Key Points
Recipient-derived IL-17A is critical for the prevention of intestinal acute GVHD.
Increased susceptibility to acute GVHD can be transferred to WT mice via cohousing with IL-17RA or IL-17RC–deficient mice.
Abstract
Donor T-cell–derived interleukin-17A (IL-17A) can mediate late immunopathology in graft-versus-host disease (GVHD), however protective roles remain unclear. Using multiple cytokine and cytokine receptor subunit knockout mice, we demonstrate that stem cell transplant recipients lacking the ability to generate or signal IL-17 develop intestinal hyper-acute GVHD. This protective effect is restricted to the molecular interaction of IL-17A and/or IL-17F with the IL-17 receptor A/C (IL-17RA/C). The protection from GVHD afforded by IL-17A required secretion from, and signaling in, both hematopoietic and nonhematopoietic host tissue. Given the intestinal-specificity of the disease in these animals, we cohoused wild-type (WT) with IL-17RA and IL-17RC–deficient mice, which dramatically enhanced the susceptibility of WT mice to acute GVHD. Furthermore, the gut microbiome of WT mice shifted toward that of the IL-17RA/C mice during cohousing prior to transplant, confirming that an IL-17–sensitive gut microbiota controls susceptibility to acute GVHD. Finally, induced IL-17A depletion peritransplant also enhanced acute GVHD, consistent with an additional protective role for this cytokine independent of effects on dysbiosis.
Introduction
Allogeneic stem cell transplantation (alloSCT) is a curative treatment of most hematologic malignancies, however the success of this treatment is limited due to major complications, principally graft-versus-host disease (GVHD). Acute GVHD affects the skin, liver, and gastrointestinal (GI) tract and is mediated by donor T cells within the transplanted graft. This occurs in 50% to 70% of SCT recipients and is the main contributor to the high mortality seen in these patients.1
The interleukin-17 (IL-17) cytokine family is complex and consists of multiple cytokine members with multiple cognate receptors. IL-17A is the most widely studied member of the family and it signals through the IL-17 receptor A (IL-17RA).2 Although IL-17 was initially reported to be produced by T helper 17 (Th17) cells,3 it has since also been shown to be produced by innate cells such as γδ T cells, natural killer T (NKT) cells, macrophages, lymphoid-tissue inducer, and T-follicular helper cells, in addition to nonimmune cells such as epithelia.4 Th17 cells arise from the differentiation of naïve T cells mediated by IL-6, transforming growth factor-β, and IL-1, with subsequent amplification by IL-21 and phenotypic stability controlled principally by IL-23.5 Donor CD4 and CD8 type-17 T-cell differentiation is now largely accepted as a pathological differentiation program following bone marrow transplantation (BMT),6,7 generating sclerotic chronic GVHD, particularly within the skin.6,8
In this study, we demonstrate that recipient IL-17A has a protective role in GVHD and that transfer of distinct intestinal microbiota from IL-17 receptor deficient to wild-type (WT) mice transfers susceptibility to hyper-acute GVHD.
Methods
Mice
B6.IL-17RA−/−, B6.IL-17RB−/−, B6.IL-17RC−/− (Amgen, Washington, DC), BALB/c.IL-17A−/− (The University of Tokyo, Japan), BALB/c luciferase,9 BALB/c 45.1+, and B6.TCRδ−/− mice were bred in-house. B6.IL-17–Cre and B6.Rosa-26–YFP mice were crossed to generate B6.IL-17–YFP reporter mice.10 B6.IL-17–YFP fate-map reporter mice were crossed with B6.Rosa26 iDTR mice11 to generate fate-map deletor mice.12 Diphtheria toxin (DT) was administered intraperitoneally at days −3 (250 ng), −1 (250 ng), and +1 (100 ng).
alloSCT
Animal procedures were undertaken using protocols approved by the Queensland Institute of Medical Research (QIMR) Berghofer Animal Ethics Committee. Mice were transplanted and monitored as described previously.13,14 All transplanted mice were housed in sterilized microisolator cages and received acidified autoclaved water. GVHD was assessed using established scoring systems15 and mice with clinical scores ≥6 were euthanized in accordance with institutional guidelines.
Cytokine analysis
Serum IL-17A, granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-6, IL-10, tumor necrosis factor (TNF), and interferon γ (IFN-γ) levels were measured using mouse Flex Array sets (BD Biosciences Pharmingen, San Diego, CA) as described previously.13
Histopathology
Formalin-fixed, paraffin-embedded tissues were sectioned (5 μm) and stained with hematoxylin and eosin for histologic assessment. Slides were examined in a blinded fashion and pathology scored using a semiquantiative scoring system as described.16
Fluorescein isothiocyanate (FITC)-dextran assay
Allogeneically transplanted mice were restricted of food and water for 4 hours prior to delivery of 8 mg FITC-dextran (molecular mass 4 kDa; Sigma-Aldrich) by oral gavage. Peripheral blood was collected 4 hours thereafter. Serum was harvested and FITC-dextran levels measured using a spectrophotofluorimeter.
16S ribosomal RNA (rRNA) sequencing and analysis
DNA was extracted from 50 to 100 mg of fecal material using the Maxwell 16 Tissue DNA Kit (Promega). The 16S rRNA gene encompassing the V6 to V8 regions was amplified and sequenced on the MiSeq Sequencing System (Illumina) using paired-end sequencing with V3 300 bp chemistry at the Australian Centre for Ecogenomics. Operational taxonomic units (OTUs) were identified using the QIIME (version 1.8.0) script pick_open_reference_otus.py17 with default parameters (97% similarity; method: uclust18 ), and assigned taxonomy using Basic Local Alignment Search Tool19 against the Greengenes reference database version 2014/0920 (http://data.ace.uq.edu.au/public/gg). Differential abundance analysis was performed on raw read counts using DESeq2.21 Full details and tables listing 16S rRNA sequence data referred to in the paper can be found in the supplemental Methods and Tables 1-3, available on the Blood Web site.
Statistical analysis
Survival curves were plotted using Kaplan-Meier estimates and compared by log-rank analysis. The parametric unpaired Student t test or the nonparametric Mann-Whitney U test (two-sided) were used for the statistical analysis of all data, where appropriate. P < .05 was considered statistically significant. Data are presented as mean ± standard error of the mean (SEM) (supplemental Methods).
Results
IL-17A signaling via host tissues protects against GVHD in the GI tract
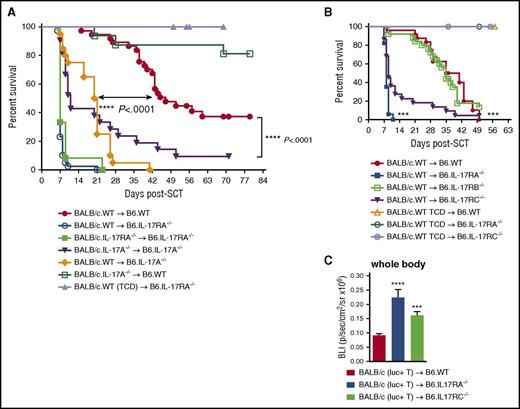
To determine the role of IL-17 in GVHD, we examined the contribution of IL-17 receptor signaling in recipient tissues following alloSCT. B6.IL-17RA–deficient mice developed hyper-acute GVHD resulting in early mortality relative to B6.WT mice (median survival of 7 vs 49 days) (Figure 1A). Cytokine levels in sera posttransplant revealed a temporal increase in Th17 (IL-17A, IL-6, and GM-CSF), Th1 (TNF and IFN-γ), and Th2 (IL-10) levels in B6.IL-17RA–deficient mice with peak differences seen at day 4 (Figure 1B). No difference in IL-17F levels was observed between the 2 groups (data not shown).
IL-17RA signaling in host tissues attenuates GVHD and donor T-cell expansion. (A) Lethally irradiated B6.WT or B6.IL-17RA−/− mice received T-cell replete or TCD splenocytes from G-CSF mobilized BALB/c.WT donors. Survival is represented by Kaplan-Meier analysis: ****P < .0001, BALB/c.WT → B6.WT (red filled circles; n = 21) vs BALB/c.WT → B6.IL-17RA−/− (blue filled squares; n = 17). BALB/c.WT (TCD) → B6.IL-17RA−/− (green open squares; n = 10). Combined data from 3 experiments are shown. (B) Serum cytokine levels over time post-alloSCT (n = 6 per group). B6.WT (red bars) vs B6.IL-17RA−/− (blue bars) recipients: IL-17A, **P = .002 (day 4); GM-CSF, *P = .02 (day 2), **P = .004 (day 4), **P = .009 (day 6); IL-6, **P = .002 (day 2), *P = .04 (day 4); IL-10, *P = .037 (day 2), **P = .002 (day 4); TNF, *P = .037 (day 2), ****P < .0001 (day 4); and IFN-γ, **P = .002 (day 2), **P = .009 (day 4), **P = .009 (day 6). (C) Grafts prepared using BM from BALB/c.WT donors and T cells from BALB/c luciferase mice were transplanted into lethally irradiated B6.WT or B6.IL-17RA−/− recipients (n = 13 per group). Animals were imaged at day 6 post-SCT and the bioluminescence intensity quantified (photons/sec/cm2/sr × 105) (Cii). Data combined from 2 replicate experiments are shown (panel Ci, WT [red bars], IL-17RA−/− [blue bars]). (D) Semiquantitative histopathology of GVHD target organs at day 6 post-alloSCT (SI, colon, lung: n = 9 to 10 per group; TCD groups: n = 4 per group, green bars; liver: n = 5 per group). SI and colon, B6.WT (red bars) vs B6.IL-17RA−/− (blue bars) recipients: ****P < .0001. Data combined from 2 experiments are shown (i). Representative images of the SI are shown (ii). (E) Intestinal barrier integrity as determined by FITC-dextran levels in serum on day 6 after alloSCT. Mice were orally gavaged with 8 mg FITC-dextran after 4 hours without food or water, and serum harvested 4 hours thereafter. Data combined from 2 replicate experiments are shown (n = 8 to 10 per group): B6.WT (red bar) vs B6.IL-17RA−/− (blue bar) recipients; **P = .004. (F) G-CSF mobilized grafts were unseparated, CD4 TCD or CD8 TCD, and transplanted into lethally irradiated B6.WT or B6.IL-17RA−/− recipients (n = 8 per group). Survival is represented by Kaplan-Meier analysis. ****P < .0001, BALB/c unseparated graft into B6.WT (red open square) vs B6.IL-17RA−/− (blue open circle) recipients; ****P < .0001, BALB/c unseparated graft (blue open circles) vs BALB/c CD4 depleted graft (green filled circles) into B6.IL-17RA−/− recipients; **P < .001, BALB/c unseparated graft (blue open circles) vs BALB/c CD8-depleted graft (purple filled triangle) into B6.IL-17RA−/− recipients. All data are presented as mean ± SEM. BLI, bioluminescence intensity; G-CSF, granulocyte CSF; GIT, gastrointestinal tract; mLN, mesenteric lymph node; ND, not detected; ns, not significant; SI, small intestine; TCD, T-cell deplete(d).
IL-17RA signaling in host tissues attenuates GVHD and donor T-cell expansion. (A) Lethally irradiated B6.WT or B6.IL-17RA−/− mice received T-cell replete or TCD splenocytes from G-CSF mobilized BALB/c.WT donors. Survival is represented by Kaplan-Meier analysis: ****P < .0001, BALB/c.WT → B6.WT (red filled circles; n = 21) vs BALB/c.WT → B6.IL-17RA−/− (blue filled squares; n = 17). BALB/c.WT (TCD) → B6.IL-17RA−/− (green open squares; n = 10). Combined data from 3 experiments are shown. (B) Serum cytokine levels over time post-alloSCT (n = 6 per group). B6.WT (red bars) vs B6.IL-17RA−/− (blue bars) recipients: IL-17A, **P = .002 (day 4); GM-CSF, *P = .02 (day 2), **P = .004 (day 4), **P = .009 (day 6); IL-6, **P = .002 (day 2), *P = .04 (day 4); IL-10, *P = .037 (day 2), **P = .002 (day 4); TNF, *P = .037 (day 2), ****P < .0001 (day 4); and IFN-γ, **P = .002 (day 2), **P = .009 (day 4), **P = .009 (day 6). (C) Grafts prepared using BM from BALB/c.WT donors and T cells from BALB/c luciferase mice were transplanted into lethally irradiated B6.WT or B6.IL-17RA−/− recipients (n = 13 per group). Animals were imaged at day 6 post-SCT and the bioluminescence intensity quantified (photons/sec/cm2/sr × 105) (Cii). Data combined from 2 replicate experiments are shown (panel Ci, WT [red bars], IL-17RA−/− [blue bars]). (D) Semiquantitative histopathology of GVHD target organs at day 6 post-alloSCT (SI, colon, lung: n = 9 to 10 per group; TCD groups: n = 4 per group, green bars; liver: n = 5 per group). SI and colon, B6.WT (red bars) vs B6.IL-17RA−/− (blue bars) recipients: ****P < .0001. Data combined from 2 experiments are shown (i). Representative images of the SI are shown (ii). (E) Intestinal barrier integrity as determined by FITC-dextran levels in serum on day 6 after alloSCT. Mice were orally gavaged with 8 mg FITC-dextran after 4 hours without food or water, and serum harvested 4 hours thereafter. Data combined from 2 replicate experiments are shown (n = 8 to 10 per group): B6.WT (red bar) vs B6.IL-17RA−/− (blue bar) recipients; **P = .004. (F) G-CSF mobilized grafts were unseparated, CD4 TCD or CD8 TCD, and transplanted into lethally irradiated B6.WT or B6.IL-17RA−/− recipients (n = 8 per group). Survival is represented by Kaplan-Meier analysis. ****P < .0001, BALB/c unseparated graft into B6.WT (red open square) vs B6.IL-17RA−/− (blue open circle) recipients; ****P < .0001, BALB/c unseparated graft (blue open circles) vs BALB/c CD4 depleted graft (green filled circles) into B6.IL-17RA−/− recipients; **P < .001, BALB/c unseparated graft (blue open circles) vs BALB/c CD8-depleted graft (purple filled triangle) into B6.IL-17RA−/− recipients. All data are presented as mean ± SEM. BLI, bioluminescence intensity; G-CSF, granulocyte CSF; GIT, gastrointestinal tract; mLN, mesenteric lymph node; ND, not detected; ns, not significant; SI, small intestine; TCD, T-cell deplete(d).
We examined donor T-cell expansion and tissue localization using grafts in which donor T cells expressed luciferase. Donor T cells expanded and infiltrated target tissues in an enhanced fashion in the B6.IL-17RA−/− recipients (Figure 1C), suggesting that IL-17 signaling in host tissue was attenuating donor T-cell expansion. To understand the principal target organs protected from GVHD, we analyzed target organ histology. This revealed severe GI tract but not liver or lung pathology in B6.IL-17RA–deficient mice that received T-cell replete grafts. Importantly, IL-17RA−/− recipients of TCD grafts had no significant GI tract pathology, confirming this was GVHD related. (Figure 1D). Gut barrier function was also significantly perturbed in IL-17RA−/− recipients (Figure 1E). Collectively, these data demonstrate that IL-17 signaling within host tissue prevents severe GVHD of the GI tract.
We next determined the contribution of CD4 and/or CD8 donor T cells to hyper-acute GVHD. Recipients of grafts containing both CD4 and CD8 donor T cells developed early acute GVHD mortality, which was only modestly delayed in recipients receiving donor CD8-depleted T cells in isolation (Figure 1F). In contrast, recipients of donor CD4-depleted T cells in isolation had enhanced survival, developing lethal GVHD >5 weeks after SCT. Thus, the hyper-acute GVHD seen in IL-17RA−/− recipients was predominantly CD4 dependent.
Recipient-derived IL-17A protects against GVHD through IL-17RA signaling in host tissues
To confirm IL-17 was acting through receptor signaling in host tissues and not as a result of exaggerated signaling in donor T cells as a consequence of increased cytokine availability, due to increased receptor uptake, B6.IL-17RA−/− recipients were transplanted with either BALB/c.WT or BALB/c.IL-17RA−/− allografts. GVHD mortality was severe and identical in IL-17RA−/− recipients, regardless of the ability of IL-17 to signal within the donor (Figure 2A), confirming that IL-17 signaling through the donor T cell was not involved in IL-17–dependent protection from GVHD. We next investigated the contribution of donor-derived IL-17A in GVHD. WT or IL-17A−/− allografts were transplanted into B6.WT recipients. As expected, recipients of IL-17A−/− allografts had improved survival compared with recipients of WT allografts, confirming that donor-derived IL-17A promotes GVHD in this system.6 We next investigated the contribution of the recipient-derived IL-17A cytokine in this effect. IL-17A−/− recipients of WT allografts developed enhanced GVHD mortality relative to WT recipients of the same graft, consistent with a protective role of host-derived IL-17A in GVHD. To further confirm this, we transplanted IL-17A−/− allografts into WT or IL-17A−/− recipients. Acute GVHD was similarly enhanced in the absence of IL-17A from both donor and host sources, further demonstrating the importance of IL-17A from host tissues in providing protection from GVHD (Figure 2A). Together, these data demonstrate that IL-17A produced by host T cells and/or tissue, signals through the IL-17 receptor in host tissues to mediate protection against GVHD of the GI tract, independently of any IL-17 signaling in donor T cells.
Recipient-derived IL-17A protects against GVHD by signaling in recipient tissues without contribution from IL-17E or IL-17B molecules. (A) G-CSF mobilized T-cell replete or TCD BALB/c.WT, BALB/c.IL-17A−/−, or BALB/c.IL-17RA−/− donor grafts were transplanted into lethally irradiated B6.WT, B6.IL-17A−/−, or B6.IL-17RA−/− mice. Survival is represented by Kaplan-Meier analysis. Combined data from 5 individual experiments are shown. BALB/c.WT → B6.WT (red filled circles; n = 37); BALB/c.WT → B6.IL-17RA−/− (blue open circles; n = 39); BALB/c.IL-17RA−/− → B6.IL-17RA−/− (green filled square; n = 12); BALB/c.IL-17A−/− → B6.IL-17A−/− (purple filled inverted triangle; n = 21); BALB/c.WT → B6.IL-17A−/− (orange filled diamond; n = 20); BALB/c.IL-17A−/− → B6.WT (green open squares; n = 16); and BALB/c.WT (TCD) → B6.IL-17RA−/− (lavender filled triangle; n = 14). ****P < .0001, WT vs IL-17A−/− recipients; ****P < .0001, WT vs IL-17A−/− recipients transplanted with donor WT or IL-17A−/−, respectively. (B) G-CSF mobilized BALB/c.WT donor grafts were transplanted into lethally irradiated (B6.WT, red filled circles; B6.IL-17RA−/−, blue filled squares; B6.IL-17RB−/−, green open squares; or B6.IL-17RC−/−, purple filled inverted triangles) recipients (n = 17 to 24 per group). TCD BALB/c.WT grafts were transplanted into lethally irradiated (WT, orange open triangle; IL-17RA−/−, green open circle; IL-17RC−/−, lavender open circle) recipients (n = 3 to 7 per TCD group). Survival is represented by Kaplan-Meier analysis. Combined data from 4 replicate experiments are shown. ***P < .0001, WT vs IL-17RA−/− recipients; ***P < .0001, WT vs B6.IL-17RC−/− recipients. (C) BALB/c.WT donor BM and luciferase-expressing T cells were transplanted into lethally irradiated B6.WT, B6.IL-17RA−/−, and B6.IL-17RC−/− recipients (n = 8 to 9 per group). Animals were imaged at day 6 post-alloSCT and the bioluminescence intensity quantified (photons/sec/cm2/sr × 106). Combined data from 2 replicate experiments are shown. ****P < .0001, WT (red bars) vs IL-17RA−/− (blue bars); ***P = .0002, WT vs IL-17RC−/− (green bars). All data are presented as mean ± SEM.
Recipient-derived IL-17A protects against GVHD by signaling in recipient tissues without contribution from IL-17E or IL-17B molecules. (A) G-CSF mobilized T-cell replete or TCD BALB/c.WT, BALB/c.IL-17A−/−, or BALB/c.IL-17RA−/− donor grafts were transplanted into lethally irradiated B6.WT, B6.IL-17A−/−, or B6.IL-17RA−/− mice. Survival is represented by Kaplan-Meier analysis. Combined data from 5 individual experiments are shown. BALB/c.WT → B6.WT (red filled circles; n = 37); BALB/c.WT → B6.IL-17RA−/− (blue open circles; n = 39); BALB/c.IL-17RA−/− → B6.IL-17RA−/− (green filled square; n = 12); BALB/c.IL-17A−/− → B6.IL-17A−/− (purple filled inverted triangle; n = 21); BALB/c.WT → B6.IL-17A−/− (orange filled diamond; n = 20); BALB/c.IL-17A−/− → B6.WT (green open squares; n = 16); and BALB/c.WT (TCD) → B6.IL-17RA−/− (lavender filled triangle; n = 14). ****P < .0001, WT vs IL-17A−/− recipients; ****P < .0001, WT vs IL-17A−/− recipients transplanted with donor WT or IL-17A−/−, respectively. (B) G-CSF mobilized BALB/c.WT donor grafts were transplanted into lethally irradiated (B6.WT, red filled circles; B6.IL-17RA−/−, blue filled squares; B6.IL-17RB−/−, green open squares; or B6.IL-17RC−/−, purple filled inverted triangles) recipients (n = 17 to 24 per group). TCD BALB/c.WT grafts were transplanted into lethally irradiated (WT, orange open triangle; IL-17RA−/−, green open circle; IL-17RC−/−, lavender open circle) recipients (n = 3 to 7 per TCD group). Survival is represented by Kaplan-Meier analysis. Combined data from 4 replicate experiments are shown. ***P < .0001, WT vs IL-17RA−/− recipients; ***P < .0001, WT vs B6.IL-17RC−/− recipients. (C) BALB/c.WT donor BM and luciferase-expressing T cells were transplanted into lethally irradiated B6.WT, B6.IL-17RA−/−, and B6.IL-17RC−/− recipients (n = 8 to 9 per group). Animals were imaged at day 6 post-alloSCT and the bioluminescence intensity quantified (photons/sec/cm2/sr × 106). Combined data from 2 replicate experiments are shown. ****P < .0001, WT (red bars) vs IL-17RA−/− (blue bars); ***P = .0002, WT vs IL-17RC−/− (green bars). All data are presented as mean ± SEM.
IL-17A and/or IL-17F but not IL-17E or IL-17B protect against GVHD
Functional IL-17RA requires the formation of a complex by subunits A and C through which both IL-17A and IL-17F molecules can bind and initiate signaling. The A subunit also forms part of the IL-17 receptor B (IL-17RB) complex, together with subunit B through which IL-17E (IL-25) can signal. In addition, a second receptor involving IL-17RB exists through which the IL-17B molecule signals. To test that protection from gut GVHD is restricted to IL-17A (or IL-17F) and is not contributed to by IL-17E or IL-17B, we performed experiments using recipients who lacked IL-17 receptor subunits A, B, or C. Grafts from BALB/c.WT donors were transplanted into lethally irradiated B6.WT, B6.IL-17RA−/−, B6.IL-17RB−/−, and B6.IL-17RC−/− recipients. Relative to WT recipients, both the IL-17RA−/− and IL-17RC−/− recipients developed enhanced GVHD, whereas that in the IL-17RB−/− recipients was identical to WT recipients (Figure 2B). The difference in survival in the IL-17RA−/− and IL-17RC−/− mice likely reflects the fact that additional (non–IL-17A/F), as yet undefined IL-17 family members play a minor but important role in GVHD pathophysiology. To date, the known cytokines able to signal through IL-17RA but not IL-17RC or IL-17RB include IL-17C.22 We confirmed that the expansion of donor T cells was significantly enhanced in IL-17RC−/− recipients (Figure 2C), consistent with the hyper-acute GVHD noted in these animals. Thus, IL-17A and IL-17F but not IL-17E (IL-25) or IL-17B, mediate protection from GVHD within host tissue.
IL-17 signaling via both hematopoietic and nonhematopoietic recipient tissue attenuates acute GVHD
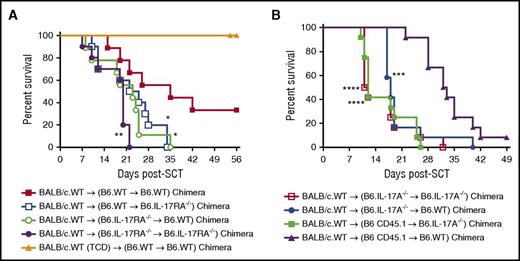
To investigate whether the protection against GVHD was mediated by cells/tissues located in either the hematopoietic or nonhematopoietic compartments of the host, chimeras were generated by transplanting TCD BM from B6.WT or B6.IL-17RA−/− mice into lethally irradiated B6.WT and B6.IL-17RA−/− recipients. Three months later, chimeras were re-transplanted and GVHD monitored thereafter. Again, recipients lacking IL-17RA signaling in both the hematopoietic and nonhematopoietic compartments developed GVHD faster than those in which the signaling was intact in both compartments (Figure 3A). In contrast, GVHD was intermediate in severity when IL-17 signaling was absent in either compartment in isolation (Figure 3A). Thus, IL-17 signaling in both hematopoietic and nonhematopoietic recipient tissues is required for maximal protection from the development of acute GVHD.
IL-17RA signaling in both hematopoietic and nonhematopoietic recipient tissue attenuates acute GVHD. (A) Lethally irradiated IL-17RA−/− chimeras were transplanted with T-cell replete (n = 13 per group) or TCD (n = 7 per group) G-CSF mobilized BALB/c.WT donor splenocytes. Survival is represented by Kaplan-Meier analysis. Combined data from 2 replicate experiments are shown. Survival: **P = .004, WT → WT chimera (red filled squares) vs IL-17RA−/− → IL-17RA−/− chimera (purple filled circles); *P = .028, WT → WT chimera (red filled squares) vs WT → IL-17RA-−/− chimera (blue open squares); *P = .024, WT → WT chimera (red filled squares) vs IL-17RA−/− → WT chimera (green open circles). (B) Lethally irradiated IL-17A−/− chimeras were transplanted with G-CSF mobilized BALB/c.WT donor splenocytes (n = 12 per group). Survival is represented by Kaplan-Meier analysis. Combined data from 2 replicate experiments are shown. Survival: ****P < .0001, WT → WT chimera (purple filled triangles) vs IL-17A−/− → IL-17A−/− chimera (red open squares); ****P < .001, WT → WT chimera (purple filled triangles) vs WT → IL-17A-−/− chimera (green filled squares); ***P = .0006, WT → WT chimera (purple filled triangles) vs IL-17A−/− → WT chimera (blue filled circles).
IL-17RA signaling in both hematopoietic and nonhematopoietic recipient tissue attenuates acute GVHD. (A) Lethally irradiated IL-17RA−/− chimeras were transplanted with T-cell replete (n = 13 per group) or TCD (n = 7 per group) G-CSF mobilized BALB/c.WT donor splenocytes. Survival is represented by Kaplan-Meier analysis. Combined data from 2 replicate experiments are shown. Survival: **P = .004, WT → WT chimera (red filled squares) vs IL-17RA−/− → IL-17RA−/− chimera (purple filled circles); *P = .028, WT → WT chimera (red filled squares) vs WT → IL-17RA-−/− chimera (blue open squares); *P = .024, WT → WT chimera (red filled squares) vs IL-17RA−/− → WT chimera (green open circles). (B) Lethally irradiated IL-17A−/− chimeras were transplanted with G-CSF mobilized BALB/c.WT donor splenocytes (n = 12 per group). Survival is represented by Kaplan-Meier analysis. Combined data from 2 replicate experiments are shown. Survival: ****P < .0001, WT → WT chimera (purple filled triangles) vs IL-17A−/− → IL-17A−/− chimera (red open squares); ****P < .001, WT → WT chimera (purple filled triangles) vs WT → IL-17A-−/− chimera (green filled squares); ***P = .0006, WT → WT chimera (purple filled triangles) vs IL-17A−/− → WT chimera (blue filled circles).
We next sought to determine whether the hematopoietic or nonhematopoietic compartment of the host was the source of IL-17A. We generated chimeras by transplanting TCD BM from B6.WT or B6.IL-17A−/− mice into lethally irradiated B6.WT or B6.IL-17A−/− recipients. Chimeras were re-transplanted as above. Recipients unable to generate any IL-17A from both hematopoietic and nonhematopoietic compartments, or from either compartment independently, succumbed to acute GVHD in an accelerated fashion relative to those in which IL-17A generation was intact in both compartments (Figure 3B). Thus, recipient IL-17A derived from both the hematopoietic and nonhematopoietic compartments ameliorates acute GVHD.
Multiple recipient hematopoietic IL-17–producing cell subsets fate-map for IL-17
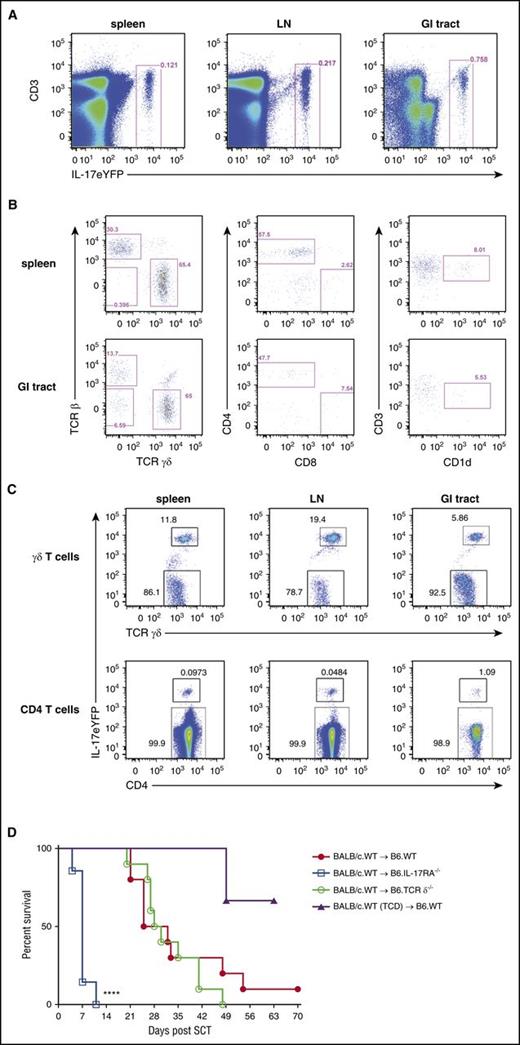
Having demonstrated that the hematopoietic compartment was important for generating IL-17A, we aimed to define which cell population(s) were the source of IL-17A and could be responsible for the observed protective effect against hyper-acute GI-tract GVHD. Using IL-17eYFP fate-map reporter mice (in which YFP is expressed continuously once a cell has made IL-17A), we isolated lymphoid and GI-tract tissue and phenotyped the IL-17A–producing cell populations therein. In naïve mice, the GI tract showed the greatest frequency of IL-17A–producing cells (0.76% vs <0.22% in lymphoid tissue; Figure 4A), which were comprised predominantly of γδ T cells and conventional CD4 T cells (and to a lesser extent, T-cell receptor negative [TCRneg] innate lymphoid T cells and NKT cells; Figure 4A-B). The proportion of γδ T cells that IL-17A fate-mapped was 5% to 19% and varied between tissues (Figure 4C). In contrast, the proportions of CD4 T cells producing IL-17A were smaller (0.05% to 1%), with the highest numbers again shown in the GI tract (Figure 4C). Because γδ T cells were the predominant IL-17A–producing cell population, we examined whether these cells were critical for the IL-17A–mediated protective effect in recipients. We performed alloSCT using TCR δ−/− or WT recipients. No difference in survival (Figure 4D) was observed between groups, demonstrating that γδ T cells were unlikely to be the IL-17A–producing cell population required to alleviate hyper-acute GVHD.
Recipient γδ T cells and conventional CD4 T cells are the predominant source of IL-17A after SCT. (A) Representative plots showing the frequency of IL-17eYFP+ cells in spleen, LN, and the GI tract of naïve B6.IL-17eYFP reporter mice. (B) Phenotype of the IL-17eYFP+ cells in the spleen and GI tract; γδ T cells (TCRγδ+), conventional CD4 T cells (TCRβ+ CD4+), conventional CD8 T cells (TCRβ+ CD8+), and NKT cells (CD3+ CD1d+) are shown. (C) Proportion of IL-17eYFP+ vs IL-17eYFPneg γδ T cells and conventional CD4 T cells in spleen, LN, and the GI tract of naïve B6.IL-17eYFP reporter mice is shown. Data combined from individual mice (n = 3) for all tissues except the GI tract where the mice were pooled. (D) Lethally irradiated B6.WT (red filled circles), B6.IL-17RA−/− (blue open squares), or B6.TCRδ−/− (green open circles) mice received either T-cell replete or TCD grafts (purple filled triangles) (n = 7 to 10 per group; TCD group, n =3) from G-CSF immobilized BALB/c.WT donors. Survival is represented by Kaplan-Meier analysis. Data combined from 2 replicate experiments are shown. ****P < .0001, WT vs IL-17RA−/− recipients. LN, lymph node.
Recipient γδ T cells and conventional CD4 T cells are the predominant source of IL-17A after SCT. (A) Representative plots showing the frequency of IL-17eYFP+ cells in spleen, LN, and the GI tract of naïve B6.IL-17eYFP reporter mice. (B) Phenotype of the IL-17eYFP+ cells in the spleen and GI tract; γδ T cells (TCRγδ+), conventional CD4 T cells (TCRβ+ CD4+), conventional CD8 T cells (TCRβ+ CD8+), and NKT cells (CD3+ CD1d+) are shown. (C) Proportion of IL-17eYFP+ vs IL-17eYFPneg γδ T cells and conventional CD4 T cells in spleen, LN, and the GI tract of naïve B6.IL-17eYFP reporter mice is shown. Data combined from individual mice (n = 3) for all tissues except the GI tract where the mice were pooled. (D) Lethally irradiated B6.WT (red filled circles), B6.IL-17RA−/− (blue open squares), or B6.TCRδ−/− (green open circles) mice received either T-cell replete or TCD grafts (purple filled triangles) (n = 7 to 10 per group; TCD group, n =3) from G-CSF immobilized BALB/c.WT donors. Survival is represented by Kaplan-Meier analysis. Data combined from 2 replicate experiments are shown. ****P < .0001, WT vs IL-17RA−/− recipients. LN, lymph node.
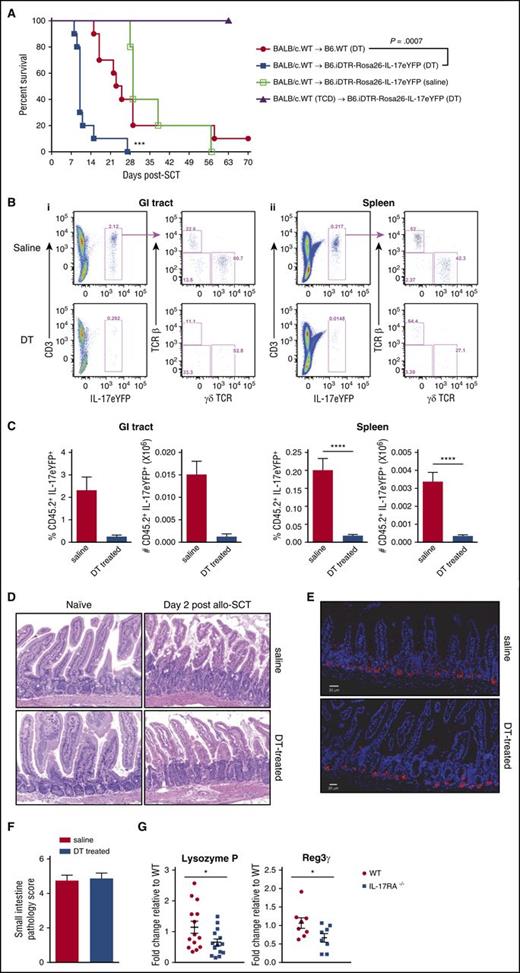
Given the technical issues encountered and the limitation of reagents to directly address the contribution of conventional CD4 T cells, innate lymphoid T cells, and NKT cells to the IL-17–mediated protective effect, we examined the effect of depleting all IL-17–producing populations from the recipient using a DT depletion strategy. We used the IL-17eYFP reporter-deletor mice (B6.iDTR-Rosa26–IL-17eYFP) that deplete all IL-17–producing cells upon DT administration. These mice, in parallel with B6.WT mice were administered 3 doses of DT (days −3, −1, and +1), transplanted with G-CSF BALB/c.WT grafts, and monitored for GVHD. This demonstrated that depletion of all IL-17–producing cells enhanced acute GVHD mortality compared with WT recipients (Figure 5A). Saline-treated IL-17eYFP reporter-deletor mice showed no difference in GVHD incidence compared with B6.WT mice administered with DT, indicating DT toxicity was not causing this effect (Figure 5A). To confirm that DT administration effectively depleted IL-17–producing cells, IL-17eYFP reporter-deletor mice were administered either saline or DT as described earlier, and the frequency and number of CD45.2+ IL-17eYFP+ cells enumerated at day 2 post-SCT in the GI tract and spleen. This demonstrated that recipient IL-17eYFP+ cells were depleted by 90% after DT administration compared with saline treatment (Figure 5B-C). To address whether the administration of DT or the depletion of IL-17A–producing populations in this system impacted on gut integrity, we examined the ileum at a time when pathology induced by DT and total body irradiation would be dominant, as opposed to GVHD. Firstly, DT administration and IL-17 deletion did not result in any overt pathology (0 to 1 apoptotic cell per 5 crypts) in nontransplanted mice (Figure 5D). Secondly, although Paneth cells showed a stressed phenotype early after transplant (day 2) with apoptosis in the base of the crypts, Paneth cell loss, and evidence of vacuolation, granule fusion, and degranulation, this appeared similar in both groups (Figure 5D). Similar numbers of Paneth cells were also confirmed with lysozyme staining of the GI tract (Figure 5E). In addition, the SI semiquantitative pathology scores at this time revealed minor damage that was similar between saline and DT-treated recipients (Figure 5F). Thus, DT administration and IL-17 depletion did not induce pathology directly in the GI tract early after BMT but did exacerbate acute GVHD, consistent with a protective role for recipient-derived IL-17 in allogeneic BMT.
Conditional depletion of recipient IL-17–producing populations exacerbates acute GVHD. (A) B6.WT or B6.iDTR-Rosa26–IL-17 mice (n = 10 per group) were lethally irradiated and received T-cell replete or TCD G-CSF mobilized BALB/c.WT grafts. Saline or DT was administered peritransplant (days −3, −1, and +1). Kaplan-Meier curves are shown with combined data from 2 replicate experiments. ***P = .0007, DT-treated WT (red filled circles) vs B6.iDTR-Rosa26-IL-17 recipients (blue filled squares). (B-C) Efficiency of depletion was determined by flow cytometry (panels Bi and Bii top, saline treated; panels Bi and Bii bottom, DT-treated). Spleen (Bi) and GI tract (Bii) were harvested from mice 2 days after SCT, and the frequency and absolute number of IL-17eYFP+ cells enumerated (n = 3 to 11 per group). Data combined from 2 replicate experiments are shown in panel C. Spleen: ****P < .0001, saline (red bars) vs DT-treated (blue bars) B6.iDTR-Rosa26–IL-17 recipients. Representative dot plots are shown in (B). (D) Representative H&E images of ileum harvested from naïve B6.iDTR-Rosa26–IL-17 mice treated with saline or DT only (no total body irradiation or SCT) or B6.iDTR-Rosa26–IL-17 mice post-alloSCT (day 2) with saline or DT administration. (E) Representative immunofluorescence images showing lysozyme expression (AF555, red) in ileum harvested on day 2 post-alloSCT from saline (top) or DT-treated (bottom) B6.iDTR-Rosa26–IL-17 recipients. DAPI nuclear stain (blue). Scale bar = 20 µm. (F) Semiquantitative histopathology of the ileum on day 2 from saline (red bar) or DT-treated (blue bar) B6.iDTR-Rosa26–IL-17 recipients (n = 8 per group). (G) Lethally irradiated B6.WT (red circles) or B6.IL-17RA−/− (blue squares) mice received 25 × 106 G-CSF immobilized BALB/c.WT grafts and the ileum harvested on day 2. Lysozyme P and Reg 3γ expression (n = 8 to 14 per group) was analyzed by RT-qPCR and normalized to WT expression. Combined data from 3 replicate experiments are shown. *P = .04, WT vs IL-17RA−/−. DAPI, 4,6, diamidino-2-phenylindole; H&E, hematoxylin and eosin; RT-qPCR, real-time quantitative polymerase chain reaction.
Conditional depletion of recipient IL-17–producing populations exacerbates acute GVHD. (A) B6.WT or B6.iDTR-Rosa26–IL-17 mice (n = 10 per group) were lethally irradiated and received T-cell replete or TCD G-CSF mobilized BALB/c.WT grafts. Saline or DT was administered peritransplant (days −3, −1, and +1). Kaplan-Meier curves are shown with combined data from 2 replicate experiments. ***P = .0007, DT-treated WT (red filled circles) vs B6.iDTR-Rosa26-IL-17 recipients (blue filled squares). (B-C) Efficiency of depletion was determined by flow cytometry (panels Bi and Bii top, saline treated; panels Bi and Bii bottom, DT-treated). Spleen (Bi) and GI tract (Bii) were harvested from mice 2 days after SCT, and the frequency and absolute number of IL-17eYFP+ cells enumerated (n = 3 to 11 per group). Data combined from 2 replicate experiments are shown in panel C. Spleen: ****P < .0001, saline (red bars) vs DT-treated (blue bars) B6.iDTR-Rosa26–IL-17 recipients. Representative dot plots are shown in (B). (D) Representative H&E images of ileum harvested from naïve B6.iDTR-Rosa26–IL-17 mice treated with saline or DT only (no total body irradiation or SCT) or B6.iDTR-Rosa26–IL-17 mice post-alloSCT (day 2) with saline or DT administration. (E) Representative immunofluorescence images showing lysozyme expression (AF555, red) in ileum harvested on day 2 post-alloSCT from saline (top) or DT-treated (bottom) B6.iDTR-Rosa26–IL-17 recipients. DAPI nuclear stain (blue). Scale bar = 20 µm. (F) Semiquantitative histopathology of the ileum on day 2 from saline (red bar) or DT-treated (blue bar) B6.iDTR-Rosa26–IL-17 recipients (n = 8 per group). (G) Lethally irradiated B6.WT (red circles) or B6.IL-17RA−/− (blue squares) mice received 25 × 106 G-CSF immobilized BALB/c.WT grafts and the ileum harvested on day 2. Lysozyme P and Reg 3γ expression (n = 8 to 14 per group) was analyzed by RT-qPCR and normalized to WT expression. Combined data from 3 replicate experiments are shown. *P = .04, WT vs IL-17RA−/−. DAPI, 4,6, diamidino-2-phenylindole; H&E, hematoxylin and eosin; RT-qPCR, real-time quantitative polymerase chain reaction.
Paneth cells in intestinal crypts are the predominant producers of the majority of antimicrobial peptides in the SI, principally α-defensin/cryptdin, which selectively kill pathogens while preserving commensal bacteria.23 Recently, naïve IL-17A−/− and IL-17RA−/− mice have been shown to have perturbed antimicrobial peptide production.24 We thus investigated the expression of Paneth cell-specific antimicrobial peptides after BMT in the absence of intact IL-17RA signaling. We allografted WT and IL-17RA−/− mice and harvested ileum tissue at day 2, and noted a significant reduction in lysozyme P and Reg 3γ messenger RNA expression (Figure 5G), suggesting that host-derived IL-17A signaling is important for the generation of antimicrobial peptides.
Cohousing with IL-17RA−/− or IL-17RC−/− mice increases susceptibility to acute GVHD
The primacy of the GI tract in dictating GVHD outcomes is well established25 and increasing recent evidence has suggested an important role for the gut microbiome in this phenomenon.26,27 We hypothesized that IL-17RA−/− mice susceptible to hyper-acute GVHD would display a unique or altered GI microbial community, and that this may contribute to the severity of GVHD in these mice. We first confirmed there were no differences in kinetics of GVHD between B6 mice bred and raised in our animal facility and those purchased from external vendors (supplemental Figure 1). We then cohoused WT and IL-17RA−/− mice to equilibrate the gut microbial community prior to transplant and confirmed that the microbiota, as measured by 16S rRNA gene amplicon profiling, had converged (Figure 6A). We observed convergence to a similar composition in 2 independent experiments despite WT mice displaying different precohousing profiles (Figure 6A). Convergence was less apparent when analysis was conducted at the family level and only a single family displayed consistent abundance shifts across the 2 experiments, suggesting opposing fluctuations were occurring within bacterial families (supplemental Table 1). Separately housed mice of each strain analyzed in parallel during the second experiment retained their initial gut community profiles, confirming the community shift was a product of the cohousing environment (Figure 6A). Critically, the transfer of the IL-17RA−/− microbiota to WT mice during cohousing was associated with the development of significantly accelerated acute GVHD in these mice relative to separately housed WT recipients (median survival of 9 vs 27.5 days), indicating transferrable members of the microbial community likely play an important role in disease development (Figure 6B; supplemental Table 1). In contrast, no change in mortality was observed in IL-17RA−/− mice following cohousing despite changes in their gut microbiota (Figure 6B; supplemental Table 2).
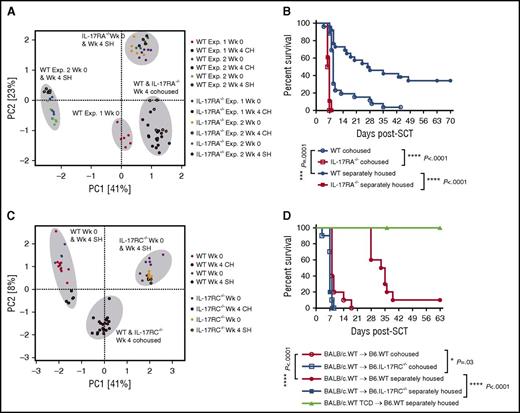
Transfer of microbiota during cohousing confers increased susceptibility to intestinal hyper-acute GVHD. (A) PCA plot of B6.WT and B6.IL-17RA−/− fecal microbiota sampled pre- and postcohousing (4 weeks) of mice. Data combined from 2 experiments (n = 12 per group) are shown with separately housed controls included within experiment 2 and sampled during the same period. (B) Cohoused or separately housed B6.WT or B6.IL-17RA−/− mice (n = 18 to 26 per group) were lethally irradiated (1000 cGy) and transplanted with G-CSF mobilized BALB/c.WT grafts. Survival is represented by Kaplan-Meier analysis: ***P = .0001, BALB/c.WT → B6.WT cohoused (blue open circle) vs B6.WT separately housed (blue filled circle); ****P < .0001, BALB/c.WT → B6.WT separately housed (blue filled circle) vs IL-17RA−/− separately housed (red filled square); ****P < .0001, BALB/c.WT → B6.WT cohoused (blue open circle) vs IL-17RA−/− cohoused (red open square). Data combined from 3 replicate experiments are shown. (C) PCA plot of B6.WT and B6.IL-17RC−/− fecal microbiota sampled pre- and postcohousing (4 weeks), and separately housed controls sampled during the same period. Data from a single experiment are shown (n = 5 to 10 per group). (D) Cohoused (4 weeks) or separately housed B6.WT or B6.IL-17RC−/− mice (n = 5 to 10 per group) were lethally irradiated (1000 cGy) and transplanted with G-CSF mobilized BALB/c.WT grafts (T-cell replete or TCD). Survival is represented by Kaplan-Meier analysis: ****P < .0001, BALB/c.WT → B6.WT cohoused (red open circle) vs B6.WT separately housed (red filled circle); ****P < .0001, BALB/c.WT → B6.WT separately housed (red filled circle) vs IL-17RC−/− separately housed (blue filled square); *P = .03, BALB/c.WT → B6.WT cohoused (red open circle) vs IL-17RC−/− cohoused (blue open square). Data combined from 2 experiments. CH, cohoused mice; PCA, principal component analysis; SH, separately housed mice; Wk, week.
Transfer of microbiota during cohousing confers increased susceptibility to intestinal hyper-acute GVHD. (A) PCA plot of B6.WT and B6.IL-17RA−/− fecal microbiota sampled pre- and postcohousing (4 weeks) of mice. Data combined from 2 experiments (n = 12 per group) are shown with separately housed controls included within experiment 2 and sampled during the same period. (B) Cohoused or separately housed B6.WT or B6.IL-17RA−/− mice (n = 18 to 26 per group) were lethally irradiated (1000 cGy) and transplanted with G-CSF mobilized BALB/c.WT grafts. Survival is represented by Kaplan-Meier analysis: ***P = .0001, BALB/c.WT → B6.WT cohoused (blue open circle) vs B6.WT separately housed (blue filled circle); ****P < .0001, BALB/c.WT → B6.WT separately housed (blue filled circle) vs IL-17RA−/− separately housed (red filled square); ****P < .0001, BALB/c.WT → B6.WT cohoused (blue open circle) vs IL-17RA−/− cohoused (red open square). Data combined from 3 replicate experiments are shown. (C) PCA plot of B6.WT and B6.IL-17RC−/− fecal microbiota sampled pre- and postcohousing (4 weeks), and separately housed controls sampled during the same period. Data from a single experiment are shown (n = 5 to 10 per group). (D) Cohoused (4 weeks) or separately housed B6.WT or B6.IL-17RC−/− mice (n = 5 to 10 per group) were lethally irradiated (1000 cGy) and transplanted with G-CSF mobilized BALB/c.WT grafts (T-cell replete or TCD). Survival is represented by Kaplan-Meier analysis: ****P < .0001, BALB/c.WT → B6.WT cohoused (red open circle) vs B6.WT separately housed (red filled circle); ****P < .0001, BALB/c.WT → B6.WT separately housed (red filled circle) vs IL-17RC−/− separately housed (blue filled square); *P = .03, BALB/c.WT → B6.WT cohoused (red open circle) vs IL-17RC−/− cohoused (blue open square). Data combined from 2 experiments. CH, cohoused mice; PCA, principal component analysis; SH, separately housed mice; Wk, week.
Mice deficient in the second IL-17A/F receptor signaling subunit, IL-17RC, also developed accelerated GVHD relative to WT (Figure 2B). Therefore, we undertook an additional cohousing of WT mice with IL-17RC−/− mice and analyzed the subsequent susceptibility to acute GVHD in these animals. Fecal community profiles confirmed a similar convergence of microbiota following 4 weeks of cohousing (Figure 6C), visible much more distinctly at the OTU level rather than at the family level (data not shown). Furthermore, once transplanted, cohoused WT mice developed significantly accelerated GVHD in comparison with their separately housed counterparts (median survival of 8 vs 34 days), as observed following cohousing with IL-17RA−/− mice (Figure 6D). Comparison across all 3 experiments revealed a number of OTUs with significantly different abundance in WT mice pre- and postcohousing; some displaying increased and others decreased abundance at the end of the cohousing period (Figure 7A; supplemental Table 3). The majority of these OTUs were from the bacterial family S24-7 (recently defined as “Candidatus Homeothermaceae”28 ), suggesting the possibility of both antagonistic and protective roles for this family within GVHD pathology.
Shifts in microbial communities to WT mice after cohousing with IL-17RA−/−or IL-17RC−/−mice are similar. (A) Heatmap displaying differentially abundant OTUs consistently increased or decreased in WT mice after cohousing with IL-17RA−/− or IL-17RC−/− mice for 4 weeks. Heatmap generated based on fecal microbiota profile from 16S rRNA sequencing of WT, IL-17RA−/−, and IL-17RC−/− mice. Each column represents an individual WT mouse. Data from 2 IL-17RA−/− cohousing experiments are shown (n = 12 mice, experiments 1 and 2) and 1 IL-17RC−/− cohousing experiment (n = 10 mice, experiment 3). Scale is based on normalized read counts generated using the regularized log transformation implemented within DESeq2. Details available in supplemental Table 3. (B) Representative fluorescence in situ hybridization images of the colon at day 6 post-alloSCT (WT [left] and IL-17RA−/− [right]) utilizing universal rRNA probes to show bacteria (red) and their translocation from the lumen (L) into the tissue (yellow arrows). Bacterial morphology is highlighted in the higher magnification image shown of the boxed area (red outline). DAPI nuclear stain (blue). Scale bar = 50 μm. MM, muscularis mucosae; SM, submucosa.
Shifts in microbial communities to WT mice after cohousing with IL-17RA−/−or IL-17RC−/−mice are similar. (A) Heatmap displaying differentially abundant OTUs consistently increased or decreased in WT mice after cohousing with IL-17RA−/− or IL-17RC−/− mice for 4 weeks. Heatmap generated based on fecal microbiota profile from 16S rRNA sequencing of WT, IL-17RA−/−, and IL-17RC−/− mice. Each column represents an individual WT mouse. Data from 2 IL-17RA−/− cohousing experiments are shown (n = 12 mice, experiments 1 and 2) and 1 IL-17RC−/− cohousing experiment (n = 10 mice, experiment 3). Scale is based on normalized read counts generated using the regularized log transformation implemented within DESeq2. Details available in supplemental Table 3. (B) Representative fluorescence in situ hybridization images of the colon at day 6 post-alloSCT (WT [left] and IL-17RA−/− [right]) utilizing universal rRNA probes to show bacteria (red) and their translocation from the lumen (L) into the tissue (yellow arrows). Bacterial morphology is highlighted in the higher magnification image shown of the boxed area (red outline). DAPI nuclear stain (blue). Scale bar = 50 μm. MM, muscularis mucosae; SM, submucosa.
To address the question of how the changed microbiota was able to lead to GVHD, we performed fluorescence in situ hybridization using universal bacterial probes to localize bacteria in the GI tract of WT and IL-17RA−/− mice 6 days post-alloSCT. Evidence of bacterial translocation was noted in the colon of IL-17RA−/− mice with bacteria normally sequestered in the lumen being detected in the submucosal layer surrounding blood vessels (Figure 7B). This likely reflects the final stimulus for hyperacute GVHD and the mortality seen in these mice.
Discussion
Here we describe that IL-17A derived from recipient cells plays a critical role in regulating T-cell activation, expansion, and subsequent donor CD4-dependent acute GVHD within the GI tract. This effect requires signaling of the IL-17RA/RC receptor complex on both hematopoietic and nonhematopoietic recipient cells by IL-17A (and potentially IL-17F). There appear to be multiple and redundant recipient sources of IL-17, including innate and CD4 lymphoid cells and undefined parenchymal sites, most likely within the GI tract. Interestingly, the gut microbiota of the IL-17A/C–deficient mice are capable of inducing susceptibility to acute GVHD in WT mice following transfer via cohousing.
The effects of donor-derived IL-17A and related cytokines in both experimental and clinical SCT is becoming increasingly clear as a pathogenic immune response, mediating relatively tissue predominant injury in the skin and lung.29 Within the constraints of experimental mouse models, these effects appear to include both early and late manifestations of GVHD with features of both acute and chronic GVHD.6-8,29 Nevertheless, at least 1 study has suggested that donor-derived IL-17 may be protective.30 In contrast, the effects of recipient-derived IL-17 and signaling thereof have not been described in the SCT setting. However, elegant work by multiple investigators has demonstrated the importance of innate-like lymphoid cells in controlling inflammation in the GI tract. These cell subtypes include invariant NKT cells, γδ T cells, and group 3 innate lymphoid cells (ILCs) that have been most recently described as lymphoid-tissue inducer and type 3 NKp46neg ILCs. In our studies, the absence of recipient invariant NKT (data not shown) and γδ T cells (Figure 4D) did not phenocopy the absence of recipient IL-17A/F signaling, making these cells an unlikely protective recipient cell population in isolation. Of note, the group 3 ILCs also make IL-22, and recipient ILC-derived sources of this cytokine have been shown to play an important role in GI-tract homeostasis after BMT.31 Nevertheless, in our model, the absence of recipient IL-22 (ie, BALB/c → B6.IL-22−/− vs B6.WT) results in only a very modest acceleration in mortality (median survival of 26 vs 35 days; P = .009; data not shown). This is in stark contrast to the acceleration in mortality in the absence of IL-17RA or IL-17RC, where the median day of mortality is accelerated from day 38 to day 8 and 9 post-BMT, respectively (Figure 2B). It would thus appear that the effects of IL-17 are largely asynchronous and independent of IL-22, and thus likely independent of group 3 ILCs. The final hematopoietic source of IL-17A examined was conventional CD4 T cells. Intriguingly, a subset of Th17 cells in the gut has been described as having regulatory properties with high levels of IL-10 and low TNF and IL-2 production,32 and is a likely regulatory cell in the GVHD scenario. IL-17A has also been demonstrated to be produced by Paneth cells within intestinal crypts in response to TNF, where it can contribute to inflammation and the generation of luminal defensins and antimicrobial peptides.33,34 Because TNF is a characteristic pro-inflammatory cytokine generated during GVHD,25 this would appear an additional important molecular pathway that may be involved in IL-17–dependent maintenance of GI-tract integrity. Nevertheless, changes in GI-tract integrity alone cannot account for the dramatic enhancement in T-cell activation seen in the absence of recipient IL-17 signaling.16 Thus there are multiple sources of recipient-derived IL-17 that together provide dramatic regulatory effects, and these include at least CD4 T cells and nonhematopoietic tissue, likely Paneth cells.
Th17 cells are key producers of IL-17 and have been shown to be induced by a number of bacteria, thereby stimulating the recruitment of neutrophils and subsequent bacterial or fungal clearance.35 Notably, colonization of the terminal ileum by commensal segmented filamentous bacteria (SFB) resulted in the induction and accumulation of Th17 cells in the lamina propria.36,37 More recently, signaling via IL-17RA in particular has been demonstrated to control SFB abundance.24 We detected very low levels (∼0.5% average relative abundance; data not shown) of a single OTU belonging to “Candidatus Savagella”38 (SFB), recently demonstrated to be controlled by IL-17R signaling24 in all our mouse strains (ie, both WT and IL-17RA/C deletion strains). However, because SFB are ileal colonizers, accurate estimates of their abundance likely require samples generated specifically from this region. IL-17 deficiency, either due to cytokine or receptor deletion, increased susceptibility to infection by extracellular microorganisms including Klebsiella pneumoniae,Toxoplasma gondii, and Candida albicans.39 IL-17 and associated transcriptional regulators has also been shown to play a role in host defense against C albicans.40-44 These studies demonstrate an important role for IL-17 in controlling opportunistic infections and by extension, the maintenance of commensal microbiota.
The majority of OTUs identified as significantly changing in abundance during cohousing of WT with IL-17RA−/− or IL-17RC−/− mice belong to the family “Ca Homeothermaceae” (S24-7). Although this likely reflects the prevalence of “Ca Homeothermaceae” within the murine host,28 the family is increasingly identified via sequencing-based approaches as being associated with perturbation of the gut environment.45,46 At the family level, “Ca Homeothermaceae” has been identified as increasing in abundance following treatment-induced remission of colitis in T-bet−/−Rag2−/− mice,45 supporting a beneficial, or at least a noninflammatory role. In contrast, we describe a potential antagonistic role for specific members of “Ca Homeothermaceae” increased during cohousing in WT mice, emphasizing the importance of fine-scale analysis of the microbiome; different genera or species within a family may elicit very different responses. “Ca Homeothermaceae” populations are identified in both immunoglobulin A+ and immunoglobulin Aneg fractions of fecal microbiota, supporting differential interaction with the immune system,47,48 and gene content variability has now been confirmed within the family.28 For example, some species were found to encode the enzyme urease, which has been shown to contribute to the deterioration of gastric barrier function associated with Helicobacter pylori colonization.49 Other differentially encoded functions within the family include putative capsule production and catalase production, which may inhibit phagocytosis by or promote survival within neutrophils, respectively.50,51 In addition, catalase-positive members of “Ca Homeothermaceae” may protect those in close proximity, including members of other families, from oxidative stress-mediated host clearance mechanisms.52 Unfortunately, the majority of published “Ca Homeothermaceae” genomes lack 16S rRNA sequences, preventing direct comparison with the differentially abundant OTUs identified in the current study. “Ca Homeothermaceae” has also only recently been cultured in the laboratory,53 and as such causative relationships between this family and host health have yet to be established.
The phenomenon of transfer to and dominance of a WT host by a disease causing microbiota is consistent with findings in other disease settings.54,55 Why WT mice seem more amenable to shifts in their gut microbiota is unclear. It may be that each genetically deficient mouse strain presents a unique environmental niche that promotes colonization by species able to exploit each respective deficiency and that increased abundance in WT mice, where regulatory mechanisms are intact, can only be achieved following a period of constant environmental exposure. Evidence to support this theory is provided by a recent study demonstrating that colitis-inducing microbiota from inflammasome-deficient mice are capable of inhibiting NLRP6 inflammasome signaling upon transfer to a WT host.56 Decreased NLRP6 signaling resulted in decreased IL-18–induced antimicrobial peptide production, which favored colonization by dysbiotic microbiota. At this point in time, a role for IL-17A in mediating inflammasome-dependent effects has not been described. Alternatively, the absence of host IL-17 signaling may promote colonization with bacteria that are typically IL-17 inducing and would normally be cleared by inflammatory responses but escape this selective pressure in an IL-17–deficient system.
Finally, IL-17 is recognized as an important pathogenic molecule driving late (predominantly chronic) GVHD6 and is an attractive candidate for inhibition. Indeed, numerous monoclonal antibodies that target the cytokine or the receptor are in late stage development and have demonstrated particular efficacy in primary cutaneous diseases such as psoriasis.57 Although these agents would appear attractive for controlling alloreactivity, our data show that the effects of IL-17 on host biology appear to trump the effects of donor IL-17 early after transplant; ie, the net effect of blocking IL-17 in both donor and host tissues early after BMT appears to worsen GVHD. Furthermore, our data demonstrate that IL-17 is critical for preventing dysbiosis in the peritransplant period, which in turn influences the severity and penetrance of GVHD. It is important to note that this microbiome effect is dominant in the IL-17–deficient mice and thus we cannot exclude additional and important immunologic regulatory effects by recipient IL-17, an effect supported by our experiments using DT-induced IL-17 deletion peritransplant (because these mice were litter mates pretransplant). Importantly, this implies that blocking IL-17 in a clinical trial could have adverse effects via dysbiosis, particularly in the early posttransplant setting, and raises caution about their potential effects if used long term.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors gratefully acknowledge Nicola Angel from the Australian Centre for Ecogenomics, University of Queensland, for assistance with sample preparation and 16S rRNA sequencing; and Clay Winterford in Histology (QIMR Berghofer) and Nigel Waterhouse in the Australian Cancer Research Foundation Centre for Comprehensive Biomedical Imaging (QIMR Berghofer) for expert technical assistance with immunofluorescence staining and imaging.
This work was supported by grants from the Australian National Health and Medical Research Council, the National Institutes of Health, National Institute of Allergy and Infectious Diseases (R01 AI34495), and the National Institutes of Health, National Heart, Lung, and Blood Institute (R01 HL56067). G.R.H. is an Australian National Health and Medical Research Council Senior Principal Research Fellow. P.H. is an Australian Research Council Laureate Fellow.
Authorship
Contribution: A.V. designed and performed experiments, analyzed data, and wrote the manuscript; M.D.B., M.K., K.H.G., R.D.K., K.R.L., C.Y.L., A.S.H., P.Z., and S.Z.H. performed experiments and contributed to helpful discussions; K.L.O. and N.L. generated microbiome data; K.L.O. and P.H. provided expert analysis and interpretation of microbiome data, and contributed to writing the manuscript; A.D.C. and M.A.M. performed blinded histologic assessment; M.A.M., B.R.B., K.P.A.M., and P.H. contributed to experimental design and/or provided intellectual input; and G.R.H. contributed to the design of experiments, provided intellectual input, and wrote the manuscript.
Conflict-of-interest disclosure: G.R.H. has received a paid consultancy from Amgen. The remaining authors declare no competing financial interests.
Correspondence: Geoffrey R. Hill, QIMR Berghofer Medical Research Institute, 300 Herston Rd, Herston, Brisbane, QLD 4006, Australia; e-mail: geoff.hill@qimrberghofer.edu.au; and Antiopi Varelias, QIMR Berghofer Medical Research Institute, 300 Herston Rd, Herston, Brisbane, QLD 4006, Australia; e-mail: antiopi.varelias@qimrberghofer.edu.au.

![Figure 1. IL-17RA signaling in host tissues attenuates GVHD and donor T-cell expansion. (A) Lethally irradiated B6.WT or B6.IL-17RA−/− mice received T-cell replete or TCD splenocytes from G-CSF mobilized BALB/c.WT donors. Survival is represented by Kaplan-Meier analysis: ****P < .0001, BALB/c.WT → B6.WT (red filled circles; n = 21) vs BALB/c.WT → B6.IL-17RA−/− (blue filled squares; n = 17). BALB/c.WT (TCD) → B6.IL-17RA−/− (green open squares; n = 10). Combined data from 3 experiments are shown. (B) Serum cytokine levels over time post-alloSCT (n = 6 per group). B6.WT (red bars) vs B6.IL-17RA−/− (blue bars) recipients: IL-17A, **P = .002 (day 4); GM-CSF, *P = .02 (day 2), **P = .004 (day 4), **P = .009 (day 6); IL-6, **P = .002 (day 2), *P = .04 (day 4); IL-10, *P = .037 (day 2), **P = .002 (day 4); TNF, *P = .037 (day 2), ****P < .0001 (day 4); and IFN-γ, **P = .002 (day 2), **P = .009 (day 4), **P = .009 (day 6). (C) Grafts prepared using BM from BALB/c.WT donors and T cells from BALB/c luciferase mice were transplanted into lethally irradiated B6.WT or B6.IL-17RA−/− recipients (n = 13 per group). Animals were imaged at day 6 post-SCT and the bioluminescence intensity quantified (photons/sec/cm2/sr × 105) (Cii). Data combined from 2 replicate experiments are shown (panel Ci, WT [red bars], IL-17RA−/− [blue bars]). (D) Semiquantitative histopathology of GVHD target organs at day 6 post-alloSCT (SI, colon, lung: n = 9 to 10 per group; TCD groups: n = 4 per group, green bars; liver: n = 5 per group). SI and colon, B6.WT (red bars) vs B6.IL-17RA−/− (blue bars) recipients: ****P < .0001. Data combined from 2 experiments are shown (i). Representative images of the SI are shown (ii). (E) Intestinal barrier integrity as determined by FITC-dextran levels in serum on day 6 after alloSCT. Mice were orally gavaged with 8 mg FITC-dextran after 4 hours without food or water, and serum harvested 4 hours thereafter. Data combined from 2 replicate experiments are shown (n = 8 to 10 per group): B6.WT (red bar) vs B6.IL-17RA−/− (blue bar) recipients; **P = .004. (F) G-CSF mobilized grafts were unseparated, CD4 TCD or CD8 TCD, and transplanted into lethally irradiated B6.WT or B6.IL-17RA−/− recipients (n = 8 per group). Survival is represented by Kaplan-Meier analysis. ****P < .0001, BALB/c unseparated graft into B6.WT (red open square) vs B6.IL-17RA−/− (blue open circle) recipients; ****P < .0001, BALB/c unseparated graft (blue open circles) vs BALB/c CD4 depleted graft (green filled circles) into B6.IL-17RA−/− recipients; **P < .001, BALB/c unseparated graft (blue open circles) vs BALB/c CD8-depleted graft (purple filled triangle) into B6.IL-17RA−/− recipients. All data are presented as mean ± SEM. BLI, bioluminescence intensity; G-CSF, granulocyte CSF; GIT, gastrointestinal tract; mLN, mesenteric lymph node; ND, not detected; ns, not significant; SI, small intestine; TCD, T-cell deplete(d).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/15/10.1182_blood-2016-08-732628/4/m_blood732628f1.jpeg?Expires=1765903377&Signature=fcVfULyXglvRZtFPJxqbOte2SqR5hldCRnvydEGDJ4u8GBxVhGr9OWMTjB0kYS1tyR9SCG44k~lWXUIrKsfPapNi~dfbgJLLMDvjdM87ktsT4tFNLHQWyr12VGKd3Ab8dmi2Dcmbt~bhOKGAx36ZEa0f8RHu0dLhFcGtFHaYYf4osqQ-EcCaJg~ISOL4chOTWpzlm8H4hFaNtQnfZfV17QnUa45nfnbIWXHeSPC1ArdpgDJNZipTVGVeq1F9~3RjMut-9vJjPczJFnaXCZDNEmy6fapK~Imw8gwuiXa6rmFY~PKxafDWlSWwMMcNV9LPZerNHIjw4W9IFkQY42NV3A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. Shifts in microbial communities to WT mice after cohousing with IL-17RA−/− or IL-17RC−/− mice are similar. (A) Heatmap displaying differentially abundant OTUs consistently increased or decreased in WT mice after cohousing with IL-17RA−/− or IL-17RC−/− mice for 4 weeks. Heatmap generated based on fecal microbiota profile from 16S rRNA sequencing of WT, IL-17RA−/−, and IL-17RC−/− mice. Each column represents an individual WT mouse. Data from 2 IL-17RA−/− cohousing experiments are shown (n = 12 mice, experiments 1 and 2) and 1 IL-17RC−/− cohousing experiment (n = 10 mice, experiment 3). Scale is based on normalized read counts generated using the regularized log transformation implemented within DESeq2. Details available in supplemental Table 3. (B) Representative fluorescence in situ hybridization images of the colon at day 6 post-alloSCT (WT [left] and IL-17RA−/− [right]) utilizing universal rRNA probes to show bacteria (red) and their translocation from the lumen (L) into the tissue (yellow arrows). Bacterial morphology is highlighted in the higher magnification image shown of the boxed area (red outline). DAPI nuclear stain (blue). Scale bar = 50 μm. MM, muscularis mucosae; SM, submucosa.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/15/10.1182_blood-2016-08-732628/4/m_blood732628f7.jpeg?Expires=1765903377&Signature=hSuH4neyCQNFMI3qFO7nv4oynJGY2kkqkJSyP81c2E9ViPzJ1BW7ZEScwQWy1c~K9LoQG2cgZvnPiMHLLZXupSnuQDG6BXJJP6W~m6O6ST-WTOIC1Q6DpbMeWT5lq98IQDKWOVyzVGlxQCAMZ3EtD8mG3Btvs8njH-dzuqucJN~TztGtJe4YU9rR-qVXw1jdd00ePDOiKXl~8NNEV98gwsiNmASOqWDfrnHFnxyHYIgVlImCh4mGxvj2DzXE8kdTI4gHbhYduKSg-kKYtiUmqTSnxVkRYJ0aJ5~aeeiVTokWoXYU700bBaQWb9EbLZXcIXoMYxHmUopKoAbKLfPaIg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal