Key Points
Differential diagnosis of pre-PMF and overt PMF by 2016 WHO criteria underscores uniqueness in disease presentation and outcome.
Patterns of driver and nondriver myeloid gene mutations contribute to prognosis in both pre-PMF and overt PMF.
Abstract
The 2016 revision of the World Health Organization (WHO) classification of myeloproliferative neoplasms defines 2 stages of primary myelofibrosis (PMF): prefibrotic/early (pre-PMF) and overt fibrotic (overt PMF) phase. In this work, we studied the clinical and molecular features of patients belonging to these categories of PMF. The diagnosis of 661 PMF patients with a bone marrow biopsy at presentation was revised according to modern criteria; clinical information and annotation of somatic mutations in both driver and selected nondriver myeloid genes were available for all patients. Compared with pre-PMF, overt PMF was enriched in patients with anemia, thrombocytopenia, leukopenia, higher blast count, symptoms, large splenomegaly, and unfavorable karyotype. The different types of driver mutations were similarly distributed between the 2 categories, whereas selected mutations comprising the high mutation risk (HMR) category (any mutations in ASXL1, SRSF2, IDH1/2, EZH2) were more represented in overt PMF. More patients with overt PMF were in higher International Prognostic Scoring System risk categories at diagnosis, and the frequency increased during follow-up, suggesting greater propensity to disease progression compared with pre-PMF. Median survival was significantly shortened in overt PMF (7.2 vs 17.6 years), with triple negativity for driver mutations and presence of HMR mutations representing independent predictors of unfavorable outcome. The findings of this “real-life” study indicate that adherence to 2016 WHO criteria allows for identification of 2 distinct categories of patients with PMF where increased grades of fibrosis are associated with more pronounced disease manifestations, adverse mutation profile, and worse outcome, overall suggesting they might represent a phenotypic continuum.
Medscape Continuing Medical Education online

In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and the American Society of Hematology. Medscape, LLC is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.00 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 3272.
Disclosures
CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, owns stock, stock options, or bonds from Alnylam, Biogen, and Pfizer. Editor Bob Löwenberg and the authors declare no competing financial interests.
Learning objectives
Upon completion of this activity, participants will be able to:
Compare clinical manifestations for patients with pre–primary myelofibrosis (pre-PMF) vs those with overt PMF according to World Health Organization criteria, based on a large clinical sample.
Compare molecular and genetic profiles for patients with pre-PMF vs those with overt PMF.
Determine outcomes for patients with pre-PMF vs those with overt PMF.
Release date: June 15, 2017; Expiration date: June 15, 2018
Introduction
The revised 2016 World Health Organization (WHO) classification of myeloid neoplasms dictated distinct criteria for prefibrotic/early primary myelofibrosis (pre-PMF) and overt fibrotic PMF (overt PMF).1 Criteria mainly rely on bone marrow (BM) morphology (with megakaryocyte proliferation and atypia in both diseases, and increased age-adjusted cellularity with granulocyte proliferation and often decreased erythropoiesis in pre-PMF) and fibrosis grade (grade 0-1 indicates pre-PMF and grade 2-3 overt PMF); accordingly, grade 1 fibrosis is included in the pre-PMF category. This contrasts with the 2008 WHO classification where criteria did not explicitly define the grade of fibrosis,2 thereby resulting in variable proportions of patients with initial fibrosis included among overt PMF. Finally, peripheral blood leukoerythroblastosis constitutes a minor diagnostic criterion of overt PMF in the 2016 WHO classification, whereas anemia, leukocytosis, increased lactate dehydrogenase (LDH) and palpable splenomegaly may be present in both diseases.1
The existence of pre-PMF as a separate entity and its differentiation from strictly WHO-defined essential thrombocythemia (ET) has been debated for the last several years,3 sometimes with contrasting results.4-7 A low interobserver concordance in applying the WHO-based histopathology criteria for pre-PMF was questioned by some experts,6-8 whereas others clearly delineated the reproducibility of those criteria and the clinical relevance of adopting the diagnostic concept of pre-PMF.9-11 In the largest multicenter study, which included 1104 patients with a diagnosis of ET who underwent revision of their diagnostic biopsies (resulting in 16% of them to be reclassified as pre-PMF), significant differences were found in the occurrence of bleeding,12 rate of death, progression to overt myelofibrosis, and transformation to leukemia, signifying the relevance of differentiating pre-PMF from ET.12
In this study, we aimed at assessing, in a real-life setting, the importance of distinguishing pre-PMF and overt PMF, as delineated by modern WHO criteria, concerning the clinical and hematologic presentation, the molecular profile, and the outcome. The data shown here indicate that pre-PMF and overt PMF are distinct diseases in terms of presentation and outcome, thereby reinforcing the appropriateness of making such a distinction in the clinical practice; at the same time, current findings support the concept that pre-PMF and overt PMF are aligned along a continuum where higher grades of fibrosis are associated with more advanced forms, more complex genetic background, and less favorable outcome.
Materials and methods
Study population
Clinical and hematologic information of 787 patients with a diagnosis of PMF was collected from 5 tertiary Italian centers (Florence, 2 in Pavia, Bergamo, Varese) belonging to the cooperative group Associazione Italiana per la Ricerca sul Cancro (AIRC) Gruppo Italiano Malattie Mieloproliferative (AGIMM). The study was performed in accordance with the Declaration of Helsinki after approval by ethical committees; informed consent was obtained. Histopathology, hematologic, and clinical data were reviewed, and diagnoses were attributed to pre-PMF and overt PMF based on the revised 2016 WHO criteria. Histopathology analysis was performed locally, blinded of patient’s history and clinical information except for sex and age. A total of 661 patients (84%) with adequate follow-up and data were finally included. Clinical and hematologic information were coincident (±6 months) with the diagnostic biopsy.
Mutation analysis
Analysis was performed on DNA from peripheral blood granulocytes collected at diagnosis or within 1 year. JAK2V617F mutation was assessed by real-time quantitative polymerase chain reaction (PCR); for MPL mutations, high-resolution melting analysis and bidirectional Sanger sequencing were used. MPL515x indicates any mutation at codon 515.13 Calreticulin (CALR) mutations were identified by capillary electrophoresis and bidirectional sequencing, and classified as type 1/type 1-like or type 2/type 2-like.14,15 Patients lacking mutations in the 3 driver genes were defined as “triple negative” (TN). A next-generation sequencing (NGS) approach with the PGM Ion Torrent platform was used to detect mutations across the entire coding region of EZH2 and ASXL1, and mutation hotspots for IDH1, IDH2, and SRSF2. A high mutation risk (HMR) and low mutation risk (LMR) status was defined, respectively, by the presence of at least 1 mutated gene or the absence of any mutation.16,17 In case of variants not previously reported, only those considered potentially damaging by Polyphen and the SIFT algorithm (http://genetics.bwh.harvard.edu/pph2/) were included in the database. Genotyping for driver mutations was performed locally, whereas analysis of HMR mutations was centralized (Florence).
Statistical analysis
Numerical variables were summarized by their median and range, and categorical variables by count and relative frequency (percentage). Patient characteristics were compared with the χ2 test or the Fisher exact test for categorical variables. Differences in the distribution of continuous variables between categories were analyzed by Mann-Whitney (2 groups) or Kruskal-Wallis (3 or more groups) tests. The cumulative incidence of anemia, thrombocytopenia, leukocytosis, transfusion dependency, acquisition of constitutional symptoms and peripheral blood blasts, and leukemic transformation was estimated with a competing risk approach. The cumulative probability of overall survival (OS) and leukemia free-survival (LFS) was estimated using the Kaplan-Meier method. Patients undergoing stem cell transplantation were censored at the time of the procedure. Differences in OS between the groups were compared by a log-rank test in univariate analysis. Multivariate analysis was carried out by Cox regression. Cox proportional hazard models were used to calculate hazard ratio (HR) and the 95% confidence interval (95% CI). A P < .05 was considered statistically significant. The IBM Statistical Package for Social Sciences (SPSS) statistics v23 was used.
Results
Clinical, hematologic, and molecular characteristics of study population
This study included 661 patients with PMF, of whom 278 (42%) were classified as pre-PMF and 383 (58%) as overt PMF according to the 2016 WHO criteria. A total of 201 patients (30.4%) previously classified as primary-MF with fibrosis grade 1 according to the WHO 2008 criteria were now included in the category of pre-PMF, whereas no patients were moved from prefibrotic-PMF to overt PMF.
The main clinical and hematologic characteristics are reported in Table 1, and differences were noted. Compared with overt PMF, patients with pre-PMF were more frequently female (44% vs 35%; P = .013), younger (median, 57 years vs 64 years; P < .0001), and had higher hemoglobin (P < .0001), leukocyte (P = .009), and platelet counts (P < .0001); accordingly, patients with anemia and thrombocytopenia, 2 adverse risk factors in the International Prognostic Scoring System (IPSS)/Dynamic International Prognostic Scoring System (DIPSS) (anemia) and DIPSS-Plus (anemia and thrombocytopenia) prognostic scores,18-20 were enriched in overt PMF. In detail, 13.3% and 6.8% of patients with pre-PMF were anemic and thrombocytopenic, respectively, compared with 35.5% and 17.5% of overt PMF (P < .0001 for both). The percentage of patients with leukocytosis (>25 × 109/L) was similar in the 2 groups, whereas patients with leukocytes <4 × 109/L (an adverse risk factor in the Lille score21 ) were more frequent in overt PMF (14.9% vs 3.6% in pre-PMF; P < .0001). Peripheral blood blasts ≥1% were found in 11.9% of pre-PMF and 25.8% overt PMF (P < .0001). Although most patients in both cohorts displayed abnormal LDH value, the median LDH level was significantly higher in overt PMF (669 U/L vs 388 U/L in pre-PMF; P < .0001). Patients with overt PMF were more frequently symptomatic (33.7% vs 20.5%) and had larger spleen (>10 cm palpable from the costal margin; 24.0% vs 10.4%) than patients with pre-PMF (P < .0001 for all contrasts). Cytogenetic information was available in 50.2% of the entire series, 54% of pre-PMF, and 49% overt PMF patients. An abnormal karyotype was twice more frequent in overt PMF (38% vs 18%; P < .0001); the unfavorable karyotype category20 (Table 1) was also more represented in overt PMF (12.0% vs 4.0%; P = .006). According to the IPSS criteria, patients with pre-PMF were included largely in the lower-risk categories (74.8%) whereas most patients with overt PMF were in the higher-risk categories (48.0%; P < .0001).
Clinical and hematologic characteristics of study patients stratified according to the diagnosis of pre-PMF and overt PMF, based on the revised 2016 WHO criteria
| Variables* . | Pre-PMF, N = 278 . | Overt PMF, N = 383 . | P . |
|---|---|---|---|
| Male patients, n (%) | 156 (56.1) | 249 (65.0) | .013 |
| Age | |||
| Median (range), y | 56.6 (18.0-90.3) | 63.6 (14.0-89.8) | <.0001 |
| >65 y, n (%) | 99 (35.6) | 179 (46.7) | .003 |
| Hemoglobin | |||
| Median (range), g/L | 129 (107-175) | 108 (47-150) | <.0001 |
| <100 g/L, n (%) | 37 (13.3) | 136 (35.5) | <.0001 |
| Leukocytes | |||
| Median (range), ×109/L | 9.1 (1.5-150) | 8.2 (1.4-109.0) | .009 |
| >25 × 109/L, n (%) | 18 (6.5) | 31 (8.1) | .252 |
| <4.0 × 109/L, n (%) | 10 (3.6) | 57 (14.9) | <.0001 |
| Platelets | |||
| Median (range), ×109/L | 488 (310-1500) | 249 (19-3279) | <.0001 |
| <100 × 109/L, n (%) | 19 (6.8) | 67 (17.5) | <.0001 |
| Circulating blasts, ≥1%, n (%) | 33 (11.9) | 99 (25.8) | <.0001 |
| LDH, N = 290 | |||
| Median (range), U/L | 388 (127-2806) | 669 (130-2643) | <.0001 |
| >Normal range, n (%) | 98 (81.7) | 159 (93.5) | .011 |
| Constitutional symptoms, n (%) | 57 (20.5) | 129 (33.7) | <.0001 |
| Splenomegaly, n (%) | 177 (63.7) | 317 (82.8) | <.0001 |
| >10 cm from LCM, n (%) | 29 (10.4) | 92 (24.0) | <.0001 |
| Patients with cytogenetic information, N= (% of total) | 150 (54.0) | 182 (47.5) | |
| Abnormal cytogenetics | 27 (18.0) | 69 (37.9) | <.0001 |
| Unfavorable karyotype | 6 (4.0) | 22 (12.1) | .006 |
| IPSS† | |||
| Low | 134 (48.2) | 89 (23.2) | <.0001 |
| Intermediate-1 | 74 (26.6) | 110 (28.7) | |
| Intermediate-2 | 36 (12.9) | 94 (24.6) | |
| High | 34 (12.3) | 90 (23.5) |
| Variables* . | Pre-PMF, N = 278 . | Overt PMF, N = 383 . | P . |
|---|---|---|---|
| Male patients, n (%) | 156 (56.1) | 249 (65.0) | .013 |
| Age | |||
| Median (range), y | 56.6 (18.0-90.3) | 63.6 (14.0-89.8) | <.0001 |
| >65 y, n (%) | 99 (35.6) | 179 (46.7) | .003 |
| Hemoglobin | |||
| Median (range), g/L | 129 (107-175) | 108 (47-150) | <.0001 |
| <100 g/L, n (%) | 37 (13.3) | 136 (35.5) | <.0001 |
| Leukocytes | |||
| Median (range), ×109/L | 9.1 (1.5-150) | 8.2 (1.4-109.0) | .009 |
| >25 × 109/L, n (%) | 18 (6.5) | 31 (8.1) | .252 |
| <4.0 × 109/L, n (%) | 10 (3.6) | 57 (14.9) | <.0001 |
| Platelets | |||
| Median (range), ×109/L | 488 (310-1500) | 249 (19-3279) | <.0001 |
| <100 × 109/L, n (%) | 19 (6.8) | 67 (17.5) | <.0001 |
| Circulating blasts, ≥1%, n (%) | 33 (11.9) | 99 (25.8) | <.0001 |
| LDH, N = 290 | |||
| Median (range), U/L | 388 (127-2806) | 669 (130-2643) | <.0001 |
| >Normal range, n (%) | 98 (81.7) | 159 (93.5) | .011 |
| Constitutional symptoms, n (%) | 57 (20.5) | 129 (33.7) | <.0001 |
| Splenomegaly, n (%) | 177 (63.7) | 317 (82.8) | <.0001 |
| >10 cm from LCM, n (%) | 29 (10.4) | 92 (24.0) | <.0001 |
| Patients with cytogenetic information, N= (% of total) | 150 (54.0) | 182 (47.5) | |
| Abnormal cytogenetics | 27 (18.0) | 69 (37.9) | <.0001 |
| Unfavorable karyotype | 6 (4.0) | 22 (12.1) | .006 |
| IPSS† | |||
| Low | 134 (48.2) | 89 (23.2) | <.0001 |
| Intermediate-1 | 74 (26.6) | 110 (28.7) | |
| Intermediate-2 | 36 (12.9) | 94 (24.6) | |
| High | 34 (12.3) | 90 (23.5) |
LCM, left costal margin.
Where information was available for some patients only, the actual number is indicated (N=). Splenomegaly indicates presence of a palpable spleen below the LCM. Unfavorable karyotype indicates any of the following: +8, −7/7q−, i(17q), inv(3), −5/5q, 12p−, or 11q23 rearrangements.
IPSS uses 5 independent predictors of inferior survival: age, >65 y; hemoglobin, <10 g/dL; leukocytes, >25 × 109/L; circulating blasts ≥1%, constitutional symptoms. The presence of 0, 1, 2, and ≥3 adverse factors defines low-, intermediate-1, intermediate-2, and high-risk disease.
We then analyzed separately patients with pre-PMF and fibrosis grade 0 or 1 and patients with overt MF, corresponding to 8.3%, 33.7%, and 57.9% of the series (supplemental Table 1, see supplemental Data available at the Blood Web site). Pre-PMF patients with absent fibrosis differed from those with fibrosis grade 1 for being more female, of younger age, with higher hemoglobin, and a prevalence of IPSS lower-risk category. Conversely, patients with grade 1 fibrosis had higher leukocytes and platelets, less common anemia, splenomegaly, symptoms, blasts, cytogenetic abnormalities, and were in lower IPSS risk categories than overt fibrosis. Similar findings were detected when comparing patients with no fibrosis vs any grade of fibrosis (supplemental Table 1).
Molecular characteristics of study population
The 3 driver mutated genes (JAK2V617F, MPLW515x, and CALR) were similarly distributed in the 2 cohorts (Table 2). JAK2V617F mutation was found in 67.2% of pre-PMF and 58.2% of overt PMF, CALR type 1 and type 2 in 12.2% and 5.8%, and 17.8% and 4.4%, respectively, of pre-PMF and overt PMF; MPLW515x-mutated patients were 4.7% and 6.0% in the 2 cohorts. The proportion of TN patients, a prognostically negative condition,22 was similar: 10.1% and 13.6% in pre-PMF and overt PMF. The allelic burden of the 3 driver mutations approximated 50% in both cohorts, suggesting a prevalence of homozygosity. The proportion of patients with abnormal karyotype was higher in the TN group compared with CALR mutation (26% vs 8% in pre-PMF and 46% vs 27% in overt PMF; P = .033); also, the unfavorable cytogenetics were enriched in TN patients with pre-PMF (11% vs 4% in CALR and 2% in JAK2V617F/MPLW515x mutation; P = .018).
Mutation profile of study patients
| Variables . | Pre-PMF, N = 278 . | Overt PMF, N = 383 . | P . |
|---|---|---|---|
| Driver mutation, n (%) | |||
| JAK2V617F | 187 (67.2) | 223 (58.2) | .091 |
| CALR | |||
| Type 1 | 34 (12.2) | 68 (17.8) | |
| Type 2 | 16 (5.8) | 17 (4.4) | |
| MPLW515x | 13 (4.7) | 23 (6.0) | |
| TN | 28 (10.1) | 52 (13.6) | |
| Allele burden, mean ± SD, % | |||
| JAK2V617F | 44.5 ± 20.9 | 47.8 ± 20.9 | .50 |
| MPLW515x | 48.9 ± 34.7 | 55.4 ± 22.6 | .60 |
| CALR | 47.8 ± 13.2 | 53.3 ± 10.3 | .16 |
| Mutated, n (%) | |||
| ASXL1 | 50 (18.0) | 129 (33.7) | <.0001 |
| EZH2 | 10 (3.6) | 46 (12.0) | <.0001 |
| SRSF2 | 25 (9.0) | 41 (10.7) | .28 |
| IDH1/2 | 6 (2.2) | 13 (3.4) | .24 |
| HMR, n (%) | |||
| =1 | 75 (27.0) | 170 (44.4) | <.0001 |
| ≥2 | 15 (5.4) | 52 (13.6) | <.0001 |
| Variables . | Pre-PMF, N = 278 . | Overt PMF, N = 383 . | P . |
|---|---|---|---|
| Driver mutation, n (%) | |||
| JAK2V617F | 187 (67.2) | 223 (58.2) | .091 |
| CALR | |||
| Type 1 | 34 (12.2) | 68 (17.8) | |
| Type 2 | 16 (5.8) | 17 (4.4) | |
| MPLW515x | 13 (4.7) | 23 (6.0) | |
| TN | 28 (10.1) | 52 (13.6) | |
| Allele burden, mean ± SD, % | |||
| JAK2V617F | 44.5 ± 20.9 | 47.8 ± 20.9 | .50 |
| MPLW515x | 48.9 ± 34.7 | 55.4 ± 22.6 | .60 |
| CALR | 47.8 ± 13.2 | 53.3 ± 10.3 | .16 |
| Mutated, n (%) | |||
| ASXL1 | 50 (18.0) | 129 (33.7) | <.0001 |
| EZH2 | 10 (3.6) | 46 (12.0) | <.0001 |
| SRSF2 | 25 (9.0) | 41 (10.7) | .28 |
| IDH1/2 | 6 (2.2) | 13 (3.4) | .24 |
| HMR, n (%) | |||
| =1 | 75 (27.0) | 170 (44.4) | <.0001 |
| ≥2 | 15 (5.4) | 52 (13.6) | <.0001 |
MPLW515x means any mutation occurring at codon 515. HMR =1 points to the presence of at least 1 mutation in any 1 of ASXL1, EZH2, SRSF2, IDH1/2. HMR ≥2 means the presence of 2 or more mutated genes among the above. Two or more mutations in the same gene are counted as 1. For the calculation of CALR allele burden, type 1 and type 2 mutations were considered together.
SD, standard deviation.
With regard to nondriver mutations, significantly more patients with overt PMF had mutations in ASXL1 (33.7%) and EZH2 (12.0%) compared with pre-PMF (18.0% and 3.6%; P < .0001), thereby resulting in more overt PMF patients being comprised in the HMR category (44.4% vs 27.0%; P < .0001); SRSF2 and IDH1/2 mutations were similarly represented. Also, the number of patients with ≥2 HMR-mutated genes, which is prognostically unfavorable,17 was greater in overt PMF (13.6%) than pre-PMF (5.4%; P < .0001).
Analysis of mutations according to fibrosis grade showed no difference in distribution and allelic burden of driver mutations, whereas any grade of fibrosis was associated with more ASXL1 and EZH2 mutations, more HMR-positive patients, and with ≥2 HMR mutations compared with absent fibrosis (supplemental Table 2).
OS and LFS
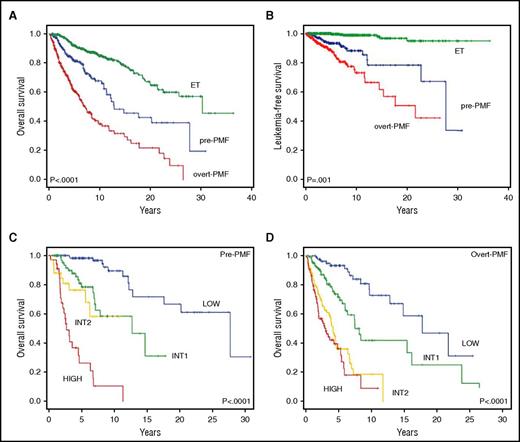
At the latest follow-up, after a median of 4.6 years and 3.1 years for pre-PMF and overt PMF, 69 patients (24.8%) and 163 patients (42.6%) had died (P < .0001). In a competitive risk analysis model, the median (range) OS was significantly shorter in overt PMF (7.2 years [5.7-8.7 years]) compared with pre-PMF (14.7 years [7.7-21.8 years]) (P < .0001) (Figure 1A). Diagnosis of overt PMF vs pre-PMF corresponded to a HR for reduced survival of 2.3 (95% CI, 1.8-3.1; P < .0001). For comparison, among 421 patients randomly selected from our database with revised diagnosis of ET according to 2016 WHO criteria, the median survival was 30.2 years (range, 23.7-31.2 years). Using ET patients as the reference category, the HR for OS was 2.7 (95% CI, 1.9-3.7; P < .0001) for pre-PMF and 5.9 (95% CI, 4.5-7.8; P < .0001) for overt PMF. Furthermore, compared with ET, survival was progressively shortened depending on fibrosis grade, with HR of 1.8, 2.8. 5.3, and 6.2 for fibrosis grade 0, 1, 2, and 3 (supplemental Table 3; supplemental Figure 1A).
OS and LFS in relation to diagnosis and IPSS risk categories in study patients population. (A-B) The Kaplan-Meier estimate of OS (A) and LFS (B) in patients with pre-MF and overt PMF using competitive risk analysis for disease-related deaths. The difference between the 2 patient populations was statistically significant at P < .0001 for OS and P = .001 for LFS. For comparison, OS and LFS curves of a population of 421 WHO 2016-defined patients with ET are also shown. (C-D) The Kaplan-Meier estimate of OS according to the 4 risk categories (low, intermediate-1, intermediate-2, high risk) in which the patients with pre-PMF (C) and overt PMF (D), respectively, were stratified at diagnosis according to the IPSS criteria. Overall, the curves were significantly different at P < .0001.
OS and LFS in relation to diagnosis and IPSS risk categories in study patients population. (A-B) The Kaplan-Meier estimate of OS (A) and LFS (B) in patients with pre-MF and overt PMF using competitive risk analysis for disease-related deaths. The difference between the 2 patient populations was statistically significant at P < .0001 for OS and P = .001 for LFS. For comparison, OS and LFS curves of a population of 421 WHO 2016-defined patients with ET are also shown. (C-D) The Kaplan-Meier estimate of OS according to the 4 risk categories (low, intermediate-1, intermediate-2, high risk) in which the patients with pre-PMF (C) and overt PMF (D), respectively, were stratified at diagnosis according to the IPSS criteria. Overall, the curves were significantly different at P < .0001.
Transformation to acute leukemia was diagnosed in 72 patients (10.9%), 23 with pre-PMF (8.3%) and 49 with overt PMF (12.8%; P = .04). The rate of leukemia transformation was 5.4%, 8.9%, 13.9%, and 10.9% in fibrosis grade 0, 1, 2, and 3. LFS was shorter in overt PMF than pre-PMF (21.6 years vs 27.6 years; P < .001; HR, 2.2 [95% CI, 1.3-3.7; P < .002]) (Figure 1B). The cumulative incidence of leukemia was 7% and 11% at 5 years, and 12% and 23% at 10 years, respectively, for pre-PMF and overt PMF (P < .0001), contrasting with 0% and 1% at 5 and 10 years, respectively, in the ET cohort (P < .001). Compared with ET, the HR significantly increased depending on the grade of fibrosis: HR, 6.4, 12.0, 24.1, and 23.6 in fibrosis grade 0, 1, 2, and 3, respectively (supplemental Table 3; supplemental Figure 1B).
Survival was predicted by the IPSS score in both pre-PMF and overt PMF (Figure 1C-D), although optimal resolution of the 4 risk categories was not achieved in all instances. In pre-PMF, curves of intermediate-1 and intermediate-2 patients did not differ (P = .205; Figure 1C); using the low risk as the reference category, the HR for intermediate-1, intermediate-2, and the high risk category was 5.3 (95% CI, 2.4-11.8), 12.2 (95% CI, 5.0-30.1), and 34.8 (95% CI, 15.7-77.2). In overt PMF, the intermediate-2 and high-risk category were superimposed (P = .170; Figure 1D); the HR was 2.9 (95% CI, 1.6-5.2), 7.8 (95% CI, 4.4-14.0), and 10.4 (95% CI, 5.8-19.0) for intermediate-1, intermediate-2, and the high-risk category. Considering patients with fibrosis grade 0, the IPSS score delineated differences only between the low- and high-risk category; in the case of fibrosis grade 1 and fibrosis grade >1, the intermediate-1 and intermediate-2 and the intermediate-2 and high-risk categories, respectively, did not result in statistical difference (supplemental Figure 1C-E).
Impact of molecular characteristics on survival and transformation to leukemia
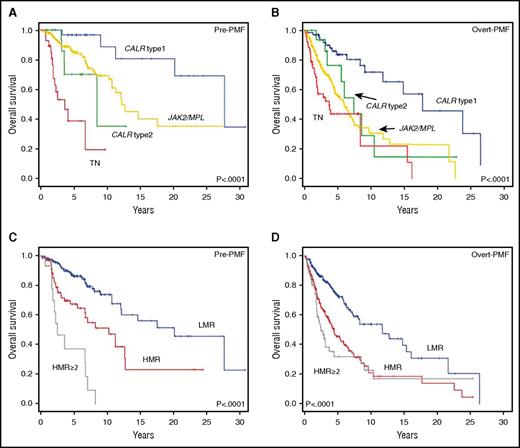
The impact of driver and nondriver mutations on OS is represented in Figure 2. In both pre-PMF and overt PMF, CALR type 1 mutation was the most favorable, with median survival of 27.7 years and 17.8 years, respectively (Figure 2A-B). Using this as the reference category, we calculated the HR for the other mutation categories; JAK2V617F and MPLW515 mutations were merged because preliminary analysis did not disclose significant differences. In pre-PMF, the HR for reduced survival was 3.8 (95% CI, 1.3-10.6; P = .013) for JAK2V617F/MPLW515 mutation, 6.1 (95% CI, 1.6-23.3; P = .008) for CALR type 2, and 22.8 (95% CI, 7.1-73.3; P < .0001) for TN. In overt PMF, the respective HRs were 3.5 (95% CI, 4.9-7.0; P < .001), 2.6 (95% CI, 1.6-5.8; P = .040), and 5.3 (95% CI, 2.8-9.9; P < .0001). In both cohorts, the survival of patients with JAK2/MPL and CALR type 2 mutations was not statistically different.
Impact of driver and HMR mutations on OS in study patients population. Kaplan-Meier estimates of OS in patients with a diagnosis of pre-PMF (A) and overt PMF (B) who were stratified according to their CALR type 1/type 1–like, CALR type 2/type 2–like, and JAK2V617F/MPLW515x mutation status. The survival curve of patients negative for the above driver mutations, that is, TN, is also shown. (C-D) The OS by Kaplan-Meier estimates in patients being stratified in a HMR (patients harboring mutation in at least 1 of ASXL1, EZH2, SRSF2, IDH1, or IDH2) and LMR (ie, no mutation in the above genes) category. (C-D) Pre-PMF and overt PMF, respectively. The survival curves of patients with 2 or more mutated genes of the HMR category are also shown.
Impact of driver and HMR mutations on OS in study patients population. Kaplan-Meier estimates of OS in patients with a diagnosis of pre-PMF (A) and overt PMF (B) who were stratified according to their CALR type 1/type 1–like, CALR type 2/type 2–like, and JAK2V617F/MPLW515x mutation status. The survival curve of patients negative for the above driver mutations, that is, TN, is also shown. (C-D) The OS by Kaplan-Meier estimates in patients being stratified in a HMR (patients harboring mutation in at least 1 of ASXL1, EZH2, SRSF2, IDH1, or IDH2) and LMR (ie, no mutation in the above genes) category. (C-D) Pre-PMF and overt PMF, respectively. The survival curves of patients with 2 or more mutated genes of the HMR category are also shown.
Then, we analyzed the impact of HMR mutations. The HMR category was associated with significantly shorter survival in both pre-PMF and overt PMF (Figure 2C-D). Median (range) survival was 8.3 years (3.6-13.0 years) and 4.5 years (3.3-5.7 years) in pre-PMF and overt PMF, respectively, compared with 20.2 years (13.1-27.3 years) and 11.8 years (7.0-16.6 years) for the LMR category (P < .0001). The HR for a HMR status was 2.5 (95% CI, 1.6-4.1; P < .0001) and 2.3 (95% CI, 1.7-3.1; P < .0001) in pre-PMF and overt PMF. All 5 genes comprising the HMR category, when mutated, individually signified for worse survival (supplemental Tables 4 and 5). The impact of ≥2 mutated genes was also evaluated (Figure 2C-D). In pre-PMF, median survival was 12.7 years vs 2.6 years in patients with 1 and ≥2 mutated genes (P < .0001); the corresponding HRs (using LMR as the reference category) were 1.7 (95% CI, 1.0-3.1) and 8.4 (95% CI, 4.3-16.3). In overt PMF, median survival was 5.3 years in patients with 1 mutated gene and 2.5 years if ≥2 mutated genes were present (P < .0001); HRs were 2.0 (95% CI, 1.4-2.9) and 3.0 (95% CI, 1.9-4.5), respectively.
Driver mutations deserved no prognostic significance for LFS, except for TN patients with pre-PMF in whom the risk to progress to leukemia was significantly higher compared with CALR type 1 (HR, 10.5; 95% CI, 2.2-49.6) (supplemental Figure 2A-B). Conversely, pre-PMF and overt PMF patients harboring HMR mutations experienced significantly shortened LFS compared with the LMR category. LFS was 22.7 years (14.0-24.6 years; P < .001) and 13.5 years (95% CI, 4.6-12.3 years; P < .001) in HMR patients with pre-PMF and overt PMF, compared with 27.6 years (95% CI, 6.1-29.2 years) and 21.6 years (95% CI, 10.6-22.7 years) for the corresponding LMR categories (supplemental Figure 2C-D).
The impact of adverse karyotype on OS and LFS was analyzed for 332 patients who had cytogenetic information. In pre-PMF, unfavorable karyotype predicted for shortened OS (median [range], 3.2 years [2.7-3.7 years]) and LFS (3.1 years [2.6-3.6 years]) (supplemental Figure 3A,C) compared with patients lacking unfavorable karyotype (median OS not reached). There was a trend for shorter survival also in overt PMF patients with unfavorable karyotype (5.9 years vs 8.4 years), but no difference concerning LFS (supplemental Figure 3B,D).
In a multivariate analysis, that included the IPSS score, diagnosis of pre-PMF or overt PMF, driver and nondriver mutations, the variables that remained significantly associated with reduced survival were the IPSS score (intermediate-1: HR, 3.1; 95% CI, 2.0-5.0) (intermediate-2: 8.6; HR, 5.3-13.9) (high risk: HR, 11.2; 95% CI, 6.8-18.3) (all P > .0001), diagnosis of overt PMF (HR, 1.5l 95% CI, 1.1-2.0; P = .008) and HMR status (HR, 1.5; 95% CI, 1.2-2.1; P = .007). For LFS, only IPSS intermediate-2 (HR, 3.7; 95% CI, 1.8-7.9) and high-risk (HR, 9.0; 95% CI, 4.5-18.3; P = .001) category and HMR status (HR, 3.0; 95% CI, 1.6-5.7; P = .001) were significant.
Disease progression
We then evaluated disease progression in the 2 patients’ cohorts. At the latest follow-up, 34.4% and 69.4% of pre-PMF and overt PMF patients, respectively, were scored as DIPSS intermediate-2 and high risk (P < .0001) (Table 3). At 3 and 5 years of follow-up, 8.0% and 14.0% of pre-PMF, and 20.5% and 31.4% of overt PMF patients, respectively, had progressed to higher-risk category (P < .0001). The proportion of patients who acquired ≥1 of the individual DIPSS-Plus adverse variables (excluding age, and adverse cytogenetics for which we did not have information) was significantly greater in overt PMF than pre-PMF (Table 3). The 3-year cumulative incidence of anemia was 29% in overt PMF vs 11% in pre-PMF (HR, 2.9; 95% CI, 2.0-4.3); leukocytosis, 10% vs 6.5% (HR, 2.2; 95% CI, 1.3-3.8); thrombocytopenia, 15% vs 6% (HR, 2.5; 95% CI, 1.5-4.3); ≥1% peripheral blood blasts, 21% vs 8% (HR, 2.7; 95% CI, 1.8-4.0); constitutional symptoms, 14% vs 5.5% (HR, 2.0; 95% CI, 1.2-3.3); transfusion dependency, 28% vs 12% (HR, 2.6; 95% CI, 1.8-3.8). Patients with absent fibrosis were less prone to develop anemia, transfusion dependency, thrombocytopenia, and ≥1% blasts in comparison with any grade of fibrosis, and the large majority of them remained in the lower DIPSS-plus categories (supplemental Table 6).
Acquisition of DIPSS-Plus variables, except unfavorable cytogenetics and age >65 years, at the latest available follow-up in study patients
| Variables . | N= . | Pre-PMF, n (%) . | Overt PMF, n (%) . | P . |
|---|---|---|---|---|
| Hemoglobin, <100 g/L | 482 | 54 (23.1) | 110 (44.4) | <.0001 |
| Leukocytes, >25 × 109/L | 587 | 35 (14.3) | 77 (22.4) | .009 |
| Platelets, <100 × 109/L | 558 | 37 (14.9) | 76 (24.6) | .006 |
| Circulating blasts, ≥1% | 525 | 56 (23.4) | 117 (40.9) | <.0001 |
| Constitutional symptoms | 334 | 21 (14.6) | 42 (22.1) | .049 |
| Transfusion dependency | 617 | 52 (20.2) | 139 (38.6) | <.0001 |
| DIPSS | 658 | |||
| Low | 79 (28.6) | 26 (6.8) | <.0001 | |
| Intermediate-1 | 102 (37.0) | 91 (23.8) | ||
| Intermediate-2 | 67 (24.3) | 179 (46.9) | ||
| High | 28 (10.1) | 86 (22.5) |
| Variables . | N= . | Pre-PMF, n (%) . | Overt PMF, n (%) . | P . |
|---|---|---|---|---|
| Hemoglobin, <100 g/L | 482 | 54 (23.1) | 110 (44.4) | <.0001 |
| Leukocytes, >25 × 109/L | 587 | 35 (14.3) | 77 (22.4) | .009 |
| Platelets, <100 × 109/L | 558 | 37 (14.9) | 76 (24.6) | .006 |
| Circulating blasts, ≥1% | 525 | 56 (23.4) | 117 (40.9) | <.0001 |
| Constitutional symptoms | 334 | 21 (14.6) | 42 (22.1) | .049 |
| Transfusion dependency | 617 | 52 (20.2) | 139 (38.6) | <.0001 |
| DIPSS | 658 | |||
| Low | 79 (28.6) | 26 (6.8) | <.0001 | |
| Intermediate-1 | 102 (37.0) | 91 (23.8) | ||
| Intermediate-2 | 67 (24.3) | 179 (46.9) | ||
| High | 28 (10.1) | 86 (22.5) |
The number (N=) of patients evaluated for each variable at the latest follow-up is shown. Becoming older than 65 y was not considered as an adverse variable for the purposes of this analysis. A few patients only had cytogenetic information at latest follow-up, therefore acquisition of adverse cytogenetics was also not included in this analysis.
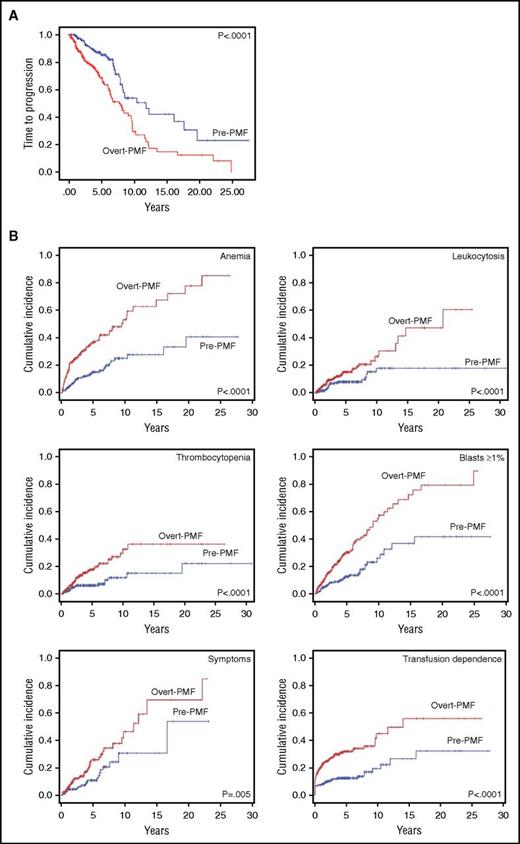
Time to progression, considered as the time to acquisition of any 1 of the above prognostically adverse variables, was assessed by a time-dependent, competitive risk analysis (Figure 3A). Median time (range) to progression was 7.7 years (6.1-9.3 years) in overt PMF compared with 11.8 years (7.9-15.7 years) in pre-PMF (P < .0001); the corresponding HR was 2.2 (95% CI, 1.5-3.1). Kaplan-Meier curves of event-free survival for each of the considered variables are shown in Figure 3B. Using pre-PMF as the reference category, the HR for acquisition of anemia in overt PMF was 2.9 (95% CI, 2.0-4.3), 2.2 for leukocytosis (95% CI, 1.3-3.8), 2.5 for thrombocytopenia (95% CI, 1.5-4.3), 2.7 for blasts ≥1% (95% CI, 1.8-4.0), 2.0 for symptoms (95% CI, 1.2-3.3), and 2.6 for transfusion dependence (95% CI, 1.8-3.8).
Time to disease progression in study patients population. Kaplan-Meier estimate of the time to disease progression in pre-PMF and overt PMF (A). Time to progression was defined as the time to acquisition of any 1 (except age) of the prognostically adverse clinical and hematologic variables included in the DIPSS-Plus score (anemia; leukocytosis; blasts ≥1% in peripheral blood; thrombocytopenia; appearance of constitutional symptoms; transfusion dependence; we did not consider adverse cytogenetics for which we had too few data). (B) Cumulative incidence for each of the individual prognostically unfavorable variables is shown. Cumulative incidence was estimated with a competing risk approach, considering death for any other cause as a competing event. Vertical tick marks indicate right-censored patients.
Time to disease progression in study patients population. Kaplan-Meier estimate of the time to disease progression in pre-PMF and overt PMF (A). Time to progression was defined as the time to acquisition of any 1 (except age) of the prognostically adverse clinical and hematologic variables included in the DIPSS-Plus score (anemia; leukocytosis; blasts ≥1% in peripheral blood; thrombocytopenia; appearance of constitutional symptoms; transfusion dependence; we did not consider adverse cytogenetics for which we had too few data). (B) Cumulative incidence for each of the individual prognostically unfavorable variables is shown. Cumulative incidence was estimated with a competing risk approach, considering death for any other cause as a competing event. Vertical tick marks indicate right-censored patients.
Impact of molecular characteristics on disease progression
We finally asked whether the molecular and cytogenetic abnormalities detected at diagnosis impacted on disease progression. Analysis of the 3-year cumulative incidence of acquisition of the individual DIPSS-Plus variables revealed that triple negativity was associated with higher rate of acquisition of anemia, transfusion dependency, and leukocytosis in both pre-PMF and overt PMF, plus thrombocytopenia in overt PMF (supplemental Table 7), whereas mutations in JAK2, MPL, and CALR were neutral. Remarkably, a HMR status was associated with statistically significant higher 3-year cumulative incidences of all of the adverse variables, except symptoms, in both cohorts compared with LMR category (supplemental Table 8).
Discussion
This analysis of a large series of patients with contemporary diagnosis of pre-PMF and overt PMF disclosed meaningful differences in clinical, hematologic, and molecular phenotype, and in outcome, thereby reinforcing the potential relevance of applying the 2016 revised WHO criteria in the clinical practice. The existence of pre-PMF distinct from ET and fibrotic overt PMF has been largely debated in the literature with regard to the reproducibility of criteria outside of pivotal studies based on centralized evaluation of diagnostic slides by a single WHO expert. In a number of reports with formal assessment of concordance degree among pathologists, the overall consensus ranged from 88% (best) to 53% (worst) (listed in supplemental Table 9). The current study was conducted in the spirit of a “real-life” approach where expert, local pathologists from 5 different tertiary centers with large accrual of myeloproliferative neoplasm patients independently formulated their diagnosis based on 2016 WHO criteria; then, we correlated histopathology with hematologic, clinical, and molecular findings. The primary aim was to assess whether stratification of patients in the 2 PMF categories, as required by modern WHO criteria, corresponded to any clinically meaningful difference that might pragmatically justify such distinction. When compared with overt PMF, patients with pre-PMF were generally females of younger age who showed a more pronounced myeloproliferative phenotype with higher leukocyte, hemoglobin, and platelet levels, whereas they less frequently had peripheral blood blasts, symptoms, and extensive splenomegaly. Conversely, overt PMF and pre-PMF did not differ regarding the driver mutations’ profile nor the variant allele frequency, whereas mutations included in the HMR category were enriched among overt PMF patients. At diagnosis, patients with pre-PMF were preferentially included in the lower IPSS risk categories, whereas 48.1% of overt MF were scored as intermediate-2 and high risk. Furthermore, patients with overt PMF had significantly shorter progression-free survival than pre-PMF.20 Notably, OS and LFS were longer in pre-PMF than overt PMF, but significantly shortened in both categories compared with patients with ET. These findings stand in support of the importance of an accurate distinction between pre-PMF and ET,12,23 as by the WHO criteria, particularly with regard to pre-PMF patients with absent fibrosis. In summary, differentiating between pre-PMF and overt PMF by adopting the 2016 revised WHO criteria proved to be clinically informative and prognostically relevant, although how this knowledge might inform a personalized therapeutic approach cannot be inferred based on this study and needs a prospectively followed series. Such prospective analyses might also include centralized evaluation of diagnostic slides to definitely assess the reproducibility of WHO criteria of pre-PMF.
Results of this study also indicate that, in spite of the uniqueness of the individual diagnostic categories with regard to characteristics and outcome, the overlapping phenotype might support the hypothesis that the 2 diseases represent a continuum where unknown individual characteristics and/or germ line or somatic gene variants eventually affect disease presentation and progression. By analyzing the individual characteristics upon the degree of fibrosis, including absent fibrosis, there appears to be a gradient in most of the clinical and hematologic variables and, mostly relevant, outcome. These findings reinforce recent observations indicating that degree of BM fibrosis is prognostically informative.24-28
One additional finding from this study concerns the appropriateness of a reappraisal of the current IPSS prognostic scores; in fact, these scores were originally developed using populations of PMF patients18 that differed from the 2 categories currently identified by the 2016 revised WHO criteria. In this regard, we found that, although the IPSS score overall predicted survival, it largely failed to accurately separate intermediate-1 and intermediate-2, and intermediate-2 and high-risk patients, respectively, in pre-PMF and overt MF, as well as in the individual groups based on the grade of fibrosis. These observations may have importance in the settings of decision-making for stem cell transplantation, which is currently indicated in patients with intermediate-2 and high-risk PMF29 ; based on our findings, pre-PMF patients with intermediate-2 disease, whose median survival was superimposable to intermediate-1 and projected at >10 years, might be inappropriately exposed to a risky procedure. Therefore, we suggest that these aspects be addressed in further studies.
Furthermore, there might be speculation that more extensive mutation profiling, compared with the 5-gene panel of the HMR category used in this study,16 might contribute to improved discrimination of patients at risk of premature death; this notwithstanding, it is noteworthy that the HMR status confirmed its predictive value in both pre-PMF and overt PMF. Of note, selected nondriver mutations are currently included in guidelines for referral to transplantation.29,30 Concerning driver mutations, studies conducted in cohorts with 2008 WHO-defined PMF identified patients with triple negativity and presence of CALR type 1 mutation, respectively, as the worst and best category for survival; patients harboring JAK2V617F, MPLW515, and CALR type 2 mutations showed intermediate survival.15,22,31,32 In this study, we confirmed that CALR type 1 mutations represent the most favorable predictor of survival among the driver mutations; in pre-PMF, the negative impact of TN and the intermediate outcome associated with JAK2V617F and MPLW515 mutations were also confirmed, whereas in overt PMF, no significant differences among the 3 mutation profiles could be ascertained. As a whole, these findings should promote efforts to critically re-evaluate current scores and, eventually, develop separate risk scores for pre-PMF and overt PMF that include the most relevant clinical, molecular, and cytogenetic variables. In a multivariate analysis performed in this series, HMR mutations, unlike driver ones, maintained their independent prognostic value.
In conclusion, in this “real-life” study of 661 molecularly annotated patients with a diagnosis of pre-PMF and overt PMF, according to the 2016 revised WHO criteria, we identified differences in patterns of presentation, survival, and disease progression, overall indicating that these criteria might help to separate clinically distinct categories of patients. Current results also suggest that accurate differentiation between pre-PMF and overt PMF is required for meaningful interpretation of results of clinical trials with novel therapeutic agents.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a grant from AIRC (Milan, Italy), Special Program Molecular Clinical Oncology 5x1000, to AGIMM project #1005. This work was supported by AIRC Investigator Grant 2014 #15967 (P.G.). G.R. was the recipient of a fellowship from “Beat Leukemia.” The research was also supported by a generous donation from Fondazione Ferragamo, Florence.
Authorship
Contribution: P.G., T.B., G.B., M.C., and A.M.V. contributed to the conception of the work; P.G., A. Pacilli, G.R., E.R., V.R., F.D., M.M., T.F., A. Pancrazzi, D.P., S.S., C.M., A.F., C.P., A.R., F.P., G.B., T.B., M.C., and A.M.V. contributed to the acquisition, analysis, or interpretation of data for the work; P.G. and A.M.V. wrote the manuscript; T.B., F.P., and M.C. contributed to manuscript drafting; and all authors critically reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the AGIMM Group appears in the online appendix.
Correspondence: Alessandro M. Vannucchi, Centro di Ricerca e Innovazione delle Malattie Mieloproliferative (CRIMM), AOU Careggi, Dipartimento di Medicina Sperimentale e Clinica, Università di Firenze, Viale Pieraccini, 6, 50134 Firenze, Italy; e-mail: amvannucchi@unifi.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal