Key Points
IL-2 induces expression of PD-1 on Tregs, and PD-1 blockade promotes Treg differentiation and apoptosis.
PD-1 regulates IL-2–induced Treg proliferation and prolongs Treg survival in murine models and in patients receiving low-dose IL-2 therapy.
Abstract
CD4+Foxp3+ regulatory T cells (Tregs) play a central role in the maintenance of immune tolerance after hematopoietic stem cell transplantation. We previously reported that low-dose interleukin-2 (IL-2) therapy increased circulating Tregs and improved clinical symptoms of chronic graft-versus-host-disease (cGVHD); however, the mechanisms that regulate Treg homeostasis during IL-2 therapy have not been well studied. To elucidate these regulatory mechanisms, we examined the role of inhibitory coreceptors on Tregs during IL-2 therapy in a murine model and in patients with cGVHD. Murine studies demonstrated that low-dose IL-2 selectively increased Tregs and simultaneously enhanced the expression of programmed cell death 1 (PD-1), especially on CD44+CD62L+ central-memory Tregs, whereas expression of other inhibitory molecules, including CTLA-4, LAG-3, and TIM-3 remained stable. PD-1–deficient Tregs showed rapid Stat5 phosphorylation and proliferation soon after IL-2 initiation, but thereafter Tregs became proapoptotic with higher Fas and lower Bcl-2 expression. As a result, the positive impact of IL-2 on Tregs was completely abolished, and Treg levels returned to baseline despite continued IL-2 administration. We also examined circulating Tregs from patients with cGVHD who were receiving low-dose IL-2 and found that IL-2–induced Treg proliferation was promptly followed by increased PD-1 expression on central-memory Tregs. Notably, clinical improvement of GVHD was associated with increased levels of PD-1 on Tregs, suggesting that the PD-1 pathway supports Treg-mediated tolerance. These studies indicate that PD-1 is a critical homeostatic regulator for Tregs by modulating proliferation and apoptosis during IL-2 therapy. Our findings will facilitate the development of therapeutic strategies that modulate Treg homeostasis to promote immune tolerance.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) provides curative therapy for patients with various hematologic malignancies, bone marrow failure syndromes, and congenital immune deficiencies. Improvements in immune suppressive therapy and supportive care have improved patient outcomes, but chronic graft-versus-host disease (cGVHD) remains a major problem affecting long-term survivors.1-3 The clinical and laboratory manifestations of cGVHD closely resemble those of autoimmune diseases, and both T- and B-cell responses play a role in disease pathogenesis, suggesting that cGVHD reflects a general loss of immune tolerance, including abnormalities in the function of CD4+Foxp3+ regulatory T cells (Tregs).4-9
Tregs are a functionally distinct subset of mature T cells, which have a critical role in the development and maintenance of immune tolerance.10-12 Tregs are physiologically primed and constitutively proliferate in the presence of interleukin-2 (IL-2) and promptly mediate active suppression to prevent excessive inflammation.13-15 Patients with cGVHD and other autoimmune diseases have a relative deficiency of Tregs,16-21 and enhancement of Treg function can prevent allograft rejection and suppress autoimmune activity,22-24 which indicates that Tregs play an essential role in the establishment of life-long tolerance between recipient tissues and donor-derived immunity after allogeneic HSCT.25
In contrast to the genetic Treg deficiency in patients with immunodysregulation polyendocrinopathy enteropathy X-linked syndrome,26 Tregs after HSCT are derived from genetically normal healthy donors. Treg deficiency in this setting seems to be a consequence of homeostatic abnormalities in the posttransplant lymphopenic environment wherein increased proliferation of Tregs is not sufficient to compensate for reduced thymic output and increased susceptibility to apoptosis.18,21,27 Importantly, abnormal Treg homeostasis in patients with cGVHD can be restored by the supplemental administration of low-dose IL-2.28-30 In previous clinical trials, daily therapy with low-dose IL-2 for 8 to 12 weeks in patients with cGVHD led to a rapid increase in circulating Tregs without a significant increase in CD4+ or CD8+ effector T cells. This was associated with improvement in clinical GVHD symptoms in more than 50% of patients.28,29 Similar results of low-dose IL-2 therapy have been reported in healthy donors as well as in patients with various autoimmune diseases.31-34
Prolonged therapeutic intervention is often needed in patients with cGVHD as well as other autoimmune-based disorders. In our previous studies, patients with clinical improvement were eligible to continue low-dose IL-2 therapy for prolonged periods, and some patients have continued IL-2 treatment for more than 1 year. In these patients, increased levels of circulating Tregs are maintained for the entire duration of IL-2 therapy and contribute to clinical improvement.29 However, the mechanisms that regulate Treg homeostasis under the pressure of exogenous IL-2 are not well understood. Because IL-2 can induce apoptosis of T cells with an activated-memory phenotype,14 cell-intrinsic inhibitory pathways seem to be needed to prevent apoptosis of activated Tregs and to maintain homeostasis during prolonged IL-2 therapy. Although inhibitory coreceptors are known to play important roles in the regulation of effector T cells,35 their functions in activated Tregs have not been well characterized.
In this study, we examined the role of inhibitory receptors on Treg homeostasis in vivo. Among these inhibitory receptors, we found that exogenous IL-2 induced Treg expression of programmed cell death 1 (PD-1) without increased expression of CTLA-4, LAG-3, or Tim-3. Expression of PD-1 was most evident in central-memory Tregs, and increased PD-1 expression was maintained during IL-2 therapy. PD-1 blockade negated these effects of IL-2, promoted Treg apoptosis, and reduced Treg numbers in vivo. In contrast, PD-1 expression did not reduce the suppressive activity of Tregs. These results demonstrate that PD-1 signaling has a critical role in maintaining Treg homeostasis and contributes to the maintenance of immune tolerance.
Methods
Mice
C57BL/6 (B6) mice were purchased from CLEA Japan (Tokyo, Japan). PD-1–deficient mice (PD-1−/−) with a B6 background were purchased from RIKEN BioResource Center.36-38 All mice were maintained under specific-pathogen-free conditions and used at the age of 8 to 12 weeks. All animal experiments were performed according to the regulations of the Animal Care and Use Committee, Okayama University Advanced Science Research Center. For in vivo experiments, recombinant IL-2 (teceleukin) and anti-mouse PD-1 antibody (RMP1-14) were used. IL-2 was administered by subcutaneous injection in either wild-type or PD-1−/− mice without transplant conditioning or transplantation of hematopoietic stem cells.
Flow cytometry for murine experiments
Single-cell suspensions were first incubated with the following directly conjugated monoclonal antibodies (obtained from eBioscience unless otherwise stated) for 20 minutes at 4°C: eFluor450-conjugated anti-CD4 (RM4-5) and anti-CD3 (17A2); fluorescein isothiocyanate (FITC)–, phycoerythrin (PE)–, or PE-Cy7–conjugated anti-CD25 (FITC, 7D4; BD Biosciences, PE and PE-Cy7, PC61.5); FITC-conjugated anti-CD62L (MEL14), anti-PD-L2 (122), and anti-LAG-3 (eBioC9B7W); PE-conjugated anti-PD-1 (RMP1-30), anti-PD-L1 (MIH5), anti-Tim-3 (RMT3-23), anti-Fas (15A7), and annexin-V; PerCP-Cy5.5-conjugated anti-CD8 (53-6.7) and anti-CD11c (N418); PE-Cy7-conjugated anti-MHCII-1A/1E (M5/114.15.2); allophycocyanin (APC)-conjugated CD19 (MB19-1); and APC-eFluor780–conjugated anti-CD8 (53-6.7) and anti-CD44 (IM7). For intracellular staining, we processed cells by using a Foxp3 staining buffer set (eBioscience); the cells were incubated with FITC-conjugated anti-Bcl-2 (10C4) and anti-CTLA-4 (UC10-4B9) and APC-conjugated anti-Foxp3 (FJK-16S) for 30 minutes at 4°C. Bcl-2 and Fas expression (measured in terms of mean fluorescence intensity) were calculated as follows: Bcl-2 ratio = Bcl-2 of IL-2–treated mice/Bcl-2 of control mice and Fas ratio = Fas of IL-2–treated mice/Fas of control mice. Samples were analyzed on MAQSQuant flow cytometer (Miltenyi Biotec), and data were analyzed by using FlowJo software (Tree Star).
In vitro suppression assay
CD4+CD25+ Tregs and CD4+CD25– conventional T cells (Tcons) were isolated from murine spleen cells by using a CD4+CD25+ regulatory T-cell isolation kit and autoMACS Pro Separator (Miltenyi Biotec). Sorted cells were confirmed to be more than 95% pure. Responder Tcons purified from naïve B6 mice were labeled with CellTrace Violet according to the manufacturer’s protocols and were cultured with suppressor Tregs in the presence of anti-CD3/anti-CD28 dynabeads (Dynal) in 96-well round-bottom plates. After 3 days, cells were harvested and incubated with PerCP-Cy5.5–conjugated anti-CD4 (RM4-5; eBioscience). Proliferations of Tcons were analyzed on a MAQSQuant flow cytometer.
Patient characteristics
Laboratory studies were undertaken in 14 patients with cGVHD who were enrolled in a phase 1 clinical trial of low-dose IL-2 at the Dana-Farber Cancer Institute in Boston, MA (Table1).28 The clinical protocol was approved by the Human Subjects Protection Committee of the Dana-Farber/Harvard Cancer Center. Written informed consent was obtained from each patient before sample collection in accordance with the Declaration of Helsinki. Recombinant IL-2 (aldesleukin) was administrated subcutaneously once per day for 8 weeks. Seven patients received IL-2 at 3.0 × 105 IU/m2 per day, and 7 received IL-2 at 1.0 × 106 to 1.5 × 106 IU/m2 per day. Blood samples were obtained before and at 1, 2, 4, 6, 8, and 12 weeks after starting IL-2.
Clinical characteristics of patients who received IL-2 (n = 14)
| Characteristic . | No. of patients . |
|---|---|
| Sex | |
| Male | 5 |
| Female | 9 |
| Diagnosis | |
| Acute myeloid leukemia | 5 |
| Chronic lymphocytic leukemia | 5 |
| Chronic myelogenous leukemia | 1 |
| Hodgkin lymphoma | 1 |
| Myelodysplastic syndrome | 1 |
| Non-Hodgkin lymphoma | 1 |
| Conditioning regimen | |
| Myeloablative | 7 |
| Nonmyeloablative | 7 |
| Stem cell source | |
| PBSCs | 13 |
| BM and PBSCs | 1 |
| Donor type | |
| Matched-related | 4 |
| Matched-unrelated | 7 |
| Mismatched-unrelated | 3 |
| aGVHD prophylaxis | |
| Contained sirolimus | 9 |
| Did not contain sirolimus | 5 |
| Grade 2-4 aGVHD | 5 |
| Median days to cGVHD (range) | 269 (130-483) |
| Immunosuppressive therapy at start of IL-2 treatment | |
| Prednisone | 13 |
| Sirolimus | 6 |
| Tacrolimus | 5 |
| MMF | 10 |
| Characteristic . | No. of patients . |
|---|---|
| Sex | |
| Male | 5 |
| Female | 9 |
| Diagnosis | |
| Acute myeloid leukemia | 5 |
| Chronic lymphocytic leukemia | 5 |
| Chronic myelogenous leukemia | 1 |
| Hodgkin lymphoma | 1 |
| Myelodysplastic syndrome | 1 |
| Non-Hodgkin lymphoma | 1 |
| Conditioning regimen | |
| Myeloablative | 7 |
| Nonmyeloablative | 7 |
| Stem cell source | |
| PBSCs | 13 |
| BM and PBSCs | 1 |
| Donor type | |
| Matched-related | 4 |
| Matched-unrelated | 7 |
| Mismatched-unrelated | 3 |
| aGVHD prophylaxis | |
| Contained sirolimus | 9 |
| Did not contain sirolimus | 5 |
| Grade 2-4 aGVHD | 5 |
| Median days to cGVHD (range) | 269 (130-483) |
| Immunosuppressive therapy at start of IL-2 treatment | |
| Prednisone | 13 |
| Sirolimus | 6 |
| Tacrolimus | 5 |
| MMF | 10 |
aGVHD, acute GVHD; BM, bone marrow; MMF, mycophenolate mofetil; PBSC, peripheral blood stem cell.
Flow cytometry for human patients
Peripheral blood mononuclear cells (PBMCs) were incubated with the following directly conjugated monoclonal antibodies for 20 minutes at 4°C: Pacific Blue–conjugated anti-CD4 (RPA-T4; BD Biosciences), PE-Cy7–conjugated anti-CD25 (M-A251; BD Biosciences), FITC-conjugated anti-CD45RA (ALB11; Beckman Coulter), PE-conjugated anti-PD-1 (eBioJ105; eBioscience), and APC-Fluor750–conjugated anti-CD127 (eBioRDR5; eBioscience). To detect intracellular Foxp3, we incubated the PBMCs with PE-conjugated anti-Foxp3 (PCH101) for 30 minutes at 4°C by using a Foxp3 staining buffer set (eBioscience). Samples were analyzed on a FACSCanto II flow cytometer (BD Biosciences), and data were analyzed by using FlowJo software.
In vitro proliferation assay
For in vitro proliferation assay of human lymphocytes, CD45RA+ Treg and Tcon cells were isolated from a patient receiving low-dose IL-2 by cell sorting with a FACSAria cell sorter. Sorted cells were confirmed to be more than 95% pure. Cells were labeled with carboxyfluorescein succinimidyl ester (CFSE; Invitrogen) according to the manufacture’s protocol and were cultured separately in the presence of anti-CD3 antibody (0.1 μg/mL) (OKT3; eBioscience), anti-CD28 antibody (1 μg/mL) (L293; BD Biosciences), and functional anti-programmed death-ligand 1 (PD-L1) antibody (MIH1; eBioscience) in 96-well round-bottom plates at a concentration of 1 × 104 T cells per well. After 4 days, cells were harvested and incubated with Pacific Blue–conjugated anti-CD4 (RPA-T4; BD Biosciences), PE-conjugated anti-PD-1 (eBioJ105; eBioscience), and PE-Cy7-conjugated anti-CD45RA (HI100; BD Biosciences). Cell death was assessed by APC-conjugated annexin-V staining and forward-side scatter profiles.
Statistical analysis
In murine experiments, results are presented as means +/– standard error of the mean. Student t test was used to assess statistical significance between 2 groups and one-way analysis of variance was used to compare more than 2 groups. P values < .05 indicated statistical significance. In analyses of human patients, the Wilcoxon signed rank test was used for paired group comparisons. All tests were two-sided at the significance level of 0.05.
Results
Low-dose IL-2 administration selectively activates Tregs in a murine model
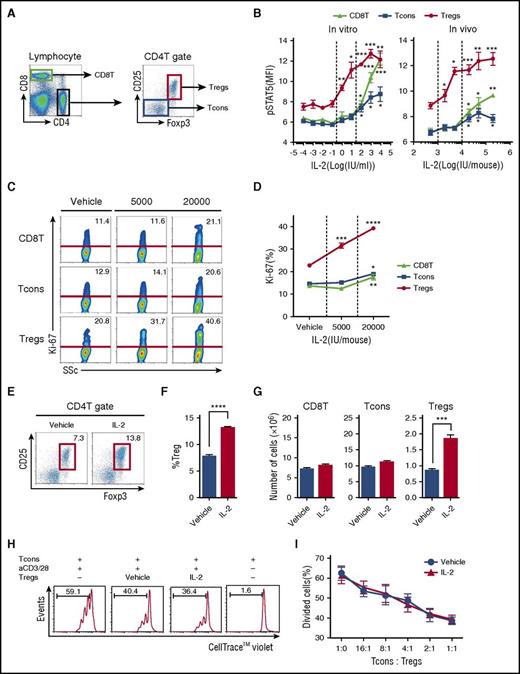
We first determined the dose of IL-2 needed to activate Tregs in a murine model. Each T-cell subset was defined as shown in Figure 1A. To compare the response of T-cell subsets to IL-2 in vitro, spleen cells were stimulated with IL-2 for 30 minutes, and phosphorylation of Stat5 was evaluated by flow cytometry. High concentrations of IL-2 (100 to 10 000 IU/mL) induced Stat5 phosphorylation all T-cell subsets. However, lower concentrations of IL-2 (1 to 10 IU/mL) induced Stat5 phosphorylation only in Tregs (Figure 1B, left). To compare the response of T-cell subsets to IL-2 in vivo, a single dose of IL-2 was administered to naïve mice, and phosphorylated Stat5 (pStat5) was measured in splenic T cells. Consistent with results from in vitro experiments, Stat5 was phosphorylated in all T-cell subsets after treatment with relatively high doses of IL-2 (>20 000 IU per mouse). In contrast, Stat5 phosphorylation was observed in Tregs only after injection of low-dose IL-2 (<5,000 IU per mouse) (Figure 1B, right). To evaluate the effects of prolonged treatment on T cells, mice received control vehicle at 5000 IU or IL-2 at 20 000 IU once per day for 14 days. Administration of IL-2 at 5000 IU/d selectively increased Treg proliferation, leading to the expansion of this subset. In contrast, administration of IL-2 at 20 000 IU/d induced proliferation of other T-cell subsets, including CD8 T cells and CD4 Tcons (Figure 1C-D). Administration of IL-2 at 5000 IU/d did not affect either CD8 T cells or Tcons (Figure 1E-G). To determine whether IL-2–expanded Tregs maintain suppressive activity, we purified Tregs from IL-2–treated mice and control mice and compared their suppressive function in vitro. As shown in Figure 1H, IL-2–expanded Tregs suppressed the proliferation of responder Tcons, and the suppressive activity was comparable to control Tregs (Figure 1I). These results indicate that administration of IL-2 at 5000 IU/d is sufficient to provide Tregs with homeostatic signals to initiate proliferation without affecting other T-cell subsets.
Selective expansion of CD4 Tregs in a murine model of low-dose IL-2 therapy. (A) Representative lymphocyte gates for identification of CD4 and CD8 T-cell subsets. Within the CD4 T-cell gate, Tregs are identified as CD4+CD25+Foxp3+ cells and Tcons are identified as CD4+CD25–Foxp3– cells. (B) IL-2 dose-dependent phosphorylation of Stat5 in T-cell subsets. Left panel: Spleen cells (5 × 105 cells per well) were cultured for 30 minutes in various concentrations of recombinant IL-2. Right panel: Wild-type C57BL/6 mice received single doses of recombinant IL-2 and spleen cells were harvested after 30 minutes. The level of intracellular pStat5 was determined by flow cytometry. (C-D) Wild-type C57BL/6 mice received control vehicle, 5000 or 20 000 IU recombinant IL-2 once per day for 14 days and spleen cells were analyzed on day 15. (C) Representative flow cytometry histograms for identification of Ki-67+ proliferating cells in CD8 T cells, Tcons, and Tregs. Percentage of Ki-67+ cells is shown for each histogram. (D) IL-2 dose-dependent increase of Ki-67+ proliferating cells in each T-cell subset. (E-G) Wild-type C57BL/6 mice received control vehicle or 5000 IU recombinant IL-2 subcutaneously once per day for 14 days, and spleen cells were analyzed on day 15. (E) Representative panel gated on CD4 T cells identifying CD4 Tregs (red box) in mice treated with vehicle control or IL-2. (F) Frequency of CD4+CD25+Foxp3+ Tregs. (G) Number of CD8 T cells, Tcons, and Tregs. (H-I) In vitro Treg suppression assay. Tcons labeled with CellTrace Violet from wild-type C57BL/6 mice were cultured at a 1:1 ratio with Tregs isolated from vehicle or IL-2–treated mice in the presence of CD3/CD28 stimulation for 3 days. (H) Representative flow cytometry histograms measuring Tcon proliferation in the presence or absence of Tregs. Percentage of divided Tcons is shown for each histogram. (I) Percentage of divided Tcons at various Tcon:Treg cell ratios. Responder Tcons (1 × 105 cells per well) were cultured with various numbers of suppressor Tregs with 4 mice per group per experiment. Data are representative of (H-I) 2 or (A-G) 3 independent experiments and expressed as means +/– standard error of the mean (SEM). *P < .05, **P < .01, ***P < .001, and ****P < .0001.
Selective expansion of CD4 Tregs in a murine model of low-dose IL-2 therapy. (A) Representative lymphocyte gates for identification of CD4 and CD8 T-cell subsets. Within the CD4 T-cell gate, Tregs are identified as CD4+CD25+Foxp3+ cells and Tcons are identified as CD4+CD25–Foxp3– cells. (B) IL-2 dose-dependent phosphorylation of Stat5 in T-cell subsets. Left panel: Spleen cells (5 × 105 cells per well) were cultured for 30 minutes in various concentrations of recombinant IL-2. Right panel: Wild-type C57BL/6 mice received single doses of recombinant IL-2 and spleen cells were harvested after 30 minutes. The level of intracellular pStat5 was determined by flow cytometry. (C-D) Wild-type C57BL/6 mice received control vehicle, 5000 or 20 000 IU recombinant IL-2 once per day for 14 days and spleen cells were analyzed on day 15. (C) Representative flow cytometry histograms for identification of Ki-67+ proliferating cells in CD8 T cells, Tcons, and Tregs. Percentage of Ki-67+ cells is shown for each histogram. (D) IL-2 dose-dependent increase of Ki-67+ proliferating cells in each T-cell subset. (E-G) Wild-type C57BL/6 mice received control vehicle or 5000 IU recombinant IL-2 subcutaneously once per day for 14 days, and spleen cells were analyzed on day 15. (E) Representative panel gated on CD4 T cells identifying CD4 Tregs (red box) in mice treated with vehicle control or IL-2. (F) Frequency of CD4+CD25+Foxp3+ Tregs. (G) Number of CD8 T cells, Tcons, and Tregs. (H-I) In vitro Treg suppression assay. Tcons labeled with CellTrace Violet from wild-type C57BL/6 mice were cultured at a 1:1 ratio with Tregs isolated from vehicle or IL-2–treated mice in the presence of CD3/CD28 stimulation for 3 days. (H) Representative flow cytometry histograms measuring Tcon proliferation in the presence or absence of Tregs. Percentage of divided Tcons is shown for each histogram. (I) Percentage of divided Tcons at various Tcon:Treg cell ratios. Responder Tcons (1 × 105 cells per well) were cultured with various numbers of suppressor Tregs with 4 mice per group per experiment. Data are representative of (H-I) 2 or (A-G) 3 independent experiments and expressed as means +/– standard error of the mean (SEM). *P < .05, **P < .01, ***P < .001, and ****P < .0001.
In vivo IL-2–expanded Tregs exhibit central-memory phenotype with enhanced PD-1 expression
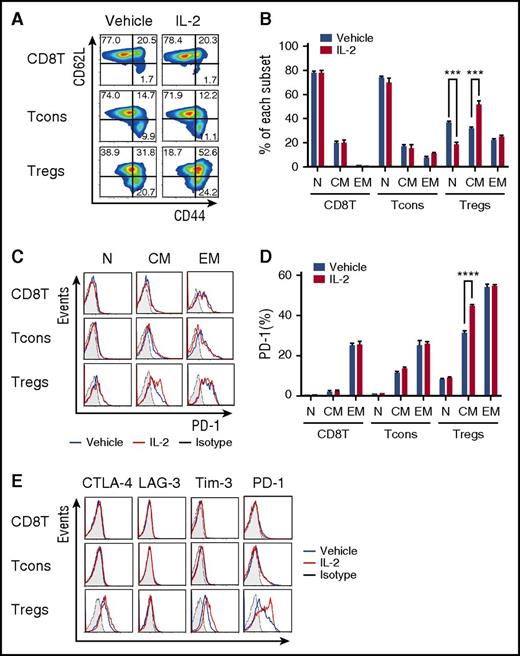
To examine the effect of low-dose IL-2 on Treg differentiation, we examined the phenotype of IL-2–expanded Tregs. Mice received control vehicle or IL-2 at 5000 IU once per day for 14 days, and spleen cells were analyzed on day 15. At this dose, IL-2 treatment did not affect the phenotype of CD8 T cells or CD4 Tcons. However, compared with control mice, IL-2–treated mice had decreased frequency of Tregs with a naïve phenotype (CD44lowCD62Lhigh) and increased frequency of Tregs with a central-memory phenotype (CD44highCD62Lhigh) (Figure 2A-B). IL-2 induced proliferation of both CD44highCD62Lhigh central-memory and CD44highCD62Llow effector-memory Tregs but expression of Ki-67 was significantly higher in the central-memory Treg subset (supplemental Figure 1A-B, available on the Blood Web site). We did not observe any change in expression of CCR7 and CCR4 chemokine receptors on central-memory Tregs, suggesting that low-dose IL-2 did not affect cell migration (supplemental Figure 1C). Interestingly, central-memory Tregs in IL-2–treated mice expressed higher levels of PD-1 compared with vehicle-treated mice (Figure 2C-D). In contrast, Treg expression of other inhibitory molecules, including CTLA-4 and LAG-3 was not increased, and TIM-3 expression was only slightly increased after IL-2 administration (Figure 2E). We also examined the effect of IL-2 therapy on PD-ligand (PD-L) expression on each T-cell subset and on antigen-presenting cells. These studies demonstrated that low-dose IL-2 resulted in a small increase in PD-L1 expression on Tregs (supplemental Figure 2A-B). PD-L1 and PD-L2 expression on CD11c+ major histocompatibility class II+ dendritic cells did not change during IL-2 administration (supplemental Figure 2C-D).
Phenotypic changes in murine Tregs after IL-2 therapy. Wild-type C57BL/6 mice received control vehicle or 5000 IU human recombinant IL-2 subcutaneously once per day for 14 days and spleen cells were analyzed on day 15. (A) Representative panels identify CD44lowCD62Lhigh naïve (N), CD44highCD62Lhigh central-memory (CM), and CD44highCD62Llow effector-memory (EM) subsets within CD8 T cells, Tcons, and Tregs. (B) Percentage of each subset in CD8 T cells, Tcons, and Tregs after in vivo treatment with control vehicle or IL-2. (C) Representative flow cytometry histograms identifying PD-1+ cells in each T-cell subset after treatment with control vehicle or IL-2. (D) Percentage of PD-1+ cells in CD8 T cells, Tcons, and Tregs after treatment with control vehicle or IL-2. (E) Representative flow cytometry histograms detecting expression of CTLA-4, LAG-3, Tim-3, and PD-1 on CD8 T cells, Tcons, and Tregs with 4 mice per group per experiment. Data are representative of 3 independent experiments and expressed as means +/– SEM. ***P < .001 and ****P < .0001.
Phenotypic changes in murine Tregs after IL-2 therapy. Wild-type C57BL/6 mice received control vehicle or 5000 IU human recombinant IL-2 subcutaneously once per day for 14 days and spleen cells were analyzed on day 15. (A) Representative panels identify CD44lowCD62Lhigh naïve (N), CD44highCD62Lhigh central-memory (CM), and CD44highCD62Llow effector-memory (EM) subsets within CD8 T cells, Tcons, and Tregs. (B) Percentage of each subset in CD8 T cells, Tcons, and Tregs after in vivo treatment with control vehicle or IL-2. (C) Representative flow cytometry histograms identifying PD-1+ cells in each T-cell subset after treatment with control vehicle or IL-2. (D) Percentage of PD-1+ cells in CD8 T cells, Tcons, and Tregs after treatment with control vehicle or IL-2. (E) Representative flow cytometry histograms detecting expression of CTLA-4, LAG-3, Tim-3, and PD-1 on CD8 T cells, Tcons, and Tregs with 4 mice per group per experiment. Data are representative of 3 independent experiments and expressed as means +/– SEM. ***P < .001 and ****P < .0001.
PD-1 blockade results in failure of durable Treg expansion after IL-2 stimulation
To examine the role of increased PD-1 expression on Tregs during low-dose IL-2 therapy, we inhibited the PD-1 pathway by using anti-PD-1 antibody. C57BL/6 mice received anti-PD-1 antibody twice per week for a total of 4 doses and/or IL-2 once per day for 14 days. Peripheral blood cells were collected at days 0, 4, 8, 11, and 15 to evaluate the proliferation of each subset. Proliferation of Tregs was significantly greater on day 7 in mice that received both IL-2 and PD-1 antibody compared with mice that received IL-2 alone. However, vigorous Treg proliferation was not maintained thereafter (Figure 3A). Similarly, combined treatment with IL-2 and PD-1 antibody induced a rapid increase in the frequency of Tregs, but this was not maintained. In contrast, IL-2 treatment without PD-1 blockade resulted in a durable increase in the frequency of Tregs (Figure 3B). These data indicate that PD-1 plays an important role in maintaining increased levels of Tregs during IL-2 administration.
Effects of combined IL-2 therapy and PD-1 blockade on Treg expansion in vivo. Wild-type C57BL/6 mice received vehicle (plus isotype antibody), IL-2 (plus isotype antibody), anti-PD-1 (aPD-1) antibody (plus vehicle control), or IL-2 plus anti-PD-1 antibody. Anti-PD-1 antibody (250 μg) was administrated intraperitoneally twice per week for a total of 4 injections beginning on the first day of IL-2 treatment. IL-2–treated groups received IL-2 at 5000 IU once per day for 14 days. Peripheral blood cells were collected and analyzed at days 0, 4, 8, 11, and 15. (A) Increase of the percentage of Ki-67+ proliferating Tregs from the baseline level of each group during therapy. (B) Increase of the percentage of Tregs during therapy from the baseline level of each group with 4 mice per group per experiment. Data are representative of 2 independent experiments and expressed as means +/– SEM. *P < .05.
Effects of combined IL-2 therapy and PD-1 blockade on Treg expansion in vivo. Wild-type C57BL/6 mice received vehicle (plus isotype antibody), IL-2 (plus isotype antibody), anti-PD-1 (aPD-1) antibody (plus vehicle control), or IL-2 plus anti-PD-1 antibody. Anti-PD-1 antibody (250 μg) was administrated intraperitoneally twice per week for a total of 4 injections beginning on the first day of IL-2 treatment. IL-2–treated groups received IL-2 at 5000 IU once per day for 14 days. Peripheral blood cells were collected and analyzed at days 0, 4, 8, 11, and 15. (A) Increase of the percentage of Ki-67+ proliferating Tregs from the baseline level of each group during therapy. (B) Increase of the percentage of Tregs during therapy from the baseline level of each group with 4 mice per group per experiment. Data are representative of 2 independent experiments and expressed as means +/– SEM. *P < .05.
PD-1 signaling prevents central-memory Tregs from differentiating into apoptosis-prone effector-memory Tregs
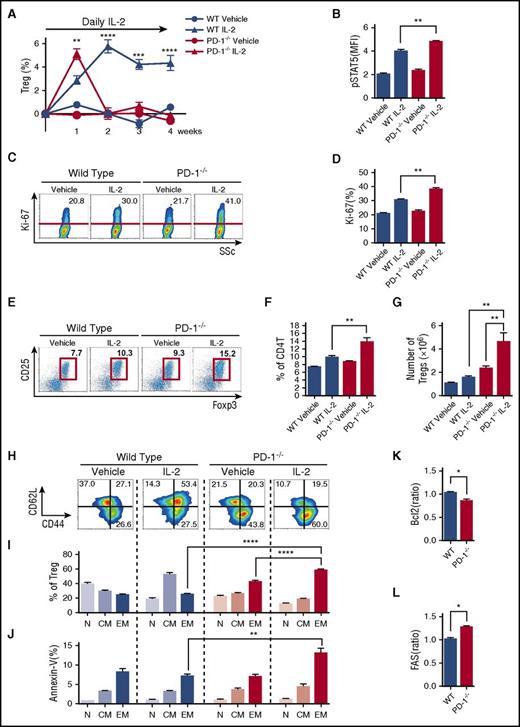
We next explored the mechanisms whereby PD-1 contributes to Treg homeostasis using PD-1−/− mice. PD-1–deficient mice and control wild-type (WT) mice received IL-2 once per day for 4 weeks, and splenic T-cell subsets were analyzed once per week. In WT mice, the percentage of Tregs continued to increase during the first 14 days and remained stable thereafter (Figure 4A). Consistent with PD-1 blocking experiments, the initial peak of Treg expansion was higher in PD-1–deficient mice. Indeed, after 1 week of IL-2 therapy, pStat5 expression in Tregs increased more in PD-1–deficient mice than in control WT mice, resulting in the rapid increase of proliferation, percentage, and absolute number of Tregs (Figure 4B-G). However, the number of Tregs in PD-1–deficient mice returned to baseline levels by day 14. In contrast, Tregs continued to increase in PD-1 WT mice (Figure 4A). On day 14, Tregs in PD-1–deficient mice treated with IL-2 were predominantly CD44highCD62Llow effector-memory type. In contrast, Tregs in PD-1 WT mice treated with IL-2 were predominantly CD44highCD62Lhigh central-memory type (Figure 4H-I). Notably, accumulating CD44highCD62Llow effector-memory Tregs in PD-1–deficient mice were annexin-V–positive (Figure 4J). IL-2–treated PD-1−/− Tregs also showed decreased expression of antiapoptotic Bcl-2 (Figure 4K) and increased expression of proapoptotic Fas (CD95) (Figure 4L). As shown in supplemental Figure 3A, IL-2–treated PD-1−/− Tregs isolated at week 2 suppressed proliferation of responder WT Tcons (supplemental Figure 3B) that was identical to that of Tregs isolated from PD-1 WT mice, indicating that IL-2–treated PD-1−/− Tregs maintain suppressive activity. These results suggest that PD-1 signaling stabilizes Treg expansion during IL-2 intervention by maintaining Tregs in a central-memory phenotype and inhibits their differentiation to an apoptosis-prone effector-memory phenotype.
PD-1 deletion alters Treg homeostasis during IL-2 therapy. C57BL/6 PD-1−/− or C57BL/6 WT mice were treated with control vehicle or IL-2 at 5000 IU once per day for 4 weeks. (A) Effect of IL-2 therapy on frequency of Tregs during treatment. Increase of percentage of Tregs during therapy from the baseline level of each group. (B-H) Spleen cells were analyzed after 1 week of IL-2 therapy. (B) pSTAT5 expression in Tregs. (C) Representative flow cytometry histograms detecting Ki-67+ proliferating Tregs. Percentage of Ki-67+ Tregs is shown for each histogram. (D) Percentage of Ki-67+ proliferating Tregs in PD-1−/− and PD-1WT mice. (E) Representative histogram identifying CD4 Tregs in PD-1−/− and PD-1WT mice receiving control vehicle or IL-2. (F) Frequency of CD4+CD25+Foxp3+ Tregs in spleen (percentage of CD4 T cells). (G) Number of CD4+CD25+Foxp3+ Tregs in spleen. (H-L) Spleen cells were analyzed after 2 weeks IL-2 therapy given once per day. (H) Representative histograms identify CD44lowCD62Lhigh naïve, CD44highCD62Lhigh central-memory, and CD44highCD62Llow effector-memory Treg subsets after IL-2 therapy. (I) Percentage of each Treg subset after IL-2 therapy. (J) Percentage of annexin-V+ apoptotic cells in each Treg subset after IL-2 therapy. (K) Mean fluorescence intensity (MFI) for the ratio of Bcl-2 expression in Tregs of IL-2–treated mice:Bcl-2 expression in Tregs of control vehicle–treated mice. (L) MFI for the ratio of Fas expression in Tregs of IL-2–treated mice:Fas expression in Tregs of control vehicle–treated mice with 4 mice per group per experiment. Data are representative of (A) 2 or (B-L) 3 independent experiments and expressed as means +/– SEM. *P < .05, **P < .01, ***P < .001, ****P < .0001.
PD-1 deletion alters Treg homeostasis during IL-2 therapy. C57BL/6 PD-1−/− or C57BL/6 WT mice were treated with control vehicle or IL-2 at 5000 IU once per day for 4 weeks. (A) Effect of IL-2 therapy on frequency of Tregs during treatment. Increase of percentage of Tregs during therapy from the baseline level of each group. (B-H) Spleen cells were analyzed after 1 week of IL-2 therapy. (B) pSTAT5 expression in Tregs. (C) Representative flow cytometry histograms detecting Ki-67+ proliferating Tregs. Percentage of Ki-67+ Tregs is shown for each histogram. (D) Percentage of Ki-67+ proliferating Tregs in PD-1−/− and PD-1WT mice. (E) Representative histogram identifying CD4 Tregs in PD-1−/− and PD-1WT mice receiving control vehicle or IL-2. (F) Frequency of CD4+CD25+Foxp3+ Tregs in spleen (percentage of CD4 T cells). (G) Number of CD4+CD25+Foxp3+ Tregs in spleen. (H-L) Spleen cells were analyzed after 2 weeks IL-2 therapy given once per day. (H) Representative histograms identify CD44lowCD62Lhigh naïve, CD44highCD62Lhigh central-memory, and CD44highCD62Llow effector-memory Treg subsets after IL-2 therapy. (I) Percentage of each Treg subset after IL-2 therapy. (J) Percentage of annexin-V+ apoptotic cells in each Treg subset after IL-2 therapy. (K) Mean fluorescence intensity (MFI) for the ratio of Bcl-2 expression in Tregs of IL-2–treated mice:Bcl-2 expression in Tregs of control vehicle–treated mice. (L) MFI for the ratio of Fas expression in Tregs of IL-2–treated mice:Fas expression in Tregs of control vehicle–treated mice with 4 mice per group per experiment. Data are representative of (A) 2 or (B-L) 3 independent experiments and expressed as means +/– SEM. *P < .05, **P < .01, ***P < .001, ****P < .0001.
Enhanced expression of PD-1 on human Tregs during low-dose IL-2 therapy modulates long-term stable homeostasis
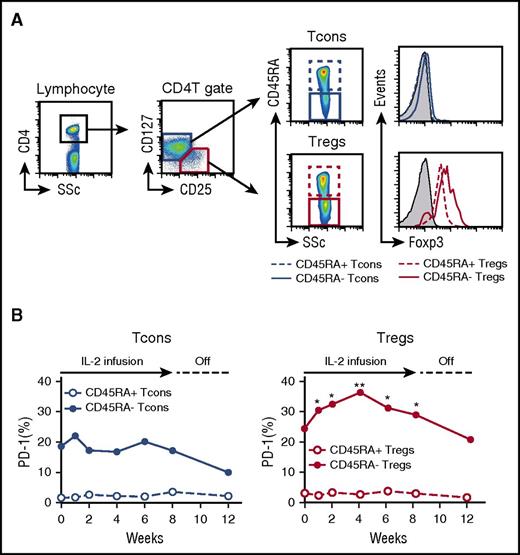
To examine the role of PD-1 expression on human Tregs, we studied peripheral blood samples from 14 patients enrolled in a phase 1 clinical trial of low-dose IL-2 for cGVHD.28 Within the CD4 T-cell gate, Tregs were identified as CD25med-highCD127low and Tcons were identified as CD25neg-lowCD127med-high. Tregs and Tcons were further divided into subsets with CD45RA+ naïve and CD45RA– activated-memory phenotype, as shown in Figure 5A. Before IL-2 therapy, both CD45RA+ naïve Tregs and Tcons showed little expression of PD-1. In contrast, both CD45RA– activated-memory Tregs and Tcons showed significantly higher expression of PD-1 than their naïve counterparts, and there was no significant difference in PD-1 expression between CD45RA– Tregs and Tcons. After starting IL-2, expression of PD-1 rapidly increased in CD45RA– Tregs, whereas PD-1 expression did not change in other CD4 T-cell subsets, including CD45RA+ Tregs, CD45RA+ Tcons, and CD45RA– Tcons (Figure 5B). PD-1 expression on CD45RA– Tregs reached maximal levels 4 weeks after starting IL-2 and remained elevated during the entire 8-week treatment period. PD-1 expression on CD45RA– Tregs returned to baseline levels 4 weeks after stopping IL-2 therapy.
Selective increase of PD-1 expression on CD45RA–activated-memory Tregs in patients with cGVHD receiving low-dose IL-2. (A) Representative flow cytometry histograms used to define CD4 T-cell subsets. (B) Median percentages of PD-1+ cells in Tcon and Treg subsets during IL-2 therapy; CD45RA– activated-memory Tregs vs baseline using Wilcoxon signed rank test. *P < .05, **P < .01.
Selective increase of PD-1 expression on CD45RA–activated-memory Tregs in patients with cGVHD receiving low-dose IL-2. (A) Representative flow cytometry histograms used to define CD4 T-cell subsets. (B) Median percentages of PD-1+ cells in Tcon and Treg subsets during IL-2 therapy; CD45RA– activated-memory Tregs vs baseline using Wilcoxon signed rank test. *P < .05, **P < .01.
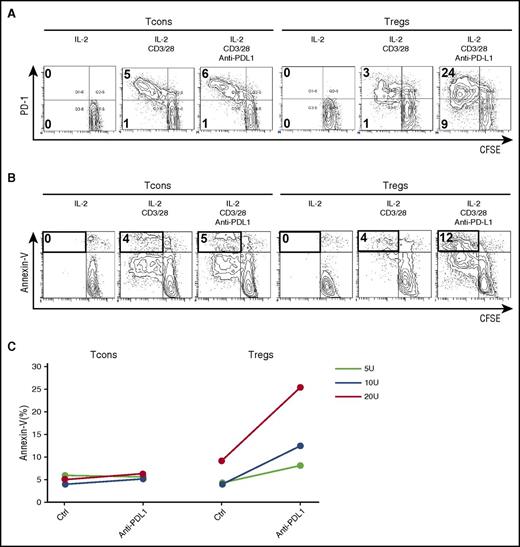
Although PD-1 expression increased in CD45RA– activated-memory Tregs, PD-1 expression did not increase in CD45RA+ naïve Tregs during IL-2 therapy. To examine the proliferative potential of CD45RA+PD-1neg naïve cells during IL-2 therapy, we used cell sorting to purify CD45RA+ Tregs and Tcons from a patient receiving IL-2 and cultured each fraction separately in the presence of CD3/CD28 stimulation with or without PD-1 blockade. CD3/CD28 stimulation resulted in limited proliferation of naïve Tregs from patients during IL-2 therapy. However, vigorous naïve Treg proliferation was observed when the PD-1 pathway was blocked (Figure 6A). Rapidly proliferating Tregs expressed high levels of annexin-V (Figure 6B-C). In contrast, the effect of PD-1 blockade on CD45RA+ naïve Tcons was relatively small (Figure 6A).
PD-1 blockade enhances IL-2–induced proliferation of expanded human Tregs and promotes apoptosis. Purified CD45RA+ naïve Tregs and Tcons labeled with carboxyfluorescein succinimidyl ester (CFSE) were stimulated with IL-2, anti-CD3/28 antibody, or anti-PD-L1 antibody for 4 days. Representative flow cytometry histograms were used to quantify CFSE dilution and (A) identify PD-1+ cells and (B) identify annexin-V+ cells. (C) Percentage of annexin-V+ apoptotic cells within Tcon and Treg populations stimulated with IL-2, anti-CD3/28 antibody, or anti-PD-L1 antibody for 4 days. Data are obtained from 1 experiment.
PD-1 blockade enhances IL-2–induced proliferation of expanded human Tregs and promotes apoptosis. Purified CD45RA+ naïve Tregs and Tcons labeled with carboxyfluorescein succinimidyl ester (CFSE) were stimulated with IL-2, anti-CD3/28 antibody, or anti-PD-L1 antibody for 4 days. Representative flow cytometry histograms were used to quantify CFSE dilution and (A) identify PD-1+ cells and (B) identify annexin-V+ cells. (C) Percentage of annexin-V+ apoptotic cells within Tcon and Treg populations stimulated with IL-2, anti-CD3/28 antibody, or anti-PD-L1 antibody for 4 days. Data are obtained from 1 experiment.
To evaluate the impact of early upregulation of PD-1 on clinical outcome, we compared expression of PD-1 in 6 patients with clinical improvement of cGVHD (clinical responders) with 5 patients with no clinical improvement (clinical nonresponders) after IL-2 therapy. Two weeks after IL-2 therapy began, PD-1 was more highly expressed on Tregs compared with Tcons in clinical responders (Figure 7A). In contrast, the level of PD-1 expression was similar on Tregs and Tcons in clinical nonresponders (Figure 7A). When PD-1 expression at 2 weeks was compared with PD-1 expression before starting IL-2, there was no change in the Treg-PD-1:Tcon-PD-1 ratio in nonresponders. In contrast, the Treg-PD-1:Tcon-PD-1 ratio increased significantly (P = .03) in clinical responders 2 weeks after beginning low-dose IL-2 therapy (Figure 7B). These results suggest that the rapid increase of PD-1 expression on Tregs after starting IL-2 therapy contributes to the maintenance of Treg expansion, which facilitates the clinical response to IL-2.
Comparison of PD-1 expression in clinical responders and nonresponders during low-dose IL-2 therapy. (A) Scatter plot of the percentage of PD-1+CD45RA– Tregs and Tcons in nonresponders and responders at week 2 during IL-2 therapy. (B) Ratio of Treg percentage of PD-1+:Tcon percentage of PD-1 in nonresponders and responders before and 2 weeks after starting IL-2 therapy. The ratio is significantly increased in clinical responders 2 weeks after IL-2 administration (P = .03, Wilcoxon signed rank test). Median values are shown in green.
Comparison of PD-1 expression in clinical responders and nonresponders during low-dose IL-2 therapy. (A) Scatter plot of the percentage of PD-1+CD45RA– Tregs and Tcons in nonresponders and responders at week 2 during IL-2 therapy. (B) Ratio of Treg percentage of PD-1+:Tcon percentage of PD-1 in nonresponders and responders before and 2 weeks after starting IL-2 therapy. The ratio is significantly increased in clinical responders 2 weeks after IL-2 administration (P = .03, Wilcoxon signed rank test). Median values are shown in green.
Discussion
In inflammatory microenvironments, activated effector T cells produce IL-2, which supports the further expansion of activated effector T cells in a positive feedback loop. However, Tregs also respond to secreted IL-2 to inhibit effector T cells and suppress inflammation.39 The constitutive expression of high-affinity IL-2 receptors enables Tregs to promptly respond to low concentrations of IL-2 without antigen-specific activation of T-cell receptors.40 But the homeostatic mechanisms that regulate the Treg response to IL-2 are not well understood.
PD-1 is a co-inhibitory receptor of the B7:CD28 family that negatively regulates T-cell activation after interaction with specific ligands, PD-L1 (B7-H1) and PD-L2 (B7-DC).41-44 Expression of PD-1 is upregulated on effector T cells during chronic antigen stimulation in the context of persistent viral infections. In addition, effector T cells often express PD-1 in the tumor microenvironment, and PD-1–mediated immune exhaustion of effector T cells is associated with immune dysfunction and disease progression.45,46 In patients with cancer, recent clinical trials have demonstrated that blockade of the PD-1 pathway by in vivo administration of anti-PD-1 or anti-PD-L1 antibodies can reverse PD-1–mediated T-cell dysfunction resulting in durable tumor regression.47-52 Although many studies have examined the role of PD-1 in the suppression of effector T cells and natural killer cells,53 the role of PD-1 in the regulation of Tregs has not been established.
To examine the role of PD-1 in Treg homeostasis in vivo, we developed a murine model of low-dose IL-2 therapy and determined that administration of IL-2 at 5000 IU/d was sufficient to selectively activate and expand Tregs in vivo without inducing expansion of other T-cell subsets. Importantly, administration of IL-2 once per day at this low dose resulted in the expansion of central-memory Tregs that was similar to the effects of once-per-day IL-2 therapy previously observed in patients with cGVHD. Further characterization of expanded Tregs demonstrated that PD-1 expression was increased on these cells whereas other immune inhibitory molecules, including CTLA-4, LAG-3, and Tim-3, remained stable. When administration of IL-2 was combined with anti-PD-1 antibody, the initial response to IL-2 was enhanced. Notably, Tregs examined after 7 days of treatment exhibited higher levels of proliferation, and the percentage of Tregs in peripheral blood was increased compared with that in mice that received IL-2 alone. However, this high level of Treg proliferation was not sustained, and by day 15, the frequency of Tregs in PBMCs was lower in mice that received IL-2 plus anti-PD-1 compared with mice that received IL-2 alone. Similar results were obtained when IL-2 was administered once per day to PD-1–deficient mice. In this setting, PD-1−/− Tregs that expanded after IL-2 therapy were predominately effector-memory Tregs with a significantly higher fraction of apoptotic cells. When compared with PD-1 WT Tregs, PD-1−/− Tregs expressed lower levels of anti-apoptotic protein Bcl-2 and increased levels of proapoptotic FAS (CD95). With continued administration of IL-2 once per day for 4 weeks, there was no expansion of PD-1−/− Tregs. Taken together, these data suggest that the PD-1 pathway plays an important role in the regulation of terminal differentiation and apoptosis of activated Tregs.
Because other inhibitory molecules such as CTLA-4 and LAG-3 are directly involved in the suppressive function of Tregs,54,55 it was important to examine the immune suppressive function of PD-1–deficient Tregs. These experiments showed that IL-2–expanded PD-1−/− Tregs exhibit normal levels of suppressive activity, indicating that PD-1 does not directly affect Treg function. Unlike effector T cells, in which PD-1 expression is associated with T-cell dysfunction and exhaustion, PD-1 expression in Tregs promotes the survival of these cells in inflammatory environments. Thus systemic PD-1 blockade acts to both enhance the function of effector T cells and limit the survival of Tregs. These results are consistent with previous reports in murine models of chronic viral infection in which combined therapy with IL-2 plus anti-PD-L1 antibody synergistically enhanced virus-specific CD8 T-cell responses and decreased viral load even though Treg numbers transiently increased.56
Examination of Tregs in patients receiving IL-2 therapy provided additional evidence that PD-1 plays an important role in Treg homeostasis. In previous clinical studies, daily administration of low-dose IL-2 rapidly expanded circulating Tregs without increasing effector T cells.28,29 Increased numbers of circulating Tregs persisted for the entire duration of low-dose IL-2 treatment. Notably, Treg proliferation dramatically increased in the first week after starting IL-2 but returned to baseline levels by the second week of treatment.30,40 In this study, analysis of cryopreserved cells from patients enrolled on this trial revealed that PD-1 expression also increased on Tregs during IL-2 therapy. Whereas Treg proliferation peaked 1 week after starting IL-2, PD-1 expression increased early but did not peak until week 4. Tregs isolated during IL-2 therapy exhibited limited proliferation in vitro in response to CD3/CD28 stimulation, but Treg proliferation increased dramatically when PD-1 blockade was added to CD3/CD28 stimulation. Consistent with results in our murine model, rapidly proliferating human Tregs also expressed high levels of annexin-V after PD-1 blockade.
The clinical relevance of these findings is suggested by the observation that objective improvement of cGVHD was more evident in those patients who expressed higher levels of PD-1 on Tregs during IL-2 therapy. Because our study included a relatively small number of patients, larger-scale studies are needed to identify the impact of PD-1 expression on Tregs on clinical response to IL-2 therapy. Clarification of the clinical significance of Treg PD-1 expression as a new biomarker will allow the development of new strategies for modulating Treg homeostasis after transplantation and potentially for developing new ways of preventing or treating cGVHD.
Taken together, these results demonstrate that the PD-1 pathway plays a critical role in the regulation of CD4 Tregs. This is most evident when Treg expansion in vivo is promoted by the exogenous administration of low-dose IL-2, but likely also occurs in the setting of chronic inflammation and endogenous activation of Tregs. In the absence of PD-1, IL-2 induces rapid proliferation and Treg expansion, but these cells also undergo terminal differentiation and become highly susceptible to apoptosis. This results in depletion of the Treg pool. In the presence of PD-1, IL-2–induced Treg proliferation is less intense, but Tregs do not undergo terminal differentiation. As a result Tregs are less susceptible to apoptosis, and expansion of the Treg pool continues as long as exogenous IL-2 is administered.
The mechanisms that regulate the expression of PD-1 by Tregs during IL-2 therapy remain to be clarified. Functional deficiency of PD-1 has been reported in a variety of human autoimmune diseases including systemic lupus erythematosis, rheumatoid arthritis, type 1 diabetes, and multiple sclerosis, and recent studies have suggested that single nucleotide polymorphisms in human PD-1 genes are associated with autoimmune diseases.57,58 Further analysis of genetic polymorphisms and PD-1 function in patients receiving IL-2 may elucidate additional mechanisms that regulate PD-1 activity in Tregs and may predict clinical response to IL-2 therapy.
Although our studies were conducted to develop approaches to promote immune tolerance and reverse symptoms of GVHD, these findings are also relevant to the role of Tregs in chronic viral infection and tumor immunity. It is now well established that activation of the PD-1 pathway causes dysfunction and exhaustion of effector T cells and prevents antigen-specific elimination of target cells. PD-1 blockade reverses the suppression of effector T cells, allowing effector cells to eliminate target cells in vivo.46,59 Our results suggest that activation of the PD-1 pathway also promotes expansion of Tregs to further suppress effector T cells. In patients with cancer, PD-1 inhibition of Tregs may provide an additional mechanism whereby PD-1 blockade promotes effective tumor immunity. Our studies suggest that in the context of PD-1 blockade, administration of low-dose IL-2 will promote the terminal differentiation of Tregs and prevent prolonged Treg expansion. Because IL-2 can also promote the expansion of activated tumor-specific effector T cells in the tumor microenvironment, administration of low-dose IL-2 in combination with PD-1 blockade may provide synergistic antitumor immunity through their combined effects on CD4 Tregs as well as effector T cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank John Daley, Suzan Lazo-Kallanian, Sean McDonough, and Gregory Bascug for excellent assistance with flow cytometric studies, Doreen Hearsey, Hiromi Nakashima, and Kyoko Maeda for help obtaining clinical samples, and all staff at the Institutional Animal Care and Research Advisory Committee, Okayama University Advanced Science Research Center and the Central Research Laboratory, Okayama University Medical School.
This work was supported by Japan Society for the Promotion of Science KAKENHI Grant No. 26461449 and the National Institutes of Health, National Cancer Institute grants P01CA142106, CA183559, and CA183560.
Authorship
Contribution: T.A. designed and performed experiments and wrote the paper; J.K. designed and supervised the clinical trial and clinical data collection and edited the paper; H.T.K. designed the clinical trial, performed statistical analysis for the clinical trial, and edited the paper; Y. Meguri, T.Y., Y.K., M.I., M.N., and Y.S. performed experiments and edited the paper; H.Y. provided monoclonal antibodies for the study and supervised the laboratory studies; Y. Maeda and M.T. supervised the laboratory studies and edited the paper; E.P.A., P.A., C.S.C., V.T.H., J.H.A., and R.J.S. enrolled patients and edited the paper; J.R. designed the clinical trial, supervised the laboratory studies, and edited the paper; and K.-i.M. designed and supervised the research and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ken-ichi Matsuoka, Department of Hematology and Oncology, Okayama University Graduate School of Medicine Dentistry and Pharmaceutical Sciences, 2-5-1 Shikata-cho, Kita-ku, Okayama 700-8558, Okayama, Japan; e-mail: k-matsu@md.okayama-u.ac.jp.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal