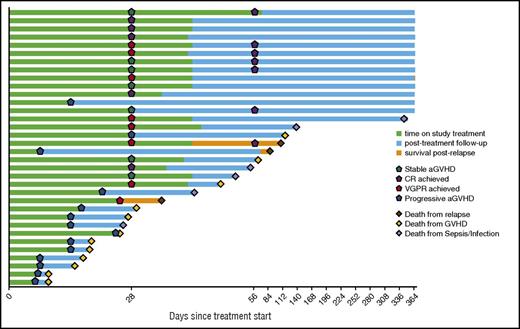
Thirty-four patients were evaluable for response (Figure 1). At day 28, 5 achieved CR and 8 achieved VGPR, yielding a formal overall response rate (CR + VGPR) of 38.2% at day 28. There were no significant differences in individual organ response rates (Table 2). An additional 7 patients achieved CR by day 56, 3 of whom had achieved VGPR and 4 of whom had stable disease at day 28. One patient who achieved CR at day 28 died of infection prior to day 56, thus, yielding a 32.4% rate of achieving CR and being alive at day 56. Of the 12 patients who achieved CR by day 56, 4 patients eventually required additional therapy when experiencing recurrent aGVHD symptoms while tapering immunosuppression.
For the patients who achieved CR by day 56, the steroid dose at day 56 was a median of 29% (range, 0, 100) of the dose at enrollment and the 6-month steroid dose was a median of 3% (range, 0, 120) of the dose at enrollment. All surviving patients were followed for 12 months after initiation of BV therapy. Of the 18 patients who ever achieved CR or VGPR to BV by day 56, 12 were alive at 12 months and 6 died (2 disease relapse, 3 infection, 1 GVHD). Of the 11 patients who achieved CR and were alive at day 56, 10 survived to 12 months and 1 experienced late death from disease relapse. Of the 16 patients who did not respond to BV therapy, only 1 remains alive at 12 months, with 11 deaths from GVHD, 3 from infection, and 1 from disease relapse. At 12 months of follow-up, 13 of 34 patients were alive, yielding an overall survival at 6 months and 12 months of 41% (95% confidence interval, 25%-57%) and 38% (95% confidence interval, 22%-54%), respectively.
Correlative studies
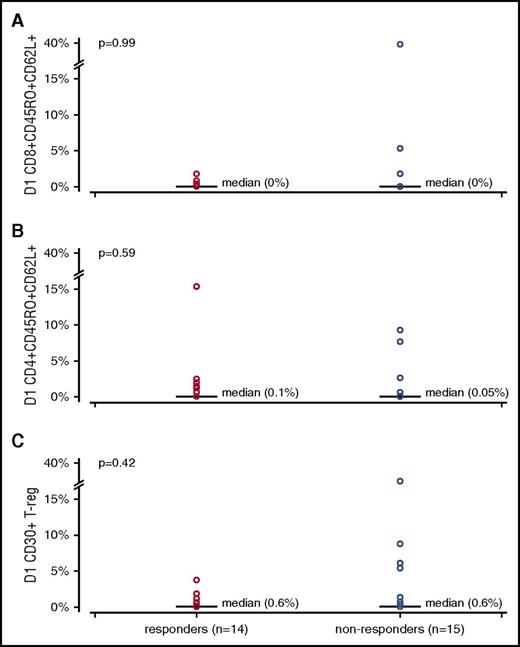
Peripheral blood mononuclear cells were collected from 29 patients at enrollment and expression of CD30 was evaluated by standard immunophenotyping. CD30 expression on central memory CD8+, central memory CD4+, and regulatory T cells was compared between responders and nonresponders to analyze whether CD30 expression correlated with response. In the central memory CD8+ population, there was no significant difference in CD30 expression between responders and nonresponders: median 0.00% (range, 0.00%-1.80%) vs median 0.00% (range, 0.00%-38.90%), P = .99, respectively. In addition, there was no correlation between CD30 expression and aGVHD response in central memory CD4+ cells or regulatory T cells (Figure 2).
Percentage of cells expressing CD30 on specific lymphocyte subsets in responders and nonresponders. Comparison done by the Wilcoxon rank sum test. (A) Central memory CD8+CD45RO+CD62L+. (B) Central memory CD4+CD45RO+CD62L+. (C) Regulatory T cells (T-reg) CD4+CD25+CD127−.
Percentage of cells expressing CD30 on specific lymphocyte subsets in responders and nonresponders. Comparison done by the Wilcoxon rank sum test. (A) Central memory CD8+CD45RO+CD62L+. (B) Central memory CD4+CD45RO+CD62L+. (C) Regulatory T cells (T-reg) CD4+CD25+CD127−.
Discussion
We report the results of a phase 1 multicenter study using BV for the treatment of SR-aGVHD. Based on our results, weekly dosing, which appeared safe in patients with lymphoma,12 resulted in profound neutropenia due to accumulation of free MMAE. With a revised dosing schedule of every other week, BV can be safely given to patients for the treatment of SR-aGVHD. The MTD was found to be 0.8 mg/kg IV every 2 weeks for 4 doses and neutropenia was only experienced in 1 of 18 patients treated on the revised dosing schedule. Among the 34 total patients treated with BV, we observed an overall response rate (CR + VGPR) of 38.2% at day 28 and a 32.4% rate of being alive and in CR at day 56.
Murine models have suggested that CD30 may help mediate aGVHD,13 and have shown that CD30 is selectively expressed on alloimmune lymphocytes.14 We recently conducted a preclinical study to assess the expression of CD30 on peripheral blood T-cell subsets in patients after allogeneic HCT. We analyzed CD30 expression on peripheral blood T-cell subsets in 26 patients at the time of presentation of aGVHD, prior to the initiation of treatment, compared with 27 HCT patients without aGVHD (none). Patients with aGVHD had a higher percentage of CD30-expressing CD8+ T cells with the difference especially pronounced in the central memory subset (CD8+CD45RO+CD62L+): GVHD median 12.4% (range, 0.8%-33.4%) vs none 2.1% (0.7%, 17.5%), P < .001. There were similar levels of CD30 expression in naive T cells, CD4+ T cells, and regulatory (CD4+CD127lowCD25+) T cells. In addition, immunohistochemical analysis of affected intestinal tissue showed many CD30+ infiltrating lymphocytes present.6 The lack of correlation of CD30 expression in central memory CD8+ T cells in this trial may be due to this population of patients who were already steroid-refractory when samples were collected. Initial GVHD therapy, specifically corticosteroids, may influence the expression of CD30. Moreover, many patients were quite lymphopenic, undoubtedly a consequence of therapy. Therefore, if expression of CD30 is to be evaluated as a potential predictor of clinical response, initial therapy may be the more appropriate setting.
Many agents have been used and investigated for therapy of SR-aGVHD. These include antithymocyte globulin (ATG),15 sirolimus,16 etanercept,17 denileukin diftitox,18 extracorporeal pheresis,19 mycophenolate mofetil,20 mesenchymal stem cells,21 and tocilizumab.22 Most of these trials have been small single-arm studies and have generally shown historic response rates of ∼30% and 6-month survival rates of around 40% to 45%,4 which are comparable to the results observed in this study. However, no agent has ever shown a benefit when tested in a phase 3 study. Ongoing trials are investigating newer therapies including α1-antitrypsin (NCT01700036), vedolizumab (NCT02993783), and ruxolitinib (NCT02953678). In addition to SR-aGVHD, BV is also being studied for several other applications after HCT including GVHD prophylaxis (NCT01700751), treatment of chronic GVHD (NCT01940796), and as maintenance therapy to prevent relapse following HCT in CD30+ lymphoid diseases (NCT02169505). Moreover, BV has already been shown to be a safe and effective treatment when given for relapsed CD30+ disease after HCT.9 The results of all of these efforts will hopefully define the appropriate role for BV after HCT, including a potential role in the treatment of aGVHD.
As this was a phase 1 study with a goal of identifying the MTD and associated toxicities of BV in patients with SR-aGVHD, there are significant limitations regarding any conclusions on efficacy. As with most trials for SR-aGVHD, patients were heterogeneous, specifically in regards to GVHD prophylaxis regimen received and which eligibility definition of SR-aGVHD each patient met. Inherent to most GVHD trials, the ability to accurately judge disease response is uncertain given that the kinetics of clinical improvement are often unknown and treating physicians make empiric decisions regarding changing therapy or waiting for response. Certainly, more research is needed to define whether BV is effective as therapy for aGVHD. Although we did observe significant activity for BV in patients with SR-aGVHD and believe there is merit to further investigation, future investigation should focus on identifying a biologically defined population most likely to respond to BV. As our menu of options for SR-aGVHD grows with no defined standard, and our patients become progressively more heterogeneous, tailoring therapy through biological testing to predict likelihood of response will become increasingly desirable.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported by Seattle Genetics, Inc, which provided BV as well as funding for this clinical trial.
Authorship
Contribution: Y.-B.C., M.-A.P., Y.A.E., S.M.D., T.R.S., R.J.S., and C.C. devised the idea, wrote the trial, enrolled patients, analyzed data, and approved the final manuscript; S.L. helped write the trial, analyzed data, and approved the final manuscript; M.K. collected data and helped to write the final manuscript; J.B. coordinated the trial and approved the final manuscript; C.R. and J.R. performed correlative analysis and approved the final manuscript; and A.E.-J. and S.L.M. enrolled patients and approved the final manuscript.
Conflict-of-interest disclosure: Y.-B.C. has received consulting fees from Incyte, Insys, Takeda, and Seattle Genetics. M.-A.P. has received consulting fees from Merck, Incyte, and Seattle Genetics. The remaining authors declare no competing financial interests.
Correspondence: Yi-Bin Chen, Massachusetts General Hospital, Yawkey 9E, Boston, MA 02114; e-mail: ychen6@partners.org.