Key Points
Conditioning is associated with better thymopoiesis, donor B-lymphocyte chimerism, cessation of immunoglobulin therapy, and normal QoL.
Abstract
Hematopoietic stem cell transplantation (HSCT) cures the T-lymphocyte, B-lymphocyte, and natural killer (NK)–cell differentiation defect in interleukin-2 γ-chain receptor (IL2RG)/JAK3 severe combined immunodeficiency (SCID). We evaluated long-term clinical features, longitudinal immunoreconstitution, donor chimerism, and quality of life (QoL) of IL2RG/JAK3 SCID patients >2 years post-HSCT at our center. Clinical data were collated and patients/families answered PedsQL Generic Core Scale v4.0 questionnaires. We performed longitudinal analyses of CD3+, CD4+ naive T-lymphocyte, CD19+, and NK-cell numbers from pretransplant until 15 years posttransplant. Thirty-one of 43 patients (72%) survived. Median age at last follow-up was 10 years (range, 2-25 years). Twenty-one (68%) had persistent medical issues, mainly ongoing immunoglobulin replacement (14; 45%), cutaneous viral warts (7; 24%), short stature (4; 14%), limb lymphoedema (3; 10%), and bronchiectasis (2; 7%). Lung function was available and normal for 6 patients. Longitudinal analysis demonstrated sustained CD3+, CD19+, and NK-cell output 15 years post-HSCT. CD4+ naive lymphocyte numbers were better in conditioned vs unconditioned recipients (P, .06). B-lymphocyte and myeloid chimerism were highly correlated (ρ, 0.98; P < .001). Low-toxicity myeloablative conditioning recipients have better B-lymphocyte/myeloid chimerism and are free from immunoglobulin replacement therapy. IL2RG/JAK3 SCID survivors free from immunoglobulin replacement have normal QoL.
Introduction
Severe combined immunodeficiencies (SCIDs) are due to defective T development or function, with variable effects on B- and natural killer (NK)–lymphocyte differentiation and development. Defects in the interleukin-2 γ-chain receptor (IL2RG) are most common.1 Less common defects in JAK3 are downstream of IL2RG signaling: both forms present with a T-lymphocyte–negative, B-lymphocyte–positive, NK-cell–negative immunophenotype. B lymphocytes are present, but intrinsic signaling defects render them nonfunctional.2,3 Since the first hematopoietic stem cell transplant (HSCT) to correct the immunodeficiency in SCID in 1968, incremental improvements in techniques and supportive care have led to better survival.4,5 However, most publications described long-term outcome of the entire SCID cohort, irrespective of the genotypic and phenotypic diversity.4-9
Detailed description of single-genotype cohorts is important because different donor sources and conditioning regimens, or use of hematopoietic stem cell infusion alone, may result in different outcomes, depending on the immunophenotype and genotype.9,10 T-lymphocyte donor chimerism and reconstitution are generally good despite various types of conditioning and donor selection. However, poor B-lymphocyte and myeloid chimerism are noted, particularly in the absence of chemotherapy conditioning.11 One study suggested that donor B-lymphocyte chimerism is required for establishment of functioning B lymphocytes in IL2RG, JAK3, and VDJ-recombinant defect SCID genotypes.12 Sustained thymopoiesis and donor B-lymphocyte chimerism may require administration of myeloablative preparative regimens7,13,14 and modified T-lymphocyte depletion techniques.15 Long-term immune function may impact on subsequent health-related quality of life (QoL) and presence or absence of ongoing medical issues, which may be dependent on prior use of a preparative regimen. Therefore, we aimed to assess the long-term immune function, health outcome, and QoL in a single-center cohort of IL2RG/JAK3 SCID patients post-HSCT.
Study design
Forty-three patients received 49 transplants between 1987 and 2012. Procedures were performed based on the European Inborn Errors Working Party guidelines current at time of transplant. Patients and families consented to data collection at time of transplant. Patients were invited to answer the previously validated PedsQL Generic Core Scale Quality of Life v4.016 as part of their routine psychological health assessment.
In the earlier patients, conditioning consisted of busulphan (8 or 16 mg/kg) and cyclophosphamide (200 mg/kg). Low-toxicity myeloablative conditioning (MAC) consisted of treosulphan and fludarabine (150 mg/m2) or cyclophosphamide (200 mg/kg). Reduced-intensity conditioning (RIC) consisted of fludarabine (150 mg/m2) and melphalan (140 mg/m2). Further details about donor types, conditioning regimens, graft sources, and serotherapy for each patient are summarized in supplemental Table 1 (available on the Blood Web site).
CD3, CD4, CD8, CD19, CD27/CD45RA,15 and CD16/56 enumeration and serum immunoglobulin G (IgG), IgA, and IgM levels were measured routinely. Donor cell chimerism was based on polymerase chain reaction amplification of short tandem repeats.
All data analysis was performed using STATA version 14.1. Multilevel mixed-effect modeling was performed for longitudinal analysis of CD3+, CD19+, NK cells, and CD4+ naive output posttransplant.
Results and discussion
Thirty-one patients survived to August 2015. Median age at last follow-up was 10 years (range, 2-25 years). Overall survival at 10 years post-HSCT was 71.9%. Transplant-related mortality (TRM) was 23.3% (1 patient died of nontransplant-related causes). There was no significant difference in 10-year survival outcome comparing unconditioned vs conditioned recipients considering first HSCT characteristics (69.8% vs 72.4%; P = .91). Details of those who received a second procedure are given in supplemental Table 2.
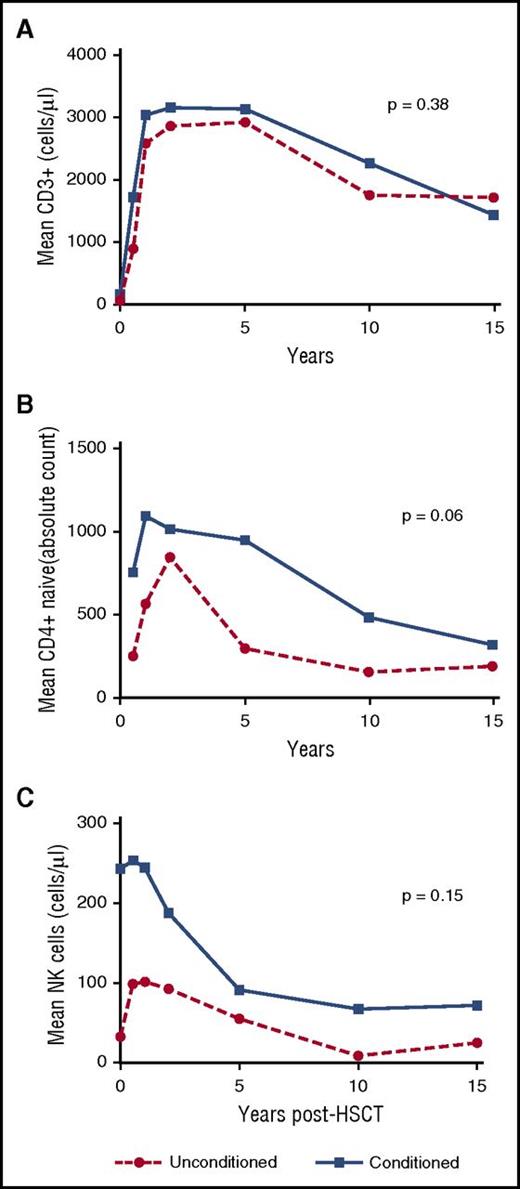
Longitudinal immune-reconstitution results were available for 29 of 31 patients. There was no significant difference in CD3+ numbers between conditioned vs unconditioned patients (P = .38) (Figure 1A). Conditioned recipients trended toward more sustained thymopoiesis, compared with unconditioned recipients (P = .06) (Figure 1B). Longitudinal NK-cell numbers were nonsignificantly higher in conditioned recipients compared with unconditioned recipients (P, .15) (Figure 1C). There was no significant difference in mean values of NK cells at latest follow-up between those with or without verrucosis (84.4 cells per microliter [standard deviation (SD), 61.2] vs 87.19 cells per microliter [SD, 83.1]; P = .53), or after comparing numbers of patients with verrucosis in conditioned vs unconditioned recipients (5 of 19 vs 2 of 10; P = .14).
The longitudinal analysis of immune parameters posttransplant according to unconditioned vs conditioned recipients of IL2RG/JAK3 SCID. (A) Mean CD3+ cells measured at different time points posttransplant. There was no significant difference in trend of circulating CD3+ numbers between conditioned vs unconditioned IL2RG/JAK3 SCID patients (P = .38) and at each time point posttransplant. (B) Mean CD4+ naive cells measured at different time points posttransplant. Naive CD4+ cell (CD3+CD4+CD45RA+CD27+) measurement was used as an indicator of the thymic output posttransplantation. Conditioned recipients demonstrated borderline higher overall trend of CD4+ naive output compared with unconditioned recipients (P, .06). Conditioned recipients had a better CD4+ naive output at early time points after transplant compared with unconditioned recipients. The data are in the format of contrast, which shows the difference between the mean of both groups and standard error (SE): 0.5 years posttransplant (contrast, 611.1; SE, 286.1; P, .03), 1 year posttransplant (contrast, 513.1; SE, 224.8; P, .02), 2 years posttransplant (contrast, 415.1; SE, 178.0; P, .01), 5 years posttransplant (contrast, 317.0; SE, 159.0; P, .04), respectively. (C) Mean NK cells measured at different time points posttransplant. The conditioned recipients had a nonsignificantly higher overall NK-cell number compared with unconditioned recipients (P, .15). However, conditioned recipients did have significantly higher NK-cell numbers compared with unconditioned recipients at time point of baseline (contrast, 126.5; SE, 66.1; P, .05) and 0.5 years posttransplant (contrast, 112.1; SE, 59.3; P, .05). Numbers of patients available for longitudinal data analysis of immunoreconstitution (CD3+, CD4+ naive, and NK cells) are shown in supplemental Table 4.
The longitudinal analysis of immune parameters posttransplant according to unconditioned vs conditioned recipients of IL2RG/JAK3 SCID. (A) Mean CD3+ cells measured at different time points posttransplant. There was no significant difference in trend of circulating CD3+ numbers between conditioned vs unconditioned IL2RG/JAK3 SCID patients (P = .38) and at each time point posttransplant. (B) Mean CD4+ naive cells measured at different time points posttransplant. Naive CD4+ cell (CD3+CD4+CD45RA+CD27+) measurement was used as an indicator of the thymic output posttransplantation. Conditioned recipients demonstrated borderline higher overall trend of CD4+ naive output compared with unconditioned recipients (P, .06). Conditioned recipients had a better CD4+ naive output at early time points after transplant compared with unconditioned recipients. The data are in the format of contrast, which shows the difference between the mean of both groups and standard error (SE): 0.5 years posttransplant (contrast, 611.1; SE, 286.1; P, .03), 1 year posttransplant (contrast, 513.1; SE, 224.8; P, .02), 2 years posttransplant (contrast, 415.1; SE, 178.0; P, .01), 5 years posttransplant (contrast, 317.0; SE, 159.0; P, .04), respectively. (C) Mean NK cells measured at different time points posttransplant. The conditioned recipients had a nonsignificantly higher overall NK-cell number compared with unconditioned recipients (P, .15). However, conditioned recipients did have significantly higher NK-cell numbers compared with unconditioned recipients at time point of baseline (contrast, 126.5; SE, 66.1; P, .05) and 0.5 years posttransplant (contrast, 112.1; SE, 59.3; P, .05). Numbers of patients available for longitudinal data analysis of immunoreconstitution (CD3+, CD4+ naive, and NK cells) are shown in supplemental Table 4.
Donor chimerism was available for 29 patients. B-lymphocyte and myeloid cell donor chimerism were highly correlated (Spearman ρ, 0.98; P < .001) (supplemental Figure 1). Low-toxicity MAC recipients had significantly better myeloid donor chimerism at last follow-up, compared with unconditioned or other conditioning recipients (P < .001) (supplemental Figure 2).
Seventeen patients (55%) with normal B-lymphocyte function were free from immunoglobulin replacement therapy. All low-toxicity MAC survivors (7 of 8) were free from immunoglobulin replacement therapy with >50% donor B-lymphocyte chimerism irrespective of donor. Of unconditioned survivors, 4 of 10 (and of survivors conditioned with 8 mg/kg busulphan, 6 of 12) did not require immunoglobulin replacement (supplemental Table 3). There was a significant association between >50% donor B-lymphocyte chimerism and the ability to cease immunoglobulin replacement therapy (P = .0001) (supplemental Figure 3).
QoL assessments were available for 20 of 31 patients (65%) and comparisons performed with published UK normal values.17 Parents reported significantly lower QoL in total, psychosocial, and school domains, but there were no significant differences between self-reporting of patients and UK published norms (Table 1). Subgroup analysis revealed that patients and parents of patients not requiring immunoglobulin replacement therapy reported no significant difference in QoL from normal, compared with those who were receiving weekly subcutaneous immunoglobulin infusions at home.
Mean PedsQL scores for IL2RG/JAK3 SCID patients posttransplantation (parent and children report)
| . | UK norm . | IL2RG/JAK3 SCID, mean (P) . | Ongoing immunoglobulin replacement, mean (P) . | No immunoglobulin replacement, mean (P) . | Ongoing medical issues (P) . | No medical issues (P) . |
|---|---|---|---|---|---|---|
| Parent report | N = 19 | N = 8 | N = 11 | N = 12 | N = 7 | |
| Total | 84.6 | 70.9 (.009) | 63.9 (.02) | 75.0 (.06) | 70.0 (.02) | 72.3 (.04) |
| Psychosocial | 82.2 | 66.5 (.007) | 56.5 (.01) | 73.8 (.08) | 64.7 (.01) | 69.5 (.06) |
| Physical | 89.1 | 82.4 (.19) | 77.7 (.12) | 85.8 (.28) | 79.9 (.10) | 86.6 (.37) |
| Emotional | 78.3 | 72.9 (.34) | 60.0 (.06) | 82.3 (.81) | 69.6 (.15) | 78.6 (.52) |
| Social | 86.8 | 77.4 (.13) | 70.0 (.09) | 82.7 (.24) | 77.5 (.15) | 77.1 (.12) |
| School | 81.5 | 63.5 (.02) | 55.3 (.01) | 68.0 (.09) | 58.8 (.01) | 70.2 (.19) |
| Child report | N = 15 | N = 5 | N = 10 | N = 8 | N = 7 | |
| Total | 83.9 | 77.8 (.23) | 71.7 (.16) | 80.8 (.28) | 77.6 (.23) | 77.9 (.17) |
| Psychosocial | 81.8 | 74.1 (.17) | 67.3 (.13) | 77.5 (.23) | 75.2 (.23) | 72.9 (.12) |
| Physical | 88.5 | 84.6 (.45) | 80.0 (.23) | 86.9 (.33) | 82.0 (.24) | 87.5 (.42) |
| Emotional | 78.5 | 79.3 (.89) | 67.0 (.24) | 85.5 (.88) | 75.0 (.37) | 84.3 (.79) |
| Social | 87.7 | 75.7 (.09) | 77.0 (.17) | 75.0 (.09) | 81.3 (.21) | 69.3 (.08) |
| School | 78.9 | 67.3 (.08) | 58.0 (.05) | 72.0 (.19) | 69.4 (.15) | 65.0 (.09) |
| . | UK norm . | IL2RG/JAK3 SCID, mean (P) . | Ongoing immunoglobulin replacement, mean (P) . | No immunoglobulin replacement, mean (P) . | Ongoing medical issues (P) . | No medical issues (P) . |
|---|---|---|---|---|---|---|
| Parent report | N = 19 | N = 8 | N = 11 | N = 12 | N = 7 | |
| Total | 84.6 | 70.9 (.009) | 63.9 (.02) | 75.0 (.06) | 70.0 (.02) | 72.3 (.04) |
| Psychosocial | 82.2 | 66.5 (.007) | 56.5 (.01) | 73.8 (.08) | 64.7 (.01) | 69.5 (.06) |
| Physical | 89.1 | 82.4 (.19) | 77.7 (.12) | 85.8 (.28) | 79.9 (.10) | 86.6 (.37) |
| Emotional | 78.3 | 72.9 (.34) | 60.0 (.06) | 82.3 (.81) | 69.6 (.15) | 78.6 (.52) |
| Social | 86.8 | 77.4 (.13) | 70.0 (.09) | 82.7 (.24) | 77.5 (.15) | 77.1 (.12) |
| School | 81.5 | 63.5 (.02) | 55.3 (.01) | 68.0 (.09) | 58.8 (.01) | 70.2 (.19) |
| Child report | N = 15 | N = 5 | N = 10 | N = 8 | N = 7 | |
| Total | 83.9 | 77.8 (.23) | 71.7 (.16) | 80.8 (.28) | 77.6 (.23) | 77.9 (.17) |
| Psychosocial | 81.8 | 74.1 (.17) | 67.3 (.13) | 77.5 (.23) | 75.2 (.23) | 72.9 (.12) |
| Physical | 88.5 | 84.6 (.45) | 80.0 (.23) | 86.9 (.33) | 82.0 (.24) | 87.5 (.42) |
| Emotional | 78.5 | 79.3 (.89) | 67.0 (.24) | 85.5 (.88) | 75.0 (.37) | 84.3 (.79) |
| Social | 87.7 | 75.7 (.09) | 77.0 (.17) | 75.0 (.09) | 81.3 (.21) | 69.3 (.08) |
| School | 78.9 | 67.3 (.08) | 58.0 (.05) | 72.0 (.19) | 69.4 (.15) | 65.0 (.09) |
All mean comparisons were made against UK norms17 using the 1 sample t test.
A number of key novel findings arise from this study. Durability of T-lymphocyte levels is confirmed, but of interest is the difference in long-term thymic output between those that received conditioning and unconditioned recipients. The difference between groups did not quite reach statistical significance, but given the small sample size, the observation was striking and likely to be real. A biological explanation may be that in conditioned patients, the thymic niche is consistently reseeded from bone marrow–derived donor stem cells leading to ongoing thymopoiesis, whereas for unconditioned recipients, initial seeding of the thymic niche at time of infusion is not generally followed by reseeding, as donor stem cell engraftment does not consistently occur in the bone marrow, and thymic seeding may have a finite lifetime, leading eventually to thymic exhaustion.18
The strong association of a preparative regimen with donor myeloid and B-lymphocyte chimerism and function is confirmed. A busulphan dose of 8 mg/kg in combination with cyclophosphamide may not be myeloablative enough to reliably ensure donor stem cell engraftment with donor B lymphopoiesis, despite a historical view that it was adequate.13 Higher doses of busulphan are associated with increased toxicity particularly in infants. The use of treosulfan-based regimens has previously been reported in patients with primary immunodeficiency with few significant short-term toxicities.19-21 It is encouraging to find that treosulfan-containing low-toxicity myeloablative regimens confer improved donor chimerism. In these patients, the goal of any conditioning regimen should be to achieve >50% donor B-lymphocyte chimerism to reliably cease immunoglobulin replacement.
Finally, we assessed health-related QoL. A previous study found decreased QoL in transplanted SCID patients.22 However, this was a heterogenous cohort with different primary immunodeficiency disorders, some of which may intrinsically affect QoL. Although our initial results confirmed these findings, a subgroup analysis showed that patients who discontinued immunoglobulin substitution appeared to have normal health-related QoL compared with normal controls, whereas those who remained on immunoglobulin had significantly worse results.
In conclusion, we have demonstrated, in a single-center cohort of IL2RG/JAK3 SCID patients, that thymopoiesis is durable over time, but better in those who received conditioning. Low-toxicity myeloablative regimens achieve better donor stem cell engraftment, with few significant short-term toxicities, although long-term follow-up will be required to assess late effects. Freedom of immunoglobulin replacement leads to normal life quality, and is most associated with preparative chemotherapy. The debate about the use of chemotherapy vs infusion is likely to continue, and we should continue to strive for safer, nontoxic regimens.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge permission for the use of PedsQL. I.J.A.H. acknowledges Universiti Sains Malaysia and Ministry of Higher Education of Malaysia for study funding in Newcastle University, United Kingdom.
Authorship
Contribution: I.J.A.H. designed the research project, collected the data and questionnaires, performed the statistical analysis and interpretation of the data, and wrote the manuscript; M.A.S., F.M., M.S.P., and A.R.G. contributed equally to the conceptualization of the research, statistical analysis, data interpretation, manuscript writing, and critical review at every level of the research stages.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Intan Juliana Abd Hamid, Pediatric Immunodeficiency Group, Institute of Cellular Medicine, Newcastle University, Newcastle upon Tyne, United Kingdom; e-mail: i.j.abd-hamid@newcastle.ac.uk or intanj@usm.my.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal