Key Points
MMB ameliorates anemia in a rodent anemia of chronic disease model by inhibiting activin receptor-like kinase-2 activity.
Hepcidin-dependent ferroportin degradation is independent of JAK2 phosphorylation.
Abstract
Patients with myelofibrosis (MF) often develop anemia and frequently become dependent on red blood cell transfusions. Results from a phase 2 study for the treatment of MF with the Janus kinase 1/2 (JAK1/2) inhibitor momelotinib (MMB) demonstrated that MMB treatment ameliorated anemia, which was unexpected for a JAK1/2 inhibitor, because erythropoietin-mediated JAK2 signaling is essential for erythropoiesis. Using a rat model of anemia of chronic disease, we demonstrated that MMB treatment can normalize hemoglobin and red blood cell numbers. We found that this positive effect is driven by direct inhibition of the bone morphogenic protein receptor kinase activin A receptor, type I (ACVR1), and the subsequent reduction of hepatocyte hepcidin production. Of note, ruxolitinib, a JAK1/2 inhibitor approved for the treatment of MF, had no inhibitory activity on this pathway. Further, we demonstrated the effect of MMB is not mediated by direct inhibition of JAK2-mediated ferroportin (FPN1) degradation, because neither MMB treatment nor myeloid-specific deletion of JAK2 affected FPN1 expression. Our data support the hypothesis that the improvement of inflammatory anemia by MMB results from inhibition of ACVR1-mediated hepcidin expression in the liver, which leads to increased mobilization of sequestered iron from cellular stores and subsequent stimulation of erythropoiesis.
Introduction
Systemic iron homeostasis is maintained by the coordinated regulation of iron absorption in the duodenum, iron recycling of senescent erythrocytes in macrophages, and mobilization of stored iron in the liver.1 One key orchestrator in this process is hepcidin, a small peptide hormone primarily synthesized in hepatocytes.2-4 Hepcidin reduces both duodenal iron absorption and iron export from monocytes/macrophages by binding to and inducing the internalization and degradation of the iron exporter ferroportin (FPN1).5-7 Thus, elevated serum hepcidin levels enhance storage of iron within the reticuloendothelial system and result in reduced iron availability and iron-restricted erythropoiesis. Inappropriately elevated hepcidin expression causes severe functional iron deficiency anemia in humans and is central to the pathophysiology of anemia of chronic disease (ACD).8
Several inputs converge to control hepcidin transcription in the liver, including body iron stores, the erythropoietic demand for iron, hypoxia, and inflammation.1 Bone morphogenic proteins (BMPs) play a central role in mediating these inputs and driving hepcidin transcriptional induction by activating BMP receptor (BMPR)-SMAD signaling.9-12 BMPR kinase activin A receptor, type I (ACVR1), which is also called activin receptor-like kinase-2 (ALK2), and BMPR1a/ALK3 have been shown to play an essential role in this process, with liver-specific deletion of either ACVR1/ALK2 or BMPR1a/ALK3 blocking the induction of hepcidin production downstream of BMP ligand binding and resulting in iron overload in mice.13 The inflammatory cytokine interleukin-6 (IL-6) has also been demonstrated to induce hepcidin expression through canonical Janus kinase (JAK)/STAT signaling; however, this activity is dependent on an intact BMP-SMAD pathway.9,14,15
A significant proportion of patients with myelofibrosis (MF) develop anemia, with many becoming dependent on frequent red blood cell (RBC) transfusions.16 Elevated serum hepcidin levels in patients with MF have recently been demonstrated to be associated with hemoglobin (Hb) levels <10 g/dL, increased requirement for RBC transfusions, and reduced survival.17 Results from the momelotinib (MMB) phase 2 study for the treatment of MF demonstrated that MMB treatment resulted in improvement of anemia.18 This anemia benefit was unexpected for a JAK2 inhibitor, because erythropoietin-mediated JAK2 signaling is essential for stimulation of erythropoiesis and because new-onset anemia has been identified as a major adverse event associated with ruxolitinib (RUX; JAK1/2 inhibitor) treatment.19-22
A possible link between JAK2 and iron metabolism was reported by De Domenico et al.23 They demonstrated that hepcidin binding to FPN1 results in stimulation of JAK2 and that this JAK2 activation is needed for the subsequent internalization and degradation of FPN1. However, Ross et al24 proposed that hepcidin-mediated FPN1 internalization and degradation do not require the action of JAK2. Instead, they suggested that ubiquitination of lysines is critical for the internalization of FPN1.
We used a well-established group A streptococcal peptidoglycan-polysaccharide fragment (PG-APS)–induced rat model of ACD to investigate the mechanism underlying the clinical anemia benefit seen with MMB treatment.7 First, by using MMB treatment and JAK2 conditional knockout (JAK2cKO) macrophages, we demonstrated that JAK2 is dispensable for the degradation of FPN1 in response to hepcidin, ruling out the possibility that MMB acts via JAK2-mediated inhibition of FPN1 degradation. We then demonstrated that in addition to primary activity on JAK-STAT signaling, MMB directly affects the control of iron metabolic pathways by inhibiting the BMPR kinase ACVR1/ALK2, which triggers the expression of the iron hormone hepcidin. Inhibition of ACVR1/ALK2 by MMB decreases the formation of hepcidin in the liver, resulting in increased release of iron from cellular stores and enhanced erythropoiesis in a rat model of ACD.
Materials and methods
Animal care
The animals had free access to food and water and were housed according to institutional and governmental guidelines in the animal facility of the Medical University of Innsbruck with a 12-hour light-dark cycle and an average temperature of 20°C ± 1°C. Animals were kept on a standard rodent diet (180-mg iron/kg; SNIFF, Soest, Germany). Design of the animal experiments was approved by the Austrian Federal Ministry of Science and Research (BMWF-66.011/0056-WF/V/3b/2015 and BMWF-66.011/0026-WF/V/3b/2015) according to the directive 2010/63/EU. All additional details on the experimental procedures performed in mice and rats are in the supplemental Materials, available on the Blood Web site.
Western blot analysis
Protein extraction and western blotting were performed exactly as described previously25 using primary antibodies and appropriate horseradish peroxidase–conjugated secondary antibodies, which were diluted with Tris-buffered saline with Tween 20 according to their dilution factor. Additional details on the preparation and antibodies used are provided in the supplemental data. For quantification, densitometry data were acquired on a ChemiDoc Touch Imaging System (Bio-Rad) and analyzed with Quantity One software (Bio-Rad).
RNA preparation from tissue, reverse transcription, and TaqMan real-time polymerase chain reaction
Total RNA was prepared from freshly isolated rat and mouse tissues using acid guanidinium thiocyanate-phenol-chloroform extraction with peqGOLD Tri-Fast (Peqlab, Erlangen, Germany). A total of 4 µg of RNA was used for reverse transcription and subsequent TaqMan real-time polymerase chain reaction for the genes of interest. Additional details on primer sequences and analysis are provided in the supplemental data.
RAW cell culture for western blot analysis
RAW 264.7 (ATCC #TIB-71) cells were cultured in Dulbecco’s modified Eagle medium (Lonza) plus 10% fetal calf serum (FCS; Biochrom) plus 1% penicillin/streptomycin plus 2-mM glutamine at 37°C, plus 5% carbon dioxide. Additional details on the different stimulations and consequent western blot analysis are provided in the supplemental data.
Complete blood count analysis
Complete blood count analysis was performed on a Vet-ABC animal blood counter (scil animal care company GmbH, Viernheim, Germany).
Serum iron measurement
Serum iron from rats and mice was measured using the QuantiChrom iron assay kit (BioAssay Systems, Hayward, CA) according to the manufacturer’s instructions.
Hepcidin enzyme-linked immunosorbent assay
Serum hepcidin was measured using the hepcidin-25 (rat) enzyme immunoassay kit extraction free (Peninsula Laboratories International, Inc., San Carlos, CA) according to the manufacturer’s protocol. Standards and samples were analyzed in duplicate, and a standard curve was obtained by performing 9-point serial twofold dilutions to cover compound (cpd) concentrations from 100 to 0.1 ng/mL. Serum samples were then diluted in a range of 1:10 to 1:40 in standard diluent to give readings within the standard curve. Optical density was measured at 650 nm in a microplate reader (Infinite M200pro; Tecan, Männedorf, Switzerland).
Flow cytometry analysis
Bone marrow from rat femurs was extruded into phosphate buffered saline (PAA Laboratories GmbH, Wagram, Austria) supplemented with 5-mM EDTA. Cell suspensions were further diluted in phosphate buffered saline supplemented with sterile 2% FCS and 0.5% bovine serum albumin (Carl Roth, Karlsruhe, Germany) and costained with fluorochrome-conjugated antibodies: allophycocyanin anti-rat erythroid cells (clone HIS49:BD) and fluorescein isothiocyanate anti-CD44 (Bio-Rad Laboratories GmbH, Vienna, Austria).
Data were acquired on a BD FACSVerse flow cytometer (BD Bioscience, San Jose, CA) equipped with a BD flow sensor that directly measures the volume during fluorescence-activated cell sorting (FACS) analysis to provide accurate absolute cell counts. Gating strategies and additional details are provided in the supplemental data.
Bone marrow cells from healthy rats and macrophages from JAK2 wild-type (JAK2Wt) and JAK2cKO mice were acquired on a Gallios flow cytometer. Preparation, staining, analysis procedures of murine macrophages, and the antibodies used are described in the supplemental data. All FACS data were further analyzed with FlowJo v8.8.6 software (Tree Star, Inc., Ashland, OR).
HepG2 cell culture for hepcidin RNA and phosphosignaling analysis
HepG2 cells were cultured in EMEM (ATCC #30-2003) plus 10% FCS plus 1% penicillin/streptomycin. Additional details on preparation and analysis procedures for hepcidin RNA measurements and phosphorylated SMAD1/5/8 (pSMAD1/5/8) and pSTAT3 analysis are provided in the supplemental data.
Biochemical IC50 measurements
JAK1, JAK2, JAK3, and TYK2 50% inhibitory concentrations (IC50s) for MMB and RUX were assessed in a commercial kinase potency screen performed by Carna Biosciences (Kobe, Japan). To obtain IC50 values, MMB and RUX were incubated as 10-point titrations to cover cpd concentrations from 0.3 nM to 10 µM.
ACVR1, BMPR1a, and transforming growth factor β receptor 1 (TGFBR1) IC50 values were obtained using the SelectScreen biochemical kinase profiling service (LanthaScreen Eu Kinase Binding Assay; Thermo Fisher). To obtain IC50 values, MMB and RUX were incubated as 10-point titration, threefold serial dilutions to cover cpd concentrations from 0.5 nM to 10 µM.
Statistics
Results are expressed as means ± standard errors of the mean. Calculations for statistical differences between various groups were carried out by the analysis of variance technique and Dunnett’s correction for multiple tests. Otherwise, a 2-tailed unpaired Student t test was used. P < .05 was used to determine statistical significance of parametric and nonparametric data.
Results
MMB ameliorates anemia in a rat model of ACD
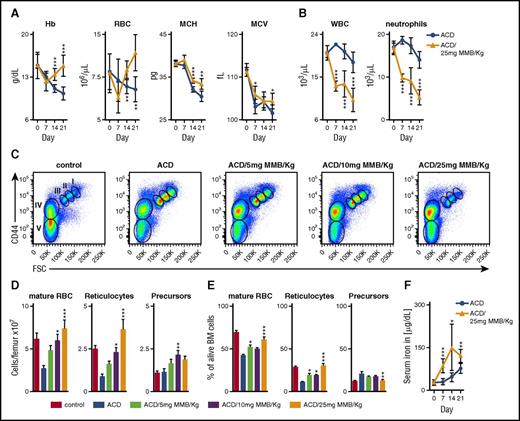
ACD was induced in female Lewis rats by intraperitoneal administration of PG-APS, resulting in anemia within 2 weeks after treatment7,25 (supplemental Figure 1A). To assess whether MMB treatment could alleviate anemia in these animals, anemic rats (2 weeks post–PG-APS) were treated once daily with either MMB (25 mg/kg) or vehicle alone. MMB treatment resulted in a significant increase in Hb concentrations and RBC count on days 14 and 21 of treatment when compared with the vehicle group (Figure 1A). In addition, we could observe an increase in mean corpuscular Hb and mean corpuscular volume, suggesting an improved supply of iron for erythroid progenitors (Figure 1A). Concomitant reductions in white blood cell and neutrophil counts were also observed (Figure 1B). Notably, these effects were also seen after 3-week treatment with lower doses of MMB (supplemental Figure 1B-C).
MMB ameliorates anemia in a rat model of ACD. (A-B) Hb, RBC count, mean corpuscular hemoglobin (MCH), mean corpuscular volume (MCV), white blood cell count (WBC), and neutrophil count in ACD rats after MMB (25 mg/kg) treatment for 21 days (day 0; corresponds to 14 days after PG-APS injection). (C-E) Representative FACS dot plots from rat bone marrow after 3 weeks of MMB (5, 10, or 25 mg/kg) treatment. FACS analysis was performed with freshly isolated bone marrow cells 6 hours after the last administration of MMB. The total numbers (D) as well as percentages (E) of the different erythropoietic populations per femur are shown as means ± standard errors of the mean (more details provided in supplemental Figure 2). (F) Serum iron levels after MMB (25 mg/kg) treatment. Unpaired Student t test was applied for comparison at each time point (A-B,F) and analysis of variance with Dunnett’s correction for multiple comparisons vs ACD (D-E). *P < .05, **P < .01, ***P < .001. FSC, forward scatter.
MMB ameliorates anemia in a rat model of ACD. (A-B) Hb, RBC count, mean corpuscular hemoglobin (MCH), mean corpuscular volume (MCV), white blood cell count (WBC), and neutrophil count in ACD rats after MMB (25 mg/kg) treatment for 21 days (day 0; corresponds to 14 days after PG-APS injection). (C-E) Representative FACS dot plots from rat bone marrow after 3 weeks of MMB (5, 10, or 25 mg/kg) treatment. FACS analysis was performed with freshly isolated bone marrow cells 6 hours after the last administration of MMB. The total numbers (D) as well as percentages (E) of the different erythropoietic populations per femur are shown as means ± standard errors of the mean (more details provided in supplemental Figure 2). (F) Serum iron levels after MMB (25 mg/kg) treatment. Unpaired Student t test was applied for comparison at each time point (A-B,F) and analysis of variance with Dunnett’s correction for multiple comparisons vs ACD (D-E). *P < .05, **P < .01, ***P < .001. FSC, forward scatter.
To further characterize the underlying hematopoietic changes being driven by MMB treatment, we analyzed rat bone marrow (gating strategy shown in supplemental Figure 2). Immunophenotypic analysis of erythropoietic progenitors and mature RBCs in the bone marrow demonstrated that MMB treatment resulted in a dose-dependent increase in the numbers of reticulocytes and mature RBCs (Figure 1C-E). As numbers of reticulocytes and mature RBCs increased, the relative percentage of erythropoietic precursors (I, II, and III) declined with increasing MMB doses. However, as total cellularity increased with MMB treatment, absolute precursor numbers also increased. MMB treatment also resulted in an increase of circulating iron concentrations (Figure 1F). In contrast, administration of MMB (5, 10, or 25 mg/kg) in healthy rats did not induce any of these effects (supplemental Figure 3A-B).
JAK2 is dispensable for FPN1 degradation in vitro and in vivo
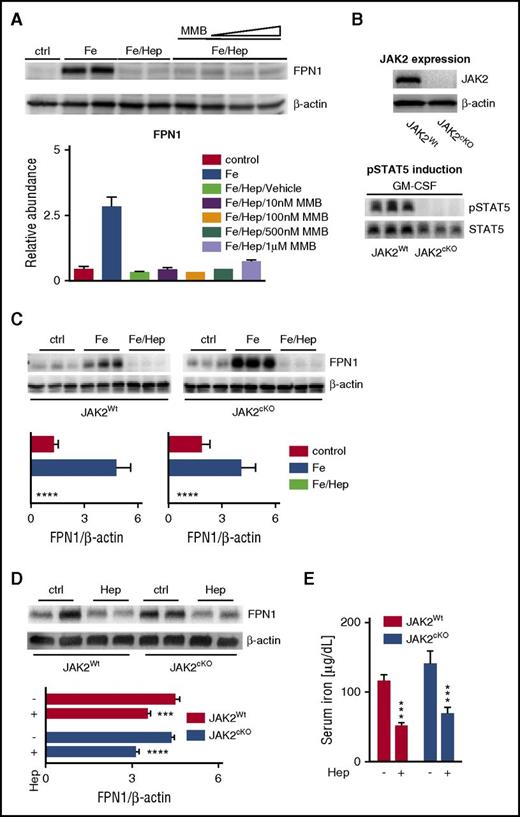
Because JAK2 activation has been demonstrated to regulate hepcidin-mediated internalization and degradation of FPN1,23 we hypothesized that the increase in circulating iron levels and the subsequent stimulation of erythropoiesis observed with MMB treatment may be caused by JAK2-mediated inhibition on FPN1 degradation. To analyze this possibility, we first used the murine macrophage cell line RAW 264.7 stimulated with iron(III) chloride (FeCl3) to induce high levels of FPN1. The cells were then exposed to synthetic hepcidin in the presence or absence of increasing concentrations of MMB (10 nM-1 µM). However, MMB treatment, even at high concentrations (up to 100-fold higher than the calculated IC50 value for JAK2), had no effect on FPN1 protein expression in this setting (Figure 2A).
JAK2 inhibition does not affect hepcidin-mediated FPN1 degradation in vitro or in vivo. (A) Representative western blot and quantification (n = 3 per group) of FPN1 protein levels in murine macrophage RAW 264.7 cells. Control-treated (ctrl) RAWs were not treated at all. All other groups were stimulated with FeCl3 (50 µM) for 20 hours to induce FPN1 expression. Where indicated, cells were further treated with hepcidin (Hep; 1 µg/mL) alone or together with increasing concentrations of MMB (10 nM, 100 nM, 500 nM, and 1 µM) for an additional 6 hours. β-actin levels were used for normalization. Each lane represents one individual replicate. (B) Western blot of JAK2 protein levels in bone marrow–derived macrophages (BMDMs) from JAK2Wt and JAK2cKO mice. β-actin levels were used as a loading control (top). Western blot of pSTAT5 protein levels in BMDMs from JAK2Wt and JAK2cKO mice after stimulation with granulocyte macrophage colony-stimulating factor (GM-CSF; 100 ng/mL) for 30 minutes (bottom). STAT5 levels were used as a loading control. Each lane represents one individual animal. (C) Representative western blots and quantification (n = 6-9 per group) of FPN1 protein levels in BMDMs from JAK2Wt and JAK2cKO mice incubated with either FeCl3 (50 µM) for 20 hours alone or with hepcidin (1 µg/mL) for 6 hours. Ctrl BMDMs received neither FeCl3 nor hepcidin. β-actin levels were used for normalization. Each lane represents one individual animal. (D-E) Representative western blot and quantification (n = 5-7) of FPN1 protein levels in whole-spleen lysates (D) and serum iron levels (E) from JAK2Wt and JAK2cKO mice at baseline (ctrl) and after intraperitoneal administration of hepcidin (4 mg/kg) for 3 hours. β-actin levels were used for normalization. Each lane represents one individual animal (D). Analysis of variance with Dunnett’s correction for multiple comparisons vs iron (Fe)/Hep-stimulated cells was applied (A,C). Unpaired Student t test was applied for comparison in each group (D-E). Results are shown as means ± standard errors of the mean. ***P < .001, ****P < .0001.
JAK2 inhibition does not affect hepcidin-mediated FPN1 degradation in vitro or in vivo. (A) Representative western blot and quantification (n = 3 per group) of FPN1 protein levels in murine macrophage RAW 264.7 cells. Control-treated (ctrl) RAWs were not treated at all. All other groups were stimulated with FeCl3 (50 µM) for 20 hours to induce FPN1 expression. Where indicated, cells were further treated with hepcidin (Hep; 1 µg/mL) alone or together with increasing concentrations of MMB (10 nM, 100 nM, 500 nM, and 1 µM) for an additional 6 hours. β-actin levels were used for normalization. Each lane represents one individual replicate. (B) Western blot of JAK2 protein levels in bone marrow–derived macrophages (BMDMs) from JAK2Wt and JAK2cKO mice. β-actin levels were used as a loading control (top). Western blot of pSTAT5 protein levels in BMDMs from JAK2Wt and JAK2cKO mice after stimulation with granulocyte macrophage colony-stimulating factor (GM-CSF; 100 ng/mL) for 30 minutes (bottom). STAT5 levels were used as a loading control. Each lane represents one individual animal. (C) Representative western blots and quantification (n = 6-9 per group) of FPN1 protein levels in BMDMs from JAK2Wt and JAK2cKO mice incubated with either FeCl3 (50 µM) for 20 hours alone or with hepcidin (1 µg/mL) for 6 hours. Ctrl BMDMs received neither FeCl3 nor hepcidin. β-actin levels were used for normalization. Each lane represents one individual animal. (D-E) Representative western blot and quantification (n = 5-7) of FPN1 protein levels in whole-spleen lysates (D) and serum iron levels (E) from JAK2Wt and JAK2cKO mice at baseline (ctrl) and after intraperitoneal administration of hepcidin (4 mg/kg) for 3 hours. β-actin levels were used for normalization. Each lane represents one individual animal (D). Analysis of variance with Dunnett’s correction for multiple comparisons vs iron (Fe)/Hep-stimulated cells was applied (A,C). Unpaired Student t test was applied for comparison in each group (D-E). Results are shown as means ± standard errors of the mean. ***P < .001, ****P < .0001.
To ascertain whether JAK2 truly plays a role in FPN1 degradation, we generated a myeloid-specific JAK2cKO mouse model by crossbreeding C57BL/6 mice harboring the LysMcre gene with Sv129 mice containing the LoxP-flanked JAK2 allele.26 BMDMs were generated from JAK2cKO mice, and JAK2 deletion was confirmed by western blot analysis (Figure 2B). To ensure complete functional JAK2 knockout in these cells, we also evaluated the effect of granulocyte macrophage colony-stimulating factor stimulation on STAT5 phosphorylation in JAK2Wt and JAK2cKO BMDMs. As expected, no pSTAT5 was detected in JAK2cKO BMDMs after cytokine stimulation (Figure 2B). Moreover, comparison of JAK2Wt and JAK2cKO tissue macrophages of different organs (spleen, bone marrow, liver, and peritoneum) by FACS analysis revealed neither quantitative nor qualitative differences (supplemental Figure 4A-C).
On the basis of these observations, we next wanted to evaluate the effect of JAK2cKO on hepcidin-mediated FPN1 internalization. BMDMs of both genotypes were stimulated with FeCl3 and then incubated with hepcidin. FeCl3 treatment resulted in increased FPN1 protein expression in both JAK2Wt and JAK2cKO BMDMs. More importantly, JAK2 deletion had no effect on the hepcidin-mediated degradation of FPN1 in vitro (Figure 2C). Because hepcidin and FPN1 regulation are influenced by an abundance of factors and because it is possible that JAK2 may play a role in FPN1 regulation on a systemic level, we sought to investigate whether JAK2 regulated FPN1 in vivo. JAK2Wt and JAK2cKO mice were injected intraperitoneally with hepcidin, and FPN1 expression in the spleen was analyzed by immunoblotting 3 hours later. In accordance with our in vitro experiments, we observed decreased FPN1 protein expression after hepcidin injection in both genotypes (Figure 2D). In addition to FPN1 protein expression in the spleen, we also studied serum iron concentrations after hepcidin injection. Again we observed diminished serum iron levels in both JAK2Wt and JAK2cKO mice (Figure 2E). Despite the experimental limitation of a cell-specific knockout model, the decrease of serum iron levels upon hepcidin injection in both genotypes further supports that JAK2 deficiency in myeloid cells has no effect on the mode of action of hepcidin.
Changes in serum iron and hepcidin are independent of MMB-mediated changes in IL-6
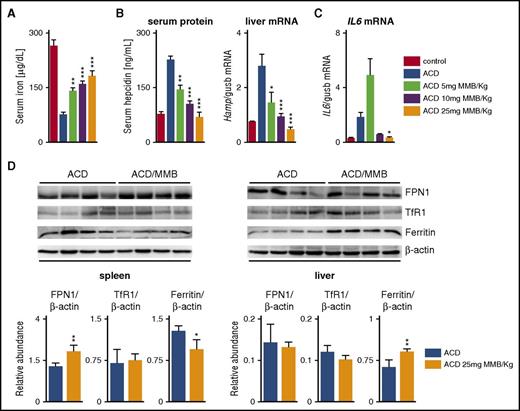
Because a direct effect of MMB on FPN1 degradation via JAK2 inhibition seemed unlikely, we investigated alternative mechanisms that might be responsible for amelioration of ACD by MMB. To study direct effects of MMB on iron homeostasis, we used ACD rats (2 weeks post–PG-APS) and healthy rats that were treated once daily with either MMB (5, 10, or 25 mg/kg) or vehicle alone for 3 days. In the case of ACD rats, this short-term MMB treatment resulted in a dose-dependent increase in serum iron (Figure 3A) that was accompanied by a dose-dependent decrease of serum hepcidin levels along with reduced liver Hamp mRNA expression (Figure 3B). Similar changes in serum hepcidin were also seen after 7-, 14-, and 21-day treatment with 25-mg/kg MMB (supplemental Figure 5).
MMB modulates hepcidin expression and iron metabolism gene expression in ACD in rats. (A-B) Serum iron, hepcidin serum protein, and liver Hamp messenger RNA (mRNA) levels in ACD rats after 3-day MMB (5, 10, or 25 mg/kg) treatment. (C) IL-6 mRNA levels in the spleen of ACD rats after 3-day MMB (5, 10, or 25 mg/kg) treatment. (D) Representative western blots and quantification (n = 4 per group) of FPN1, transferrin receptor 1 (TfR1), and ferritin in the spleens and livers of ACD rats after 3-day MMB (5, 10, or 25 mg/kg) treatment. β-actin levels were used for normalization. Each lane represents one individual animal. Analysis of variance with Dunnett’s correction for multiple comparisons vs ACD was applied (A-C). Unpaired Student t test was applied for comparison in each group (D). Results are shown as means ± standard errors of the mean. *P < .05, **P < .01, ***P < .001.
MMB modulates hepcidin expression and iron metabolism gene expression in ACD in rats. (A-B) Serum iron, hepcidin serum protein, and liver Hamp messenger RNA (mRNA) levels in ACD rats after 3-day MMB (5, 10, or 25 mg/kg) treatment. (C) IL-6 mRNA levels in the spleen of ACD rats after 3-day MMB (5, 10, or 25 mg/kg) treatment. (D) Representative western blots and quantification (n = 4 per group) of FPN1, transferrin receptor 1 (TfR1), and ferritin in the spleens and livers of ACD rats after 3-day MMB (5, 10, or 25 mg/kg) treatment. β-actin levels were used for normalization. Each lane represents one individual animal. Analysis of variance with Dunnett’s correction for multiple comparisons vs ACD was applied (A-C). Unpaired Student t test was applied for comparison in each group (D). Results are shown as means ± standard errors of the mean. *P < .05, **P < .01, ***P < .001.
Of note, MMB treatment modulated splenic IL-6 cytokine mRNA levels (especially at 25-mg/kg MMB; Figure 3C). However, this did not parallel the changes in serum hepcidin and hepatic Hamp mRNA levels that were observed at all MMB dose levels. Thus, although IL-6 has been demonstrated to positively regulate hepcidin induction,27 our results provide circumstantial evidence that this mechanism is not driving the MMB-mediated effects on hepcidin expression.
In accordance with higher serum iron availability, ferritin protein levels, as a marker of cellular iron retention, decreased in the spleen of ACD rats treated with 25-mg/kg MMB, and this was accompanied by an increase in FPN1 expression (Figure 3D). In contrast, healthy rats showed no changes in serum iron levels, Hamp mRNA expression in the liver, or FPN1 protein levels in the spleen at any MMB dose (supplemental Figure 3C-E).
MMB inhibits BMP-induced pSMAD1/5/8 signaling and hepcidin expression
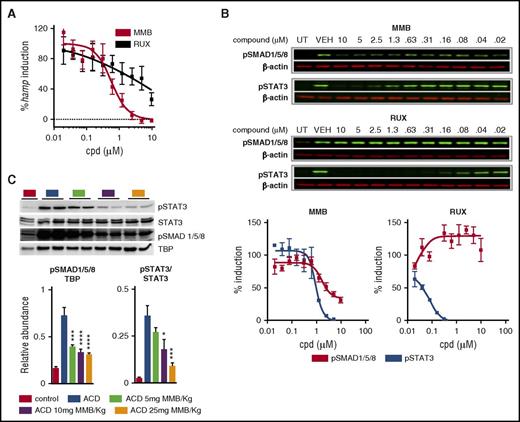
BMPs play a central role in mediating hepcidin transcriptional induction in hepatocytes by activating the BMPR-SMAD1/5/8 signaling pathway.9-12,25 Thus, we investigated the possibility that the effects of MMB on serum iron and hepcidin levels were driven through JAK-independent inhibition of BMPR-SMAD signaling. We performed hepcidin gene expression analysis on HepG2 cells (a hepatoma cell line) stimulated with 10 ng/mL of the BMP ligand BMP6 for 6 hours in the presence or absence of MMB. MMB treatment resulted in a dose-dependent reduction in Hamp mRNA levels, with a 50% effective concentration of 0.65 ± 0.2 µM (n = 3 independent experiments), whereas the JAK1/2 inhibitor RUX only suppressed hepcidin induction at high concentrations (50% effective concentration of >10 µM; Figure 4A). BMP ligand binding facilitates the association of the constitutively active type II BMPR kinases with type I BMPR kinase, resulting in phosphorylation and activation of type I BMPR kinase, initiation of downstream activation of effector SMADs (SMAD1/5/8), and their nuclear translocation in association with SMAD4.28 We thus looked at the ability of MMB to inhibit BMP6-induced phosphorylation of SMAD1/5/8 in HepG2 cells. IL-6–induced phosphorylation of STAT3 in HepG2 cells was included as a control for JAK pathway inhibition. MMB inhibited both IL-6–pSTAT3 and BMP6-pSMAD1/5/8, whereas RUX blocked IL-6–pSTAT3 but not BMP6-pSMAD1/5/8 signaling (Figure 4B). We extended these findings in vivo and demonstrated that MMB treatment for 3 days resulted in a dose-dependent reduction in both pSTAT3 and pSMAD1/5/8 in the livers of ACD rats (Figure 4C). Interestingly, pSTAT3 as well as pSMAD1/5/8 protein levels were not affected in healthy rats receiving MMB (5, 10, or 25 mg/kg) treatment (supplemental Figure 3F).
MMB inhibits SMAD1/5/8 phosphorylation and hepcidin expression in vitro and in vivo. (A) Quantitative reverse transcription polymerase chain reaction analysis of Hamp mRNA levels in HepG2 cells stimulated with 10-ng/mL BMP6 for 6 hours. Results are expressed as percentages of hepcidin induction normalized to vehicle-treated cells (50% effective concentration: MMB = 0.65 µM; RUX > 10µM; n = 3). (B) Representative western blots of pSMAD1/5/8 and pSTAT3 levels after 30-minute stimulation with 10-ng/mL BMP6 (for pSMAD1/5/8 induction) or 20-minute stimulation with 10-ng/mL IL-6 (for pSTAT3 induction; top and middle). β-actin was used as a loading control. Compound (cpd) was always added 2 hours before BMP6 stimulation. Percentages of pSMAD1/5/8/pSTAT3 induction (normalized to vehicle control-treated cells; n = 5 for pSMAD1/5/8; n = 2 for pSTAT3) ± standard errors of the mean (bottom). (C) Representative western blot and quantification (n = 5 per group) of hepatic nuclear extracts for pSTAT3 and pSMAD1/5/8 from ACD rats treated for 3 days with MMB (5, 10, or 25 mg/kg). TATA-binding protein (TBP) and STAT3 levels were used for normalization. Each lane represents one individual animal. Analysis of variance with Dunnett’s correction for multiple comparisons vs ACD was applied (C). Results are shown as means ± standard errors of the mean. *P < .05, ***P < .001, ****P < .001.
MMB inhibits SMAD1/5/8 phosphorylation and hepcidin expression in vitro and in vivo. (A) Quantitative reverse transcription polymerase chain reaction analysis of Hamp mRNA levels in HepG2 cells stimulated with 10-ng/mL BMP6 for 6 hours. Results are expressed as percentages of hepcidin induction normalized to vehicle-treated cells (50% effective concentration: MMB = 0.65 µM; RUX > 10µM; n = 3). (B) Representative western blots of pSMAD1/5/8 and pSTAT3 levels after 30-minute stimulation with 10-ng/mL BMP6 (for pSMAD1/5/8 induction) or 20-minute stimulation with 10-ng/mL IL-6 (for pSTAT3 induction; top and middle). β-actin was used as a loading control. Compound (cpd) was always added 2 hours before BMP6 stimulation. Percentages of pSMAD1/5/8/pSTAT3 induction (normalized to vehicle control-treated cells; n = 5 for pSMAD1/5/8; n = 2 for pSTAT3) ± standard errors of the mean (bottom). (C) Representative western blot and quantification (n = 5 per group) of hepatic nuclear extracts for pSTAT3 and pSMAD1/5/8 from ACD rats treated for 3 days with MMB (5, 10, or 25 mg/kg). TATA-binding protein (TBP) and STAT3 levels were used for normalization. Each lane represents one individual animal. Analysis of variance with Dunnett’s correction for multiple comparisons vs ACD was applied (C). Results are shown as means ± standard errors of the mean. *P < .05, ***P < .001, ****P < .001.
In view of the effects of MMB on hepcidin levels and SMAD1/5/8 signaling in vivo, we also investigated possible changes in the activin B pathway by assessing the expression of Inhibin BB mRNA, which codes for the activin βB subunit of activin B, in the livers of rats treated with MMB (5, 10, or 25 mg/kg). Activin B, belonging to the TGF-β protein superfamily, has been reported to activate the SMAD1/5/8 pathway and induce Hamp expression, at least in vitro.29 Although Inhibin BB mRNA levels in the liver of ACD rats decreased with MMB treatment for 3 days, levels remained above baseline and did not show a clear dose-dependent correlation compared with the effects of MMB on hepcidin levels (supplemental Figure 6). These results are in accordance with data recently published by Besson-Fournier et al,30 which demonstrated that hepcidin regulation by inflammation is independent of inhibin BB–dependent changes in SMAD1/5/8 signaling.
MMB is a JAK1/2 and ACVR1/ALK2 inhibitor
To further explore the mechanism by which MMB inhibited pSMAD1/5/8 signaling and hepcidin production both in vitro and in vivo, we screened MMB for activity against type I BMPR kinases (ACVR1/ALK2 and BMPR1a/ALK3) in biochemical competitive binding assays. MMB effectively inhibited ACVR1/ALK2 with an IC50 of 8.4 nM but was >12-fold weaker on BMPR1a/ALK3, and the TGF-β receptor TGFBR1/ALK5 (Table 1). In contrast, RUX showed no relevant inhibitory activity in these assays. JAK1, JAK2, JAK3, and TYK2 IC50 values for both MMB and RUX are also included in Table 1.
MMB inhibits BMPR kinase ACVR1/ALK2
| Kinase . | MMB IC50 (nM) . | RUX IC50 (nM) . |
|---|---|---|
| JAK1 | 26.9 | 1.2 |
| JAK2 | 1.4 | <0.3 |
| JAK3 | 6.1 | 3.1 |
| TYK2 | 19.9 | 8.5 |
| ACVR1 (ALK2) | 8.4 | 6100 |
| BMPR1a (ALK3) | 405 | >10K |
| TGFBR1 (ALK5) | 205 | 6907 |
| Kinase . | MMB IC50 (nM) . | RUX IC50 (nM) . |
|---|---|---|
| JAK1 | 26.9 | 1.2 |
| JAK2 | 1.4 | <0.3 |
| JAK3 | 6.1 | 3.1 |
| TYK2 | 19.9 | 8.5 |
| ACVR1 (ALK2) | 8.4 | 6100 |
| BMPR1a (ALK3) | 405 | >10K |
| TGFBR1 (ALK5) | 205 | 6907 |
JAK1, JAK2, JAK3, and TYK2 IC50s for MMB and RUX were assessed in a commercial kinase potency screen performed by Carna Biosciences. ACVR1, BMPR1a, and TGFBR1 IC50s for MMB and RUX were generated at SelectScreen Biochemical Kinase Profiling Service (Thermo Fisher Scientific) using a 10-point dose titration of compound.
Discussion
MMB, a JAK1/JAK2 inhibitor, has attracted interest because it has been reported that a significant number of patients with MF treated with MMB in a phase 2 trial became transfusion independent. This anemia benefit seen with MMB was an unexpected outcome for a JAK1/2 inhibitor, because erythropoietin-mediated JAK2 signaling is essential for erythropoiesis and because new-onset anemia is a major adverse event associated with RUX treatment.19-22
JAK2 has been reported to play an important role in hepcidin-dependent FPN1 degradation, a mechanism by which JAK2 activity may contribute to the development of ACD by mediating iron restriction within the reticuloendothelial system and functional iron-deficient anemia.23 Interestingly, although not fully understood, anemia in patients with MF shows typical signs of ACD.16,17,31 Therefore, we speculated whether MMB exerts its positive anemia effects via a JAK2-dependent pathway on FPN1 degradation and whether MMB may also ameliorate anemia in an inflammation-driven rat model of ACD.
Here we report that although MMB was effective in ameliorating anemia in ACD rats, we did not find any mechanistic link to a JAK2-dependent effect on FPN1 degradation. Using a myeloid-specific conditional knockout of JAK2, we were unable to confirm the data published by De Domenico et al.23 The systematic experiments presented here clearly show that JAK2 is not required for hepcidin-induced internalization of FPN1. Although cell-specific knockout models have limitations, it is worth noting that Ross et al24 have also demonstrated that hepcidin-mediated FPN1 internalization does not require JAK2 activity. Instead, they showed that this is dependent on lysine residues within the protein that may be targets of ubiquitination.24
Anemia in patients with MF has been reported to be driven, at least in part, by elevated hepcidin levels. Moreover, increased hepcidin levels in patients with MF have been shown to be associated with inferior overall survival.17 We demonstrated that MMB significantly increases Hb values and strongly reduces serum and liver Hamp mRNA expression levels in our rat ACD model, an observation that served as a starting point to understand the mechanism of action driving the anemia benefit observed in patients treated with MMB. MMB-mediated reduction of hepcidin allows the demand of iron for erythropoiesis to be met as iron becomes sufficiently exported. Accordingly, MMB treatment also resulted in an increase in both mean corpuscular volume and MCH, 2 RBC parameters that change as a function of iron availability. In fact, clinical studies showed that only 3% of patients treated with MMB experienced grade 3 or 4 anemia, in contrast with 45.2% of patients treated with RUX, currently the only JAK2 inhibitor approved by the FDA for therapy in myeloproliferative neoplasms.18,20
It could be speculated that the anemia benefit of MMB is a result of the known anti-inflammatory effects of MMB.32 IL-6 is a driver of hepcidin transcription in inflammation via the JAK1/JAK2-STAT3 pathway, because the hepcidin promoter has a pSTAT3 binding side. However, this mechanism is unlikely, because MMB and RUX have similar intra-JAK selectivity profiles, and both cause similar reduction of serum levels of inflammatory cytokines in treated patients.18,19 We also did not see a direct link between reduced IL-6 cytokine expression and hepcidin regulation in MMB-treated ACD rats. In addition, considering the potential of activin B to induce hepcidin levels, the relation between activin B expression and MMB treatment was investigated. Our analysis did not show any clear correlation between changes in Inhibin BB mRNA expression, SMAD1/5/8 phosphorylation, and hepcidin expression. Of note, while this report was in preparation, Besson-Fournier et al30 published data showing that hepcidin upregulation seen in inflammatory models is independent of differences in SMAD1/5/8 levels between animals deficient in activin B βB subunit and Wt mice.
In conclusion, both the IL-6/JAK/STAT and BMP/BMPR/SMAD pathways stimulate hepcidin expression; however, a basal BMP/SMAD signal is needed for transcriptional induction of hepcidin by IL-6.9 Therefore, we hypothesized that MMB—and not RUX—might somehow mediate an effect on this signaling pathway. We demonstrated that MMB is a potent ACVR1 inhibitor able to inhibit both BMP6-mediated pSMAD1/5/8 signaling and hepcidin production in HepG2 cells. We further demonstrated that this in vitro activity is applicable to our in vivo ACD model, because MMB treatment also resulted in decreased pSMAD1/5/8 signaling in the liver of ACD rats. Our results provide evidence of a direct role of MMB in regulating iron homeostasis and suggest a therapeutic rationale for MMB treatment in patients with ACD.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by a scientific grant from Gilead Sciences, Inc. (Foster City, CA). Editorial support was provided by AlphaBioCom, LLC, and funded by Gilead Sciences, Inc. This work was further supported by the Austrian Science Fund (FWF) project P 28302-B30 (I.T.) and by the FWF doctoral program HOROS-W1253 (G.W. and V.P.).
Authorship
Contribution: M.A., V.P., and M.R.W. conceived the project, designed and performed experiments, analyzed and interpreted data, and wrote the manuscript; D.H., P.T., E.D., M.S., W.P., M.N., P.M., and P.F. performed experiments; C.J.B., G.S., and K.-U.W. provided materials and intellectual input; G.W. and J.A.W. provided intellectual input and edited the manuscript; I.T. conceived the project, designed experiments, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: M.R.W., P.M., P.F., C.J.B., G.S., and J.A.W. are employees of Gilead Sciences, Inc. The remaining authors have no conflicts of interest to declare.
Correspondence: Igor Theurl, Department of Internal Medicine VI (Infectious Diseases, Immunology, Rheumatology and Pneumology), Medical University of Innsbruck, Anichstr 32, 6020 Innsbruck, Austria; e-mail: igor.theurl@i-med.ac.at.
References
Author notes
M.A., V.P., and M.R.W. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal