In this issue of Blood, Abd Hamid and colleagues describe the long-term follow-up of patients who underwent hematopoietic stem cell transplantation (HSCT) for IL2RG/JAK3 severe combined immunodeficiency (SCID) and demonstrate improved B-cell function and improved quality of life (QoL) in patients who received pretransplantation conditioning.1
Since the first successful HSCT for SCID in 1968, HSCT has been the standard, and until recent years, the only, definitive therapy for SCID.2 However, the best manner of transplantation for classical SCID has remained unclear, with ongoing debate about the risks of preconditioning chemotherapy vs the degree of immune reconstitution necessary for sustained cure. With the widespread use of newborn screening for SCID in the United States and other countries that has permitted rapid diagnosis and increased usage of HSCT in early infancy, the need for long-term, genotype-specific outcomes data is critical.3
In their study, Abd Hamid et al describe the outcomes of a sizeable cohort of 43 patients who have undergone HSCT for IL2RG or JAK3 SCID over a 25-year period at a single institution. The authors grouped patients based on their preconditioning regimen (if given) and analyzed data regarding immune reconstitution, including lymphocyte enumeration, B-cell and myeloid chimerism, need for immunoglobulin replacement, and thymic function based on the presence of recent thymic emigrant T cells. In addition, the QoL of patients and parents was evaluated using the PedsQL survey.
The authors found that overall survival in conditioned vs unconditioned patients was equivalent. They further found that use of a treosulfan-based conditioning regimen correlated with improved myeloid and B-cell chimerism following HSCT, and that all surviving patients in this category did not need long-term immunoglobulin replacement. In addition, all patients with ≥50% B-cell donor chimerism were free of immunoglobulin. The investigators also highlight a trend toward increased recent thymic emigrants and natural killer cells over time in patients who received preconditioning vs those who received no preconditioning. Although nonsignificant, this trend is notable and suggests that preconditioning may permit broader and more lasting immune reconstitution following HSCT.
Subgroup analysis of QoL surveys showed a decreased mean score in patients still receiving immunoglobulin, which reached significance in school (as reported by both parents and patients) and psychosocial categories (as reported by parents).
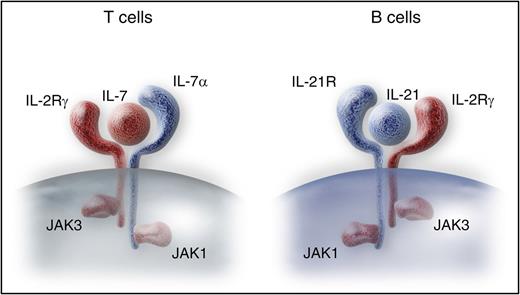
IL2RG and JAK3 SCID has previously been described to cause B-cell dysfunction despite detectable B cells, due to their dependence on cytokine signaling downstream of γc and JAK3 (see figure).4 A previous meta-analysis suggested that donor B-cell engraftment was necessary for antibody function in this SCID subcategory.5 In this study, Abd Hamid and colleagues provide evidence tying B-cell chimerism to the ability to discontinue immunoglobulin therapy after HSCT for IL2RG and JAK3 SCID. Furthermore, they show that the need for immunoglobulin replacement has an impact on QoL in this population. Although prior studies have examined QoL in patients with antibody deficiency,6 the results of this study provide notable evidence of the impact of inadequate B-cell reconstitution. Although randomized, prospective studies are needed to determine the ideal transplantation regimen for the different SCID genotypes, these results support the premise that preconditioning improves B-cell chimerism and accordingly improves QoL. Future trials may help to strike a balance between the risk of toxicities and improvement of long-term immune reconstitution in transplantation for SCID and accordingly focus on maximizing both survival and QoL when selecting definitive therapies for this disorder.
IL2RG and JAK3 are essential for T-cell development via the IL-7 receptor, and for B-cell maturation due to their function in the IL-21 receptor. Professional illustration by Somersault 18:24.
IL2RG and JAK3 are essential for T-cell development via the IL-7 receptor, and for B-cell maturation due to their function in the IL-21 receptor. Professional illustration by Somersault 18:24.
Conflict-of-interest disclosure: The author declares no competing financial interests.