Key Points
Endogenous p53 within myeloid cells regulates venous thrombus resolution, intrathrombus macrophage polarization, and fibrosis.
The p53 agonist quinacrine accelerates resolution of established venous thrombus, a potential translational benefit in patients with DVT.
Abstract
Deep venous thrombosis (DVT) remains a common and serious cardiovascular problem with both fatal and long-term consequences. The consequences of DVT include the development of postthrombotic syndrome in 25% to 60% of DVT patients. Despite the clinical importance of venous thrombus resolution, the cellular and molecular mediators involved are poorly understood, and currently there is no molecular therapy to accelerate this process. Several lines of evidence suggest that a complex and interrelated array of molecular signaling processes are involved in the inflammatory vascular remodeling associated with the resolution of DVT. Here, we have identified a role for the tumor suppressor gene p53 in regulating venous thrombus resolution. Using the stasis model of venous thrombosis and resolution in mice, we found that genetic deficiency of p53 or pharmacologic inhibition by pifithrin impairs thrombus resolution and is associated with increased fibrosis and altered expression of matrix metalloproteinase-2. The effect of p53 loss was mediated by cells of the myeloid lineage, resulting in enhanced polarization of the cytokine milieu toward an M1-like phenotype. Furthermore, augmentation of p53 activity using the pharmacological agonist of p53, quinacrine, accelerates venous thrombus resolution in a p53-dependent manner, even after establishment of thrombosis. Together, these studies define mechanisms by which p53 regulates thrombus resolution by increasing inflammatory vascular remodeling of venous thrombi in vivo, and the potential therapeutic application of a p53 agonist as a treatment to accelerate this process in patients with DVT.
Introduction
Deep venous thrombosis (DVT) is a significant clinical problem that affects ∼1 per 1000 individuals annually1 and can cause fatal pulmonary embolism. The standard treatment of DVT is anticoagulants,2,3 such as heparin, thrombin inhibitors, and Xa inhibitors, which prevent further thrombus propagation and pulmonary embolism but do not accelerate the resolution of the existing thrombus.4 Despite the widespread use of anticoagulant therapy, ∼25% to 50% of patients with DVT eventually develop postthrombotic syndrome (PTS),5 characterized by chronic pain, swelling, and leg ulceration. The development of PTS is related to the effectiveness of venous thrombus resolution, as patients with more rapid endogenous thrombus resolution have a lower likelihood of subsequent PTS and better prognosis.6 Furthermore, the majority of patients with PTS have a residual obstructive component to their disorder, suggesting incomplete or maladaptive thrombus resolution.7 Our lack of effective therapy for this common disorder reinforces the need for both improved understanding of the biology of thrombus resolution as well as translational therapy targeting this process.
A number of mechanistic insights into the process of venous thrombus resolution have come from experimental models of venous thrombosis, principally in rodents and mice,8-11 and from the application of elaborate imaging modalities, such as confocal and 2-photon excitation laser-scanning microscopy.12 In mouse models, resolution of a venous thrombus formed after vena cava ligation involves fibrinolysis and infiltration of leukocytes that mediate clot retraction, tissue clearing, fibrosis, and vessel wall remodeling.13 Inflammatory cells are the major source of the proteases, cytokines, and other effector molecules that mediate the resolution process.8 The process is similar to wound healing, with neutrophils present at the early stages14 and macrophages predominating at later stages.15-18 The emerging picture is one of a complex and interrelated array of signaling events involved in the inflammatory vascular remodeling processes required for the resolution of DVT.
The p53 protein orchestrates major signaling events as a transcriptional activator of hundreds of protein-coding target genes via direct binding to nearby p53 response elements and recruitment of transcriptional coactivators. Since its discovery in 1979, the role of the p53 protein as a tumor suppressor in cancer has been studied intensively,19 where it functions as a suppressor of cancer proliferation through the induction of cell-cycle-arrest or apoptosis programs in response to a plethora of different cellular stress signals.20,21 However, it is now apparent that p53’s functions can be manifested in diverse aspects of health and disease, and the consequences of p53 activation can be dramatically different depending on numerous factors and tissue contexts.21 For instance, p53 regulates multiple forms of vascular remodeling, including neointimal hyperplasia,22 atherosclerosis,23,24 and hypoxic pulmonary artery remodeling.25 The emerging roles of p53 in mediating vascular remodeling and in mechanisms of wound healing26 prompted us to explore the involvement of p53 in the processes of DVT and its resolution with the stasis model of venous thrombosis using p53-deficient (Tp53−/−) mice.
We found that targeted p53 deletion or exogenous inhibition of p53 caused impaired venous thrombus resolution, and was associated with increased fibrosis, altered metalloprotease expression, and changes in the local cytokine milieu. This effect of p53 deletion on thrombus resolution was mediated by cells of the myeloid lineage, resulting in enhanced polarization of the cytokine milieu toward an M1-like phenotype. Using a pharmacological agonist of p53, we demonstrate that augmentation of p53 activity accelerates inflammatory vascular remodeling and venous thrombus resolution in a p53-dependent manner, even when administered after establishment of thrombosis, demonstrating a potential translational use to accelerate thrombus resolution in patients with DVT.
Methods
Mouse models
All animal procedures were performed in accordance with federal and institutional animal care and use committee requirements. Detailed methods for mouse models are provided in supplemental Methods (see supplemental Data available on the Blood Web site).
Stasis-induced model of DVT
Stasis-induced venous formation was induced in mice as described previously10 and in supplemental Methods.
Pifithrin treatment
Mice were treated with either vehicle (dimethyl sulfoxide: phosphate-buffered saline) or pifithrin-α (PFT; 2.5 mg/kg per day) daily via intraperitoneal injections. For additional information, see supplemental Methods.
Quinacrine treatment
Mice were treated with quinacrine or sterile water using implanted microinfusion pumps implanted subcutaneously at a dose of 5.0 mg/kg per day. For additional information, see supplemental Methods.
RNA analyses
Experiments were conducted using conventional quantitative polymerase chain reaction [PCR] methodology. For details, see supplemental Methods.
Immunoblot and enzyme-linked immunosorbent assay analyses
Thrombus protein lysates were prepared using T-PER Tissue Protein Extraction Reagent (Pierce) and analyzed as detailed in supplemental Methods.
Gelatin zymography
Thrombus protein lysates were analyzed on Novex 10% Gelatin Zymogram gels (Invitrogen). For additional detail, see supplemental Methods.
Immunohistological analysis
Venous thrombi were prepared for histological analyses, and analyzed by immunostaining and signal quantitation using conventional methods. For additional detail, see supplemental Methods.
Statistics
All data are presented as mean ± standard error. The Student t test was used to compare groups and P < .05 was defined as statistical significance.
Results
Impaired venous thrombus resolution in the absence of p53
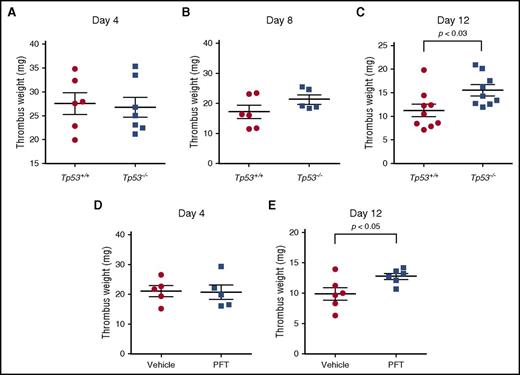
We investigated the role of p53 in the development of stasis-induced venous thrombosis by comparing thrombus formation in Tp53+/+ and Tp53−/− mice after vena cava ligation. This stasis-induced mouse model accurately mimics many of the clinical and pathophysiological features observed in human venous thrombosis (see detailed discussion, supplemental Methods), with the formed thrombi having a laminar structure that is fibrin and red cell rich, and platelet poor.13,27 Venous thrombi developed progressively in both Tp53+/+ and Tp53−/− mice, forming thrombi of similar weights by day 4 after vena cava ligation (Figure 1A). Venous thrombus resolution naturally occurs after day 4 in this model, resulting in a progressive decrease in thrombus weight. At day 8 after vena cava ligation, thrombus weights of both strains showed a progressive decrease, with a trend toward relatively larger (24%) thrombi present in the Tp53−/− mice, indicating a possible delay in thrombus resolution. By day 12, Tp53−/− mice showed significantly larger (38%) thrombi compared with Tp53−/− mice (Figure 1C), indicating that loss of p53 impairs the process of thrombus resolution. To explore this effect further, we used the small-molecule inhibitor of p53 function, PFT.28 C57BL/6 mice were treated daily with vehicle or PFT for 3 days prior to the vena cava ligation and continued to either day 4 or day 12 after surgery. PFT treatment did not affect thrombus formation measured at day 4 after vena cava ligation (Figure 1D), whereas PFT treatment resulted in significantly larger thrombi by day 12 (Figure 1E), similar to the effect observed in the mice lacking the Tp53 gene. These data indicated that impairment of thrombus resolution was a direct result of inhibition of p53 function and was not due to possible physiological or hematological abnormalities that might have arisen in mice lacking p53 since birth.
p53 deficiency enhances venous thrombus resolution. Thrombus weights of Tp53−/− mice compared with Tp53+/+ mice at (A) day 4 (n = 6-7 per group), (B) day 8 (n = 5-6 per group), and (C) day 12 (n = 8-9 per group) after vena cava ligation. Comparison of thrombus weights of C57BL/6 mice treated with vehicle or PFT at day 4 (D) and day 12 (E) after vena cava ligation (n = 5-6 per group).
p53 deficiency enhances venous thrombus resolution. Thrombus weights of Tp53−/− mice compared with Tp53+/+ mice at (A) day 4 (n = 6-7 per group), (B) day 8 (n = 5-6 per group), and (C) day 12 (n = 8-9 per group) after vena cava ligation. Comparison of thrombus weights of C57BL/6 mice treated with vehicle or PFT at day 4 (D) and day 12 (E) after vena cava ligation (n = 5-6 per group).
The absence of p53 does not affect early leukocyte numbers and apoptosis marker expression
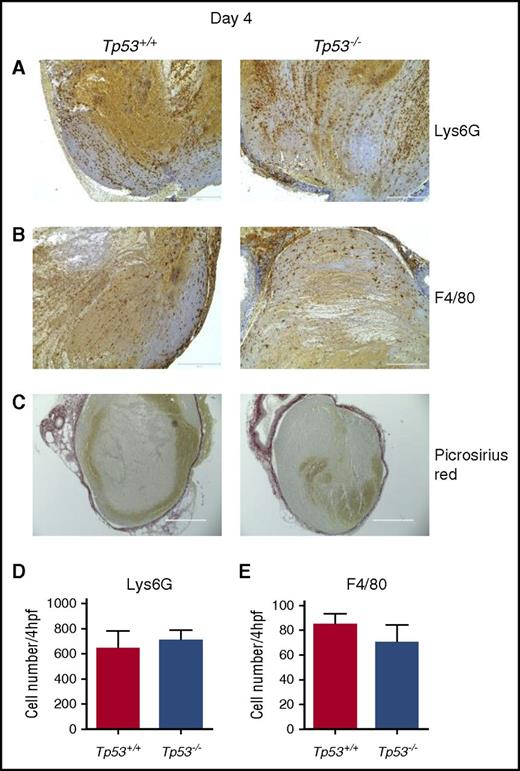
Leukocytes recruited to venous thrombi are key regulators of the inflammatory response important for thrombus resolution.29 Given the strong association between early impaired DVT resolution and decreased leukocyte influx,18 the 4-day time point was examined in detail. The numbers of Lys6G+ neutrophils (Figure 2A,D) and F4/80+ macrophages (Figure 2B,E) in thrombi of both Tp53+/+ and Tp53−/− mice were similar at day 4 after the vena cava ligation. Picrosirius Red staining for collagen showed similar staining surrounding the thrombi in both Tp53+/+ and Tp53−/− mouse strains (Figure 2C), indicating that early collagen deposition, consistent with the formation of a healing matrix, was not impaired by loss of p53. As p53 is a major physiological regulator of apoptosis after tissue injury,21 and apoptotic events ensue immediately after tissue injury and then slowly subside, we examined the expression of proapoptotic markers at the 4-day time point. No differences in the expression of the p53-regulated gene, Bax or changes in poly ADP ribose polymerase cleavage were detected in thrombi from Tp53+/+ and Tp53−/− mice (supplemental Figure 1), indicating that absence of the p53 gene did not significantly affect apoptotic events in the resolving thrombus, but was a result of a p53 function that is independent of its proapoptotic activity.
Characterization of venous thrombi from Tp53+/+and Tp53−/−mice at 4 days after vena cava ligation. Immunohistochemical analysis of intrathrombotic neutrophil accumulation was performed using anti-Lys6G antibodies (A) and macrophage accumulation was analyzed using anti-F4/80 antibodies (B) in venous thrombus samples from Tp53+/+ and Tp53−/− mice. (C) Collagen content in resolving Tp53+/+ and Tp53−/− thrombi at day 4 was determined in histological sections after Picrosirius Red staining; original magnification ×200. Scale bar, 200 μm. Representative results from 4 to 5 independent animals are shown. The numbers of Lys6G+ cells (neutrophils) (D) and F4/80+ cells (macrophages) (E) were determined as described in “Methods.” All values represent the mean ± standard error of the mean (SEM) (n = 4 Tp53+/+ and 5 Tp53−/−).
Characterization of venous thrombi from Tp53+/+and Tp53−/−mice at 4 days after vena cava ligation. Immunohistochemical analysis of intrathrombotic neutrophil accumulation was performed using anti-Lys6G antibodies (A) and macrophage accumulation was analyzed using anti-F4/80 antibodies (B) in venous thrombus samples from Tp53+/+ and Tp53−/− mice. (C) Collagen content in resolving Tp53+/+ and Tp53−/− thrombi at day 4 was determined in histological sections after Picrosirius Red staining; original magnification ×200. Scale bar, 200 μm. Representative results from 4 to 5 independent animals are shown. The numbers of Lys6G+ cells (neutrophils) (D) and F4/80+ cells (macrophages) (E) were determined as described in “Methods.” All values represent the mean ± standard error of the mean (SEM) (n = 4 Tp53+/+ and 5 Tp53−/−).
Reduced MMP-2 expression in thrombi from Tp53−/− mice
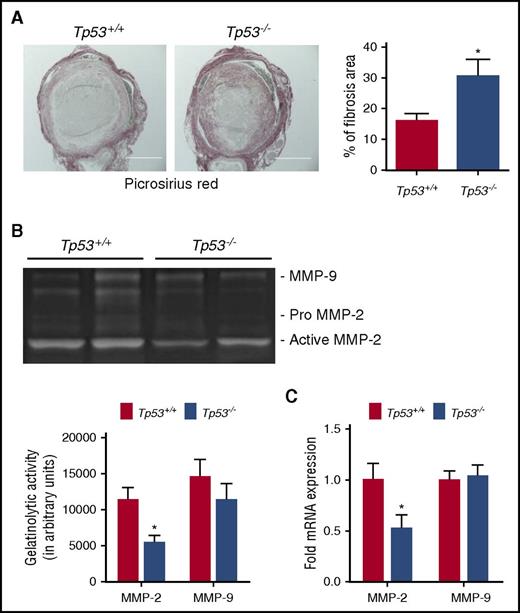
Measurement of active urokinase-type plasminogen activator as an indicator of fibrinolytic activity in thrombi revealed no significant differences between Tp53+/+ and Tp53−/− mice at day 4 or day 8 after vena cava ligation (supplemental Figure 2). As fibrosis and collagen remodeling occur in the inflammatory vascular remodeling of venous thrombi during venous thrombus resolution, we evaluated collagen content in resolving thrombi at the day 12 time point by histopathological analysis. Intrathrombotic collagen was significantly increased at the day 12 time point in Tp53−/− mice compared with thrombi from Tp53+/+ mice (Figure 3A), indicative of enhanced fibrosis. The matrix metalloproteinases 2 and 9 (MMP-2 and MMP-9) play key roles in collagenolysis and matrix remodeling during thrombus resolution. Densitometric analysis of MMP-2 and MMP-9 total gelatinolytic activity indicated significantly reduced levels of MMP-2 in the thrombus of Tp53−/− compared with Tp53+/+ controls at day 8 after vena cava ligation (Figure 3B). Quantitation of MMP-2 messenger RNA (mRNA) expression at day 8, shows a significant reduction in MMP-2 mRNA in the thrombi from Tp53−/− mice compared with Tp53+/+ mice (Figure 3C). Although p53 is known to repress MMP-9 activity in several cell types,30,31 there was no significant difference in MMP-9 activity (Figure 3B), or MMP-9 gene expression (Figure 3C), in thrombi from Tp53−/− compared with Tp53+/+ mice. These observations suggest that p53 might contribute to thrombus resolution by enhancing the expression of MMP-2.
Effect of p53 deficiency on collagen remodeling. (A) Collagen content in resolving Tp53+/+ and Tp53−/− thrombi at day 12 was determined in histological sections after Picrosirius Red staining; original magnification ×100. Scale bar, 400 μm. Representative images from 5 independent mice from each genotype are shown. Collagen content was quantified as a measure of fibrosis as described in “Methods.” All values represent the mean ± SEM (n = 5 Tp53+/+ and 5 Tp53−/−). *P < .05, Tp53+/+ vs Tp53−/−. (B) Representative gel images of intrathrombotic MMP-2 and MMP-9 activities in venous thrombus samples from Tp53+/+ and Tp53−/− mice as measured by gelatin gel zymography (n = 5 and 4 for Tp53+/+ and Tp53−/−, respectively). Gel images were subjected to semiquantitative analysis as described in “Methods.” All values represent the mean ± SEM (n = 4-5 per group). *P < .03, Tp53+/+ vs Tp53−/−. (C) Intrathrombotic mRNA expression of MMP-2 and MMP-9 in Tp53+/+ and Tp53−/− mice at day 8 was determined by quantitative PCR (qPCR). All values represent the mean ± SEM (n = 4 per group). *P < .03, Tp53+/+ vs Tp53−/−.
Effect of p53 deficiency on collagen remodeling. (A) Collagen content in resolving Tp53+/+ and Tp53−/− thrombi at day 12 was determined in histological sections after Picrosirius Red staining; original magnification ×100. Scale bar, 400 μm. Representative images from 5 independent mice from each genotype are shown. Collagen content was quantified as a measure of fibrosis as described in “Methods.” All values represent the mean ± SEM (n = 5 Tp53+/+ and 5 Tp53−/−). *P < .05, Tp53+/+ vs Tp53−/−. (B) Representative gel images of intrathrombotic MMP-2 and MMP-9 activities in venous thrombus samples from Tp53+/+ and Tp53−/− mice as measured by gelatin gel zymography (n = 5 and 4 for Tp53+/+ and Tp53−/−, respectively). Gel images were subjected to semiquantitative analysis as described in “Methods.” All values represent the mean ± SEM (n = 4-5 per group). *P < .03, Tp53+/+ vs Tp53−/−. (C) Intrathrombotic mRNA expression of MMP-2 and MMP-9 in Tp53+/+ and Tp53−/− mice at day 8 was determined by quantitative PCR (qPCR). All values represent the mean ± SEM (n = 4 per group). *P < .03, Tp53+/+ vs Tp53−/−.
p53 regulates expression of inflammatory cytokines and fibrosis in resolving thrombi
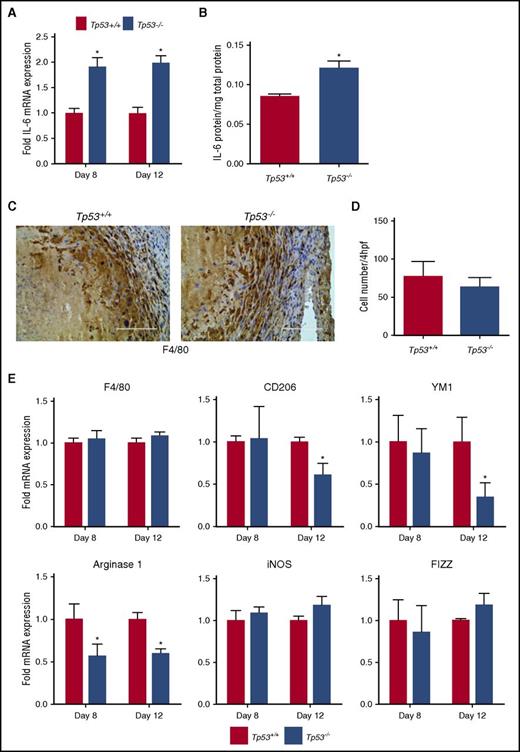
Because p53 regulates the transcription of a number of cytokine genes32,33 that could potentially affect inflammatory vascular remodeling of resolving thrombi, we compared the mRNA expression of various cytokine genes in thrombi from Tp53−/− and Tp53+/+ mice at the 8-day time point. Although there were no significant differences in the expression of interleukin-1β (IL-1β), monocyte chemotactic protein 1 (MCP-1), or tumor necrosis factor α (TNF-α) mRNAs between the 2 groups (supplemental Figure 3), IL-6 mRNA expression was significantly elevated in thrombi from Tp53−/− mice (Figure 4A) and there was a trend for higher expression of interferon γ (IFN-γ) compared with Tp53+/+ controls (supplemental Figure 3), indicative of a proinflammatory environment associated with the Tp53−/− thrombi. Expression of IFN-γ and IL-6 has been identified previously to have detrimental effects on thrombus resolution in animal models, with genetic deletion of IFN-γ resulting in accelerated venous thrombus resolution34 and enhancement of venous thrombus resolution via administration of anti-IL-6 antibodies. IL-6 mRNA expression remained elevated in Tp53−/− thrombi through day 12 (Figure 4A), and was associated with significantly increased IL-6 protein levels in the resolving day 12 thrombus (Figure 4B). Collectively, our data showed that genetic deletion of p53 results in enhanced expression of proinflammatory genes and increased fibrosis that potentially impairs vascular remodeling of venous thrombi.
Effect of absence of p53 on intrathrombotic cytokine expression. (A) Intrathrombotic expression of IL-6 mRNA at day 8 and day 12 after vena cava ligation was determined by qPCR. Values represent the mean ± SEM (n = 3 Tp53+/+ and 4 Tp53−/−). *P < .05, Tp53+/+ vs Tp53−/−. (B) Intrathrombotic expression of IL-6 protein at day 12 was determined by enzyme-linked immunosorbent assay (ELISA). All values represent the mean ± SEM (n = 4 Tp53+/+ and 5 Tp53−/−). **P < .02, Tp53+/+ vs Tp53−/−. (C) Enumeration of intrathrombotic macrophages at day 12 after vena cava ligation by immunohistochemical staining using anti-F4/80 antibody; original magnification ×200. Scale bar, 200 μm. Representative images from 4 independent animals are shown. (D) The number of macrophages was quantified as described in “Methods.” All values represent the mean ± SEM (n = 4 Tp53+/+ and 4 Tp53−/−). (E) Intrathrombotic gene expression of macrophage polarization markers at 8 days (A) and 12 days (B) after vena cava ligation in Tp53+/+ and Tp53−/− animals were determined by qPCR. All values represent the mean ± SEM (n = 3 Tp53+/+ and 4 Tp53−/−). *P < .05, Tp53+/+ vs Tp53−/−. hpf, high-powered field; iNOS, inducible NO synthase.
Effect of absence of p53 on intrathrombotic cytokine expression. (A) Intrathrombotic expression of IL-6 mRNA at day 8 and day 12 after vena cava ligation was determined by qPCR. Values represent the mean ± SEM (n = 3 Tp53+/+ and 4 Tp53−/−). *P < .05, Tp53+/+ vs Tp53−/−. (B) Intrathrombotic expression of IL-6 protein at day 12 was determined by enzyme-linked immunosorbent assay (ELISA). All values represent the mean ± SEM (n = 4 Tp53+/+ and 5 Tp53−/−). **P < .02, Tp53+/+ vs Tp53−/−. (C) Enumeration of intrathrombotic macrophages at day 12 after vena cava ligation by immunohistochemical staining using anti-F4/80 antibody; original magnification ×200. Scale bar, 200 μm. Representative images from 4 independent animals are shown. (D) The number of macrophages was quantified as described in “Methods.” All values represent the mean ± SEM (n = 4 Tp53+/+ and 4 Tp53−/−). (E) Intrathrombotic gene expression of macrophage polarization markers at 8 days (A) and 12 days (B) after vena cava ligation in Tp53+/+ and Tp53−/− animals were determined by qPCR. All values represent the mean ± SEM (n = 3 Tp53+/+ and 4 Tp53−/−). *P < .05, Tp53+/+ vs Tp53−/−. hpf, high-powered field; iNOS, inducible NO synthase.
Deletion of p53 alters the intrathrombi macrophage phenotype
Macrophages are a major source of inflammatory gene expression in the resolving thrombus and produce inflammatory cytokines such as IL-6 and IFN-γ, which orchestrate tissue remodeling and revascularization.34,35 The numbers of F4/80+ macrophages present in the resolving thrombi of Tp53−/− mice and Tp53+/+ mice were similar at the day 12 time point (Figure 4C-D) and there were no significant differences in F4/80 mRNA expression between the 2 groups (Figure 4E first panel), suggesting that loss of p53 does not influence macrophage infiltration into the resolving thrombus. The increased IL-6 levels suggested that macrophage function in the resolving thrombus may be affected by the loss of p53. Indeed, examination of expression of markers indicative of macrophage polarization in the resolving thrombi at 8 and 12 days after vena cava ligation showed significantly reduced expression of Arginase 1, as well as reduced CD206 and YM1 gene expression by day 12 in Tp53−/− compared with Tp53+/+ thrombi (Figure 4E). Together, these data suggested that loss of p53 alters macrophage polarization away from the M2-like phenotype during venous thrombus resolution.
Myeloid-specific deletion of p53 impairs venous thrombus resolution and alters macrophage polarization
To explore the functional consequences of loss of p53 in the myeloid lineage during venous thrombus resolution, we crossed mice with a floxed Tp53 allele with mice carrying the Cre transgene under the control of the myeloid-specific LysM promoter to generate Tp53fl/flLysMCre+/+ mice (referred to here as Mp53−/−) and their associated wild-type littermate control Tp53fl/flLysMCre−/− mice (referred to here as Mp53+/+). This strategy resulted in the efficient deletion of p53 from the myeloid lineage, as shown by the presence of the recombined allele only in fully differentiated bone marrow–derived macrophages (supplemental Figure 4A) and by the decreased p53 mRNA in thioglycolate-elicited peritoneal macrophages (supplemental Figure 4B).
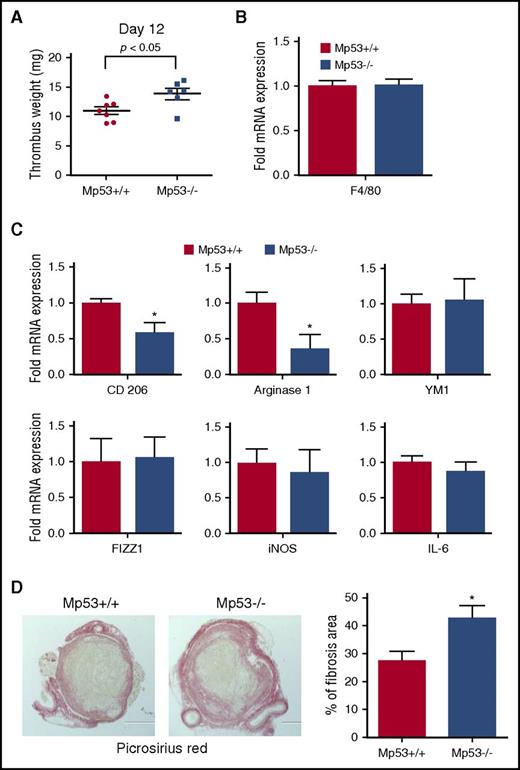
The role of myeloid-specific p53 in the development of stasis-induced venous thrombosis was investigated by vena cava ligation in Mp53−/− and Mp53+/+ animals. Mp53−/− mice, which lack p53 expression in the myeloid lineage-specific cells, had significantly larger thrombi (29%) by day 12 after vena cava ligation compared with the wild-type littermate controls (Figure 5A), similar to the larger thrombi found in mice with global deletion of the p53 gene (Figure 1C). Macrophage recruitment to the resolving thrombi in Mp53+/+ and Mp53−/− animals was similar (Figure 5B). Examination of the expression of markers of macrophage polarization in the thrombi of the mice at day 12 showed significantly reduced expression of the M2-like macrophage polarization marker genes, CD206 and Arginase 1 (Figure 5C), indicating that deletion of p53 in myeloid cells repressed the M2-like phenotype, similar to global deletion of p53. We did not detect any difference in the levels of IL-6 between the thrombi from Mp53+/+ and Mp53−/− animals (Figure 5C last panel), suggesting that the increased IL-6 associated with global deletion of p53 may originate in a nonmyeloid cell compartment. Picrosirius Red staining revealed increased collagen content in the Mp53−/− thrombi (Figure 5D), similar to the increased collagen content in thrombi of mice with global deletion of p53 (Figure 3C). CD206+ M2-like macrophages are implicated as the major cell type involved in collagen turnover in vivo.36 The reduction in CD206+ macrophages in the resolving Mp53−/− thrombi and in mice with global deletion of p53 may thus be responsible for the enhanced fibrosis accompanying the impaired venous thrombus resolution associated with p53 deficiency.
Myeloid cell-specific p53 deficiency impairs venous thrombus resolution. (A) Thrombus weights of Mp53+/+ and Mp53−/− mice 12 days after vena cava ligation (n = 7 Tp53+/+ and 6 Tp53−/−). *P < .05, Mp53+/+ vs Mp53−/− mice. (B) Intrathrombotic F4/80 mRNA expression at 12 days after vena cava ligation in Mp53+/+ and Mp53−/− mice, determined by qPCR. All values represent the mean ± SEM (n = 4 Tp53+/+ and 4 Tp53−/−). (C) Intrathrombotic mRNA expression of macrophage polarization markers at 12 days after vena cava ligation in Mp53+/+ and Mp53−/− mice, as determined by qPCR. All values represent the mean ± SEM (n = 4 Tp53+/+ and 4 Tp53−/−). *P < .05, Mp53+/+ vs Mp53−/−. (D) Histochemical analysis of intrathrombotic collagen content by Picrosirius Red staining at day 12 after vena cava ligation; original magnification ×100. Representative images from 4 to 5 independent mice of each genotype are shown. The intrathrombus collagen area was quantitated as a measure of fibrosis as described in “Methods.” All values represent the mean ± SEM (n = 5 Tp53+/+ and 4 Tp53−/− n= 4-5). *P < .05, p53 Mp53+/+ vs Mp53−/−.
Myeloid cell-specific p53 deficiency impairs venous thrombus resolution. (A) Thrombus weights of Mp53+/+ and Mp53−/− mice 12 days after vena cava ligation (n = 7 Tp53+/+ and 6 Tp53−/−). *P < .05, Mp53+/+ vs Mp53−/− mice. (B) Intrathrombotic F4/80 mRNA expression at 12 days after vena cava ligation in Mp53+/+ and Mp53−/− mice, determined by qPCR. All values represent the mean ± SEM (n = 4 Tp53+/+ and 4 Tp53−/−). (C) Intrathrombotic mRNA expression of macrophage polarization markers at 12 days after vena cava ligation in Mp53+/+ and Mp53−/− mice, as determined by qPCR. All values represent the mean ± SEM (n = 4 Tp53+/+ and 4 Tp53−/−). *P < .05, Mp53+/+ vs Mp53−/−. (D) Histochemical analysis of intrathrombotic collagen content by Picrosirius Red staining at day 12 after vena cava ligation; original magnification ×100. Representative images from 4 to 5 independent mice of each genotype are shown. The intrathrombus collagen area was quantitated as a measure of fibrosis as described in “Methods.” All values represent the mean ± SEM (n = 5 Tp53+/+ and 4 Tp53−/− n= 4-5). *P < .05, p53 Mp53+/+ vs Mp53−/−.
The p53 agonist quinacrine enhances venous thrombus resolution by inflammatory vascular remodeling
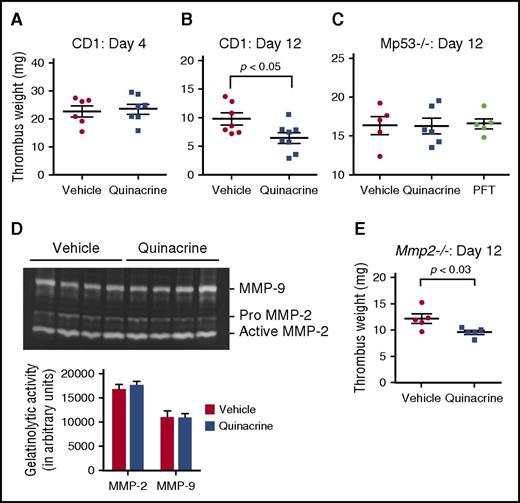
These data identify a key role for p53 in the stimulation of inflammatory processes important for thrombus resolution by, and further suggest the possibility that pharmacological activation of p53 could be beneficial for, enhancing this process. Based on studies that the antimalarial drug and p53 agonist quinacrine stabilizes p53 and induces p53-dependent and p53-independent tumor cell death,37 we examined whether quinacrine could potentially activate p53 function to enhance venous thrombus resolution. Outbred CD-1 mice were continuously infused with quinacrine or vehicle alone starting at 3 days prior to the vena cava ligation surgery through 12 days postligation. Venous thrombi developed progressively in both quinacrine- and vehicle-pretreated cohorts, forming thrombi of similar weights at day 4 after vena cava ligation (Figure 6A). At day 12 after vena cava ligation, quinacrine-treated animals had significantly smaller thrombi (by ∼40%) compared with the animals treated with the vehicle control (Figure 6B), indicating that quinacrine treatment was highly effective in accelerating the inflammatory vascular remodeling processes important for venous thrombus resolution.
Quinacrine enhances venous thrombus resolution through myeloid p53-dependent and MMP-2-independent manners. (A) Thrombus weights of vehicle- and quinacrine-treated CD1 mice at (A) day 4 (n = 6-7 per group) and (B) day 12 (n = 5-6 per group), after vena cava ligation. (C) Thrombus weights of Mp53−/− mice at 12 days after vena cava ligation are unaffected after pretreatment with quinacrine (n = 6) or PFT (n = 5) compared with vehicle (n = 5). (D) Representative gel images of intrathrombotic MMP-2 and MMP-9 activities measured by gelatin gel zymography in day 8 venous thrombus samples from CD1 mice pretreated with vehicle (n = 4) or quinacrine (n = 4). Gel images were subjected to semiquantitative analysis as described in “Methods.” All values represent the mean ± SEM. (E) Thrombus weights of Mmp2−/− mice at 12 days after vena cava ligation following pretreatment with vehicle or quinacrine (n = 5 vehicle and 5 quinacrine).
Quinacrine enhances venous thrombus resolution through myeloid p53-dependent and MMP-2-independent manners. (A) Thrombus weights of vehicle- and quinacrine-treated CD1 mice at (A) day 4 (n = 6-7 per group) and (B) day 12 (n = 5-6 per group), after vena cava ligation. (C) Thrombus weights of Mp53−/− mice at 12 days after vena cava ligation are unaffected after pretreatment with quinacrine (n = 6) or PFT (n = 5) compared with vehicle (n = 5). (D) Representative gel images of intrathrombotic MMP-2 and MMP-9 activities measured by gelatin gel zymography in day 8 venous thrombus samples from CD1 mice pretreated with vehicle (n = 4) or quinacrine (n = 4). Gel images were subjected to semiquantitative analysis as described in “Methods.” All values represent the mean ± SEM. (E) Thrombus weights of Mmp2−/− mice at 12 days after vena cava ligation following pretreatment with vehicle or quinacrine (n = 5 vehicle and 5 quinacrine).
Enhanced venous thrombus resolution by quinacrine is dependent on myeloid-derived p53
To determine the specificity of the quinacrine treatment of activation of p53 produced by myeloid cells in the venous thrombi, Mp53−/− mice were infused similarly with quinacrine or vehicle alone starting at 3 days prior to vena cava ligation surgery through 12 days postligation. Analysis of thrombus weights at day 12 after vena cava ligation showed no difference in thrombus weights from Mp53−/− mice treated with quinacrine or vehicle alone, demonstrating that loss of myeloid-derived p53 abrogated the effect of quinacrine on acceleration of venous thrombus resolution (Figure 6C). Similarly, any nonspecific effects of the p53 inhibitor PFT in Figure 1E were assessed by treating Mp53−/− mice with PFT as before. There was no difference in thrombus weights at day 12 (Figure 6C), demonstrating the action of PFT is p53-specific.
Intrathrombus expression of the macrophage marker F4/80 (supplemental Figure 5A) and macrophage polarization markers (supplemental Figure 5B) were not altered by quinacrine treatment compared with vehicle alone in the Mp53−/− mice. These data demonstrate that the effect of quinacrine on acceleration of venous thrombus resolution is dependent on myeloid-derived p53. Thus, it is likely that myeloid-derived p53-regulated molecular mediators play a significant role in quinacrine-induced acceleration of inflammatory vascular remodeling during venous thrombus resolution.
Acceleration of venous thrombus resolution by quinacrine is MMP-2 independent and associated with increased intrathrombus M2-like macrophage phenotype
MMP-2 is a potential downstream effector of p53 function38 and we found reduced MMP-2 activity associated with thrombi from Tp53−/− compared with Tp53+/+ mice (Figure 3D-E). However, gelatin zymography showed no significant difference in day 8 intrathrombus MMP-2 activity between quinacrine-treated and vehicle-treated CD-1 mice (Figure 6D). Moreover, quinacrine treatment of Mmp2−/− mice resulted in accelerated thrombus resolution relative to vehicle-treated mice by ∼21% (Figure 6E), similar to quinacrine treatment of CD-1 mice. These data indicate that quinacrine activates p53 and downstream mediators other than MMP-2 that mediate its beneficial effects on vascular remodeling during venous thrombus resolution.
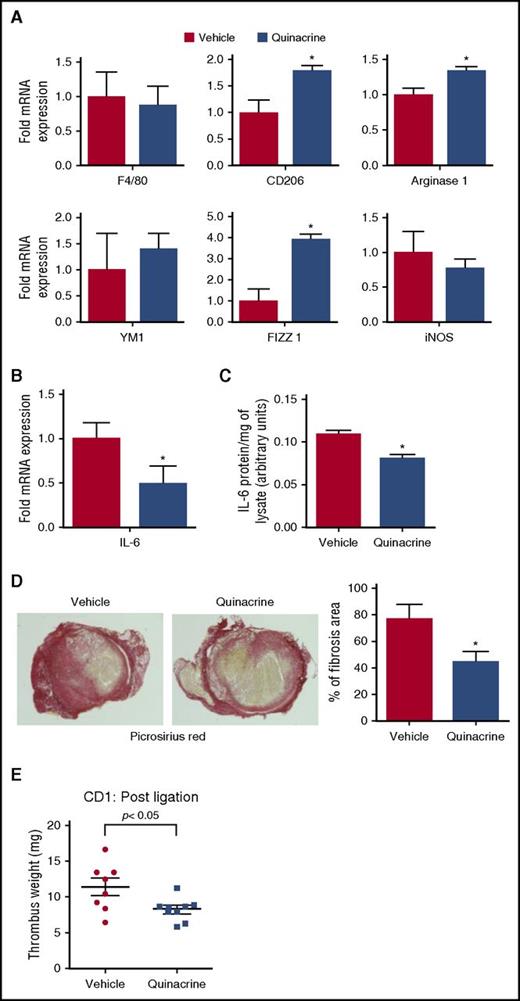
Thrombi from CD-1 mice treated with infused quinacrine were further analyzed for cytokine expression, fibrosis, macrophage numbers, and macrophage polarization markers. The mRNA levels of various cytokines (TNF-α, IFN-γ, IL-1β, IL-4) at day 12 after vena cava ligation were not significantly different between the vehicle- and quinacrine-pretreated groups (supplemental Figure 6) and macrophage recruitment, as assessed by F4/80 mRNA expression, did not differ between the 2 groups (Figure 7A first panel). In contrast, mRNA expression of macrophage polarization markers revealed that quinacrine-pretreated animals had significantly higher expression of CD206, FIZZ 1, and Arginase 1 at day 12 after vena cava ligation, indicating an increased M2-like macrophage phenotype (Figure 7A). Furthermore, quinacrine treatment resulted in significantly lower levels of IL-6 mRNA and protein in the resolving thrombi (Figure 7B-C), in direct contrast to the elevated levels of IL-6 observed with the impaired thrombus resolution associated with global p53 deficiency in Tp53−/− mice (Figure 4A-B). Collagen content, as measured by Picrosirius Red staining in thrombi of quinacrine-treated mice was significantly reduced compared with vehicle-treated mice (Figure 7D), indicating decreased fibrosis that is consistent with elevated CD206 expression and reduced collagen deposition and/or increased collagen turnover. These data show that the acceleration of venous thrombus resolution via p53 activation by quinacrine is mediated through increased M2-like macrophage polarization.
Quinacrine treatment alters intrathrombotic macrophage polarization and fibrosis. (A) Intrathrombotic mRNA expression of macrophage polarization markers at day 12 after vena cava ligation following treatment with vehicle or quinacrine, as determined by qPCR. All values represent the mean ± SEM (n = 4 vehicle and 4 quinacrine). *P < .05, vehicle vs quinacrine. (B) Intrathrombotic expression of IL-6 mRNA at day 12 after vena cava ligation following treatment with vehicle or quinacrine by qPCR. Values represent the mean ± SEM (n = 4 vehicle and 4 quinacrine). *P < .05, vehicle vs quinacrine. (C) Intrathrombotic expression of IL-6 protein at day 12 was determined by ELISA. All values represent the mean ± SEM (n = 4 vehicle and 4 quinacrine). **P < .05, vehicle and vs quinacrine. (D) Histochemical analysis of intrathrombotic collagen content by Picrosirius Red staining after treatment with vehicle or quinacrine at day 12 after vena cava ligation; original magnification ×100. Representative images from 4 to 5 independent mice of each group. The intrathrombus collagen area was quantified as a measure of fibrosis as described in “Methods.” All values represent the mean ± SEM (n = 4 vehicle and 5 quinacrine). *P < .05, vehicle vs quinacrine treated. (E) Effect of quinacrine treatment on established thrombi. CD-1 mice were treated with vehicle or quinacrine from 3 days post–vena cava ligation, and thrombus weights measured at 12 days after vena cava ligation (n = 8 vehicle and 9 quinacrine). *P < .05, vehicle vs quinacrine.
Quinacrine treatment alters intrathrombotic macrophage polarization and fibrosis. (A) Intrathrombotic mRNA expression of macrophage polarization markers at day 12 after vena cava ligation following treatment with vehicle or quinacrine, as determined by qPCR. All values represent the mean ± SEM (n = 4 vehicle and 4 quinacrine). *P < .05, vehicle vs quinacrine. (B) Intrathrombotic expression of IL-6 mRNA at day 12 after vena cava ligation following treatment with vehicle or quinacrine by qPCR. Values represent the mean ± SEM (n = 4 vehicle and 4 quinacrine). *P < .05, vehicle vs quinacrine. (C) Intrathrombotic expression of IL-6 protein at day 12 was determined by ELISA. All values represent the mean ± SEM (n = 4 vehicle and 4 quinacrine). **P < .05, vehicle and vs quinacrine. (D) Histochemical analysis of intrathrombotic collagen content by Picrosirius Red staining after treatment with vehicle or quinacrine at day 12 after vena cava ligation; original magnification ×100. Representative images from 4 to 5 independent mice of each group. The intrathrombus collagen area was quantified as a measure of fibrosis as described in “Methods.” All values represent the mean ± SEM (n = 4 vehicle and 5 quinacrine). *P < .05, vehicle vs quinacrine treated. (E) Effect of quinacrine treatment on established thrombi. CD-1 mice were treated with vehicle or quinacrine from 3 days post–vena cava ligation, and thrombus weights measured at 12 days after vena cava ligation (n = 8 vehicle and 9 quinacrine). *P < .05, vehicle vs quinacrine.
Quinacrine treatment enhances thrombus resolution in already formed thrombi
The ability of quinacrine to enhance the inflammatory vascular remodeling associated with venous thrombus resolution and suppress fibrosis makes it an attractive drug candidate for the treatment of DVT in human patients. Patients with DVT are diagnosed after the thrombosis is established and any treatment begins thereafter, and thus we investigated the effect of quinacrine treatment on established thrombi. CD-1 mice were treated with quinacrine from 3 days post–vena cava ligation, a time when thrombus formation is near complete. Measurement of thrombus weights showed that quinacrine treatment resulted in significantly accelerated thrombus resolution similar to the findings obtained after pretreatment with quinacrine, with significantly smaller thrombi (by ∼27%) by day 12 compared with the vehicle-treated group (Figure 7E). These data show that transient pharmacological activation of p53 function in formed thrombi is sufficient to accelerate inflammation-mediated vascular remodeling of venous thrombi to enhance thrombus resolution.
Discussion
Venous thrombus resolution is a critically important multistep process involving fibrinolysis, tissue clearing, angiogenesis, and vessel wall remodeling. Despite recent advancements in studies of venous thrombus formation and resolution, our understanding of the molecular mediators involved in these processes is limited. Here, using an experimental stasis model of venous thrombosis, we have identified a novel function for p53 in thrombus resolution. We find that p53 activity in cells of the myeloid lineage is required for thrombus resolution, and that absence of p53 induces a decrease in the M2-like proinflammatory state required for effective thrombus resolution. Pharmacological augmentation of p53 activity using the p53 agonist quinacrine accelerates thrombus resolution even of established thrombi in a p53-dependent manner.
Collagenolysis is a key event in the inflammatory vascular remodeling processes associated with venous thrombus resolution.8 The increased collagen content in thrombi of Tp53−/− compared with the Tp53+/+ mice would indicate that enhanced collagen synthesis and/or decreased collagen turnover contribute to the impaired thrombus resolution associated with p53 deficiency. Elevated IL-6 expression in Tp53−/− thrombi is consistent with increased collagen synthesis, as p53 is reported to repress the IL-6 gene promoter39 and the inhibition of IL-6 using an anti-IL-6 antibody reduces thrombus size and vein wall collagen deposition in stasis venous thrombosis.40 In addition, CD206+ M2-like macrophages play a critical role in mediating collagen turnover.36 The significant reduction in intrathrombus CD206 expression associated with myeloid-specific deletion of p53 as well as global deletion of p53, and the increased CD206 associated with quinacrine treatment, implicates p53 in the regulation of CD206 expression in macrophages that control collagen content and fibrosis during inflammatory vascular remodeling of venous thrombi.
Depletion of p53 in myeloid lineage cells was responsible for impaired venous thrombus resolution, suggesting that p53 loss from macrophages may be responsible for the observed phenotype. Several lines of evidence indicate that monocytes are recruited into venous thrombi and increasing macrophage numbers are associated with enhanced thrombus resolution,17 but the contribution of macrophage polarization to venous thrombus resolution is unknown. Macrophages can exist in dynamic proinflammatory (M1) or anti-inflammatory (M2) polarization states depending on the tissue environment.41 Whereas M1-polarized macrophages are associated with the initial stage of tissue injury and secrete cytokines and other factors that amplify the inflammatory response, M2-polarized macrophages are generally involved in the tissue clearing and dissipation of inflammation. Our data demonstrate that deletion of p53 reduces the M2-like environment in the resolving thrombus, and the opposite effect is observed when p53 activity is augmented by quinacrine. Although p53 has been shown recently to modulate the polarization of macrophages in a murine model of renal ischemia-reperfusion,42 to our knowledge, this is the first observation of macrophage phenotype changes during the vascular remodeling associated with venous thrombosis resolution.
Augmentation of p53 activity with quinacrine starting 3 days prior to vena cava ligation or 3 days post–vena cava ligation significantly enhanced venous thrombus resolution. Although quinacrine is reported to have multiple activities independent of p53,43 the absence of an effect of quinacrine in Tp53−/− mice indicates that its activity in this context is p53-specific. There are only very few compounds that have been shown to enhance venous thrombus resolution. Oral inhibition of P-selectin by the small molecule PSI-421 resulted in greater vein reopening in a stasis-induced DVT model,44 and oral as well as subcutaneous delivery of the PAI-1 inhibitor, tiplaxtinin, in a rat stenosis model of venous thrombosis showed a reduction in thrombus weight.45 Clinical trials testing these molecules in human patients would be required to determine their safety and efficacy in resolving human venous thrombi. In contrast, quinacrine and other acridine derivatives are used currently in human patients for treatment of multiple diseases. Quinacrine has been used as an antimalarial agent since the 1930s and has also been used for the treatment of tapeworm infections,46 giardiasis,47 and lupus erythematosus.48 Most recently, quinacrine has shown promise in the treatment of Creutzfeldt-Jakob disease.49 Thus, treatment of patients presenting with DVT with quinacrine for a short period to minimize the side effects represents a clinically viable option.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Oussama Benalla for assistance with collagen staining and quantitation, Grazyna Zaidel of the VA Baltimore Research Histology Core Laboratory for the immunohistochemical staining of tissue specimens, and Abatar Paudel for general technical assistance.
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grant R01 HL083917 (R.S.), and also in part by National Institutes of Health, National Cancer Institute grant T32 CA154274-01 (M.H.H.) and award number 1I01BX001921 (T.M.A.) from the Biomedical Laboratory Research & Development Service of the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development.
Authorship
Contribution: R.S., S.M., and T.M.A. were involved in the study design; S.M., M.H.H., and K.P.N. conducted the research; S.M. analyzed the data and planned and conducted the statistical analysis; S.M., T.M.A., and R.S. wrote and critically revised the manuscript; and all authors approved of the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Rajabrata Sarkar, Department of Surgery, University of Maryland School of Medicine, Room S10B00, 22 S. Greene St, Baltimore, MD 21201; e-mail: rsarkar@smail.umaryland.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal