Key Points
The monotypic and polytypic PC compartments assessed by MFC are prognostic in AL amyloidosis.
MFC may play a role in the definition of hematologic response to treatment.
Abstract
Multiparametric flow cytometry (MFC) in amyloid light-chain (AL) amyloidosis has not been widely adopted and, consequently, there is little information on its clinical relevance. We studied 173 patients with AL amyloidosis who underwent MFC immunophenotyping of bone marrow sample at diagnosis and 82 patients at the end of the first line of treatment (EOT). The number of monotypic plasma cells (PCs) and the polytypic PCs/bone marrow PCs (pPCs/BMPCs) ratio were analyzed. At diagnosis, ≥2.5% monotypic PCs was associated with a shorter progression-free survival (PFS) and overall survival (OS) compared with patients with <2.5% monotypic PCs (2-year PFS 41% vs 56%, P = .007; 2-year OS 55% vs 70%; P = .01). Additionally, patients with a pPCs/BMPCs ratio of ≤5% had a shorter PFS compared with patients with pPCs/BMPCs ratio >5% (2-year PFS 43% vs 55%; P = .02), but without OS difference (2-year OS 60% vs 67%; P = .19). In a multivariate analysis, the monotypic PCs retained an independent prediction for PFS/OS, whereas the pPCs/BMPCs ratio retained significance only for PFS. At EOT, ≥0.1% monotypic PCs was associated with a shorter PFS and OS compared with patients with <0.1% monotypic PCs (2-year PFS 31% vs 87%; P < .0001; 2-year OS 87% vs 98%, P = .02). In a subgroup analysis among patients who attained a very good partial response or better, the monotypic PCs at the 0.1% cutoff was predictive for progression rate but not for PFS/OS. MFC is prognostic for AL amyloidosis at diagnosis and at EOT. MFC may have a role in the definition of hematologic response.
Introduction
Multiparameter flow cytometry (MFC) has been used for diagnosis and disease monitoring in several hematologic malignancies.1 Underestimation of the plasma cell (PC) compartment by flow cytometry, caused by hemodilution and/or lack of lipid-enriched spicules in the liquid bone marrow sample,2,3 has led to its underutilization in PC disorders. In multiple myeloma, its use has focused on the assessment of minimal residual disease. A lower level of clonal plasma cells detected by MFC correlates with longer survival.4 Studies on MFC utilization in AL amyloidosis are limited,5-11 with only a few addressing the clinical relevance of MFC in this rare disorder.11,12 Paiva et al reported MFC results in 35 patients with AL amyloidosis and found that the presence of >1% monotypic PCs in the bone marrow was associated with a shorter survival.12 Additionally, <5% polytypic PCs (pPCs) of bone marrow PCs (pPCs/BMPCs) was also associated with a shorter survival. We assessed the prognostic implications of MFC in a large cohort of patients with AL amyloidosis, both at diagnosis and at the end of first-line treatment (EOT).
Methods
All patients with biopsy-confirmed AL amyloidosis, who underwent MFC immunophenotyping of a bone marrow sample between May 2012 and January 2016, were included. Relevant clinical data were extracted from a prospectively maintained database. The Mayo Foundation Institutional Review Board (IRB) approved the study. All patients gave written informed consent to have their medical records reviewed according to IRB requirements and federal regulations.
MFC immunophenotyping was assessed before initiation of therapy in 173 consecutive patients and at EOT in 82 patients. Of the 82 EOT studies, 78% were analyzed both at diagnosis and EOT, whereas the remaining 22% were analyzed only at EOT.
Erythrocyte-lysed BM aspirate samples were examined using a single 7-color tube with the following antibodies: CD138-PerCPCy5, CD38-APC-5, CD19-PE-7, and CD45-APC-H7. After cell fixation and permeabilization, κ-FITC and λ-PE cytoplasmic reagents were introduced followed by commercial RNAse reagent and fluorescent 4′,6′-diamidino-2-phenylindole (DAPI) stain. Data were obtained on surface immunophenotype, cytoplasmic immunoglobulin light-chain restriction, and DNA content and S phase through analysis of DAPI staining. The distinction between monotypic and polytypic PCs was established by differential expression of CD138, CD38, CD19, CD45, and DAPI (in cases of hyper- or hypodiploidy) and light-chain restriction (κ: λ expression ratio of either >4:1 [κ] or <1:2 [λ]) on gated plasma cells (examples of specimen analysis are provided in supplemental Figure 1 [pretreatment sample] and supplemental Figure 2 [EOT sample], available on the Blood Web site). A total of 500 000 live cellular events were set as a target per exam (median gated events achieved 488 379, 25%-75% IQR 476 115-492 653). The percentage of monotypic and polytypic plasma cells of all events was calculated as well as the ratio of pPCs/BMPCs, where BMPCs is the sum of monotypic and polytypic PCs (eg, a patient with 0.1% polytypic PCs and 2.3% monotypic PCs will have a ratio of 0.1/[2.3+0.1] = 4.2%). The cell surface–conjugated antibodies and the DAPI stain were purchased from BD Biosciences (San Jose, CA) and the κ/λ reagents from DAKO (Carpinteria, CA). The samples were analyzed using a BD FacsCanto II instrument (Becton Dickinson, Franklin Lakes, NJ). The data were analyzed using Kaluza software (Beckman Coulter).
The level of BMPCs by morphology was considered the highest estimate from the aspirate or the biopsy. The 2004 Mayo staging system13 and the 2012 revised Mayo staging system14 were used to stratify patients into risk categories.
The Pearson χ2 test and the Kruskal-Wallis test were used to ascertain differences between nominal and continuous variables, respectively. The thresholds used for response and survival analysis were based on the median values. Hematologic response to treatment, progression-free survival (PFS), and overall survival (OS) were evaluated in accordance with consensus criteria.15 For PFS calculation, progression was defined as hematologic and/or organ progression (of 47 progressions in the diagnosis cohort, 40 were hematologic and 7 were organ progression). For response at EOT, we used the response achieved when EOT MFC was performed. For survival analysis in the EOT cohort, day 0 was set as the day of EOT MFC examination.
Survival analysis was performed using the Kaplan-Meier method, with the log-rank test used to compare groups. Multivariate analysis was conducted using the Cox proportional hazards regression model to determine independent predictors for PFS and OS. For construction of the multivariate model, we used variables with P < .05 on the univariate analysis. As the monotypic and the polytypic PC compartments were intercorrelated, we used each compartment separately in a multivariate model, if applicable. P < .05 was considered significant. All statistical analyses were performed on JMP software (SAS, Cary, NC).
Results
The baseline characteristics of patients at diagnosis (n = 173) and at EOT (n = 82) are listed in Table 1. Treatment distribution can be viewed in Table 1. ASCT was administrated to 38% of the newly diagnosed patients and to 84% of patients at EOT. The median follow-up from diagnosis was 18 months (range 0.1-52) for the newly diagnosed patients and 32 months (range 8-55) for patients with EOT MFC.
Baseline characteristics at presentation for newly diagnosed patients and for patients at EOT
| . | Before treatment initiation (n = 173) . | End of first-line treatment (n = 82) . |
|---|---|---|
| Age in years, median (range) | 64 (40-88) | 61 (43-77) |
| Male sex, n (%) | 112 (65) | 56 (68) |
| No. of involved organs | ||
| Median (range) | 2 (1-4) | 2 (1-4) |
| Cardiac involvement | 128 (74%) | 42 (51%) |
| Renal involvement | 90 (52%) | 51 (62%) |
| Peripheral/autonomic neuropathy | 48 (28%) | 17 (26%) |
| Liver involvement | 29 (17%) | 10 (12%) |
| GI involvement | 19 (11%) | 6 (9%) |
| λ restriction, n (%) | 125 (72) | 55 (67) |
| dFLC (mg/dL), median (range) | 24.8 (0.2-1498) | 20.9 (0.7-1498) |
| Monotypic PCs percentage, median (range) | ||
| By MFC | 2.3 (0-41.8) | 2 (0-41.8) |
| By aspiration/biopsy | 10 (3-80) | 10 (2-80) |
| 2004 Mayo stage, n (%) | ||
| I | 39 (22) | 37 (45) |
| II | 67 (39) | 32 (39) |
| III | 67 (39) | 13 (16) |
| 2012 Mayo stage, n (%) | N = 172 | |
| I | 43 (25) | 34 (42) |
| II | 40 (23) | 27 (33) |
| III | 38 (22) | 6 (7) |
| IV | 51 (30) | 15 (18) |
| FISH abnormalities, n (%)* | N = 147 | N = 70 |
| t (11;14) | 83 (56) | 35 (50) |
| del (13q) | 60 (41) | 28 (40) |
| Any trisomy(ies) | 46 (31) | 17 (24) |
| First-line treatment, n (%) | ||
| ASCT | 65 (38) | 69 (84) |
| Bortezomib-based | 74 (43) | 10 (12) |
| Melphalan-based | 18 (10) | 3 (4) |
| IMiD-based | 2 (1) | — |
| No treatment | 8 (5) | — |
| Unknown | 6 (3) | — |
| . | Before treatment initiation (n = 173) . | End of first-line treatment (n = 82) . |
|---|---|---|
| Age in years, median (range) | 64 (40-88) | 61 (43-77) |
| Male sex, n (%) | 112 (65) | 56 (68) |
| No. of involved organs | ||
| Median (range) | 2 (1-4) | 2 (1-4) |
| Cardiac involvement | 128 (74%) | 42 (51%) |
| Renal involvement | 90 (52%) | 51 (62%) |
| Peripheral/autonomic neuropathy | 48 (28%) | 17 (26%) |
| Liver involvement | 29 (17%) | 10 (12%) |
| GI involvement | 19 (11%) | 6 (9%) |
| λ restriction, n (%) | 125 (72) | 55 (67) |
| dFLC (mg/dL), median (range) | 24.8 (0.2-1498) | 20.9 (0.7-1498) |
| Monotypic PCs percentage, median (range) | ||
| By MFC | 2.3 (0-41.8) | 2 (0-41.8) |
| By aspiration/biopsy | 10 (3-80) | 10 (2-80) |
| 2004 Mayo stage, n (%) | ||
| I | 39 (22) | 37 (45) |
| II | 67 (39) | 32 (39) |
| III | 67 (39) | 13 (16) |
| 2012 Mayo stage, n (%) | N = 172 | |
| I | 43 (25) | 34 (42) |
| II | 40 (23) | 27 (33) |
| III | 38 (22) | 6 (7) |
| IV | 51 (30) | 15 (18) |
| FISH abnormalities, n (%)* | N = 147 | N = 70 |
| t (11;14) | 83 (56) | 35 (50) |
| del (13q) | 60 (41) | 28 (40) |
| Any trisomy(ies) | 46 (31) | 17 (24) |
| First-line treatment, n (%) | ||
| ASCT | 65 (38) | 69 (84) |
| Bortezomib-based | 74 (43) | 10 (12) |
| Melphalan-based | 18 (10) | 3 (4) |
| IMiD-based | 2 (1) | — |
| No treatment | 8 (5) | — |
| Unknown | 6 (3) | — |
ASCT, autologous stem cell transplantation; dFLC, difference between involved and uninvolved light chains; FISH, fluorescence in situ hybridization; GI, gastrointestinal; IMiDs, immunomodulatory drugs.
Patients may have more than one abnormality.
Plasma cells characteristics by MFC at diagnosis
The median percentage of monotypic plasma cells by MFC was 2.3% (range 0%-41.8%), whereas the median percentage of monotypic PCs in the S-phase was 0.3% (range 0%-2.6%). A correlation (r2 = 0.48, P < .001) was seen between the percentage of monotypic plasma cells by MFC and their percentage by morphology in bone marrow aspirate and/or biopsy (highest value) (supplemental Figure 3).
The DNA ploidy index was available for 160 patients. Seventy-five percent were diploid, 17% hyperdiploid, 6% hypodiploid, and 2% tetraploid. Ploidy status by MFC was correlated with fluorescence in situ hybridization abnormalities performed on the same sample. Compared with patients with a hyperdiploid DNA, patients with a diploid or hypodiploid DNA content were more likely to harbor t(11;14) (4% vs 73% vs 67%; P < .001). As expected, trisomies were more commonly seen in patients with hyperdiploid DNA assessed by MFC (93%) compared with diploid (15%) or hypodiploid DNA (11%; P < .001).
The median percentage of polytypic PCs was 0.1% (range 0.003%-0.91%) and the median pPCs/BMPCs ratio was 4.9% (range 0.07%-100%).
The fraction of patients by their monotypic PCs (using a 2.5% cutoff) and by the pPCs/BMPCs ratio (using a 5% cutoff) can be viewed in Table 2. The concordance between the 2 variables was 73%. Baseline characteristics of patients by the monotypic PCs and the pPCs/BMPCs ratio subgroups can be seen in supplemental Table 1.
Distribution and concordance between the monotypic PCs and the pPCs/BMPCs at diagnosis
| . | pPCs/BMPCs ratio ≤5% (n = 89) . | pPCs/BMPCs ratio >5% (n = 84) . | Total . |
|---|---|---|---|
| ≥2.5% monotypic PCs (n = 76) | 34% | 10% | 44% |
| <2.5 monotypic PCs (n = 97) | 17% | 39% | 56% |
| Total | 51% | 49% | 100% |
| . | pPCs/BMPCs ratio ≤5% (n = 89) . | pPCs/BMPCs ratio >5% (n = 84) . | Total . |
|---|---|---|---|
| ≥2.5% monotypic PCs (n = 76) | 34% | 10% | 44% |
| <2.5 monotypic PCs (n = 97) | 17% | 39% | 56% |
| Total | 51% | 49% | 100% |
Response and survival by MFC parameters in newly diagnosed AL amyloidosis
Response data were available for 73% of patients (n = 127, evaluated only for patients who were treated and response evaluation was available). Patients with ≥2.5% monotypic PCs (n = 52) had a trend toward a reduced likelihood of attaining greater than or equal to very good partial response (VGPR) to first-line treatment compared with patients with <2.5% monotypic PCs at diagnosis (n = 75) (60% vs 73%; P = .1). Patients with pPCs/BMPCs ratio ≤5% (n = 63) were less likely to achieve greater than or equal to VGPR to first-line treatment compared with those with pPCs/BMPCs ratio >5% at diagnosis (n = 64) (59% vs 77%; P = .03).
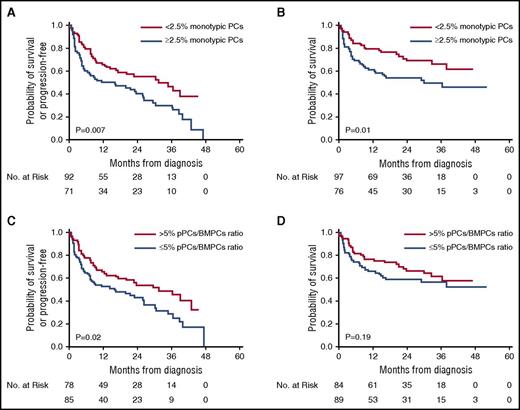
PFS was inferior in patients with ≥2.5% monotypic PCs compared with patients with monotypic PCs below this threshold (2-year PFS 41% vs 56%; P = .007) (Figure 1A), as well as shorter OS (2-year OS 55% vs 70%; P = .01) (Figure 1B). Similarly, patients with pPCs/BMPCs ratio ≤5% had a shorter PFS compared with patients with pPCs/BMPCs ratio >5% (2-year PFS 43% vs 55%; P = .02) (Figure 1C), but no OS difference was seen between the pPCs/BMPCs ratio subgroups (2-year OS 60% vs 67%; P = .19) (Figure 1D). In comparison, no difference was seen in 2-year PFS/OS by using the morphologic assessment of BMPCs between patients with <10% BMPCs to those with ≥10% BMPCs (2-year PFS 48% vs 50%, P = .9; 2-year OS 60% vs 65%; P = .9).
Survival curves from diagnosis using the Kaplan-Meier method for patients with MFC immunophenotyping at diagnosis. (A) PFS stratified by monotypic PCs cutoff at 2.5%. (B) Overall survival stratified by monotypic PCs cutoff at 2.5%. (C) PFS stratified by pPCs/BMPCs ratio cutoff at 5%. (D) Overall survival stratified by pPCs/BMPCs ratio cutoff at 5%.
Survival curves from diagnosis using the Kaplan-Meier method for patients with MFC immunophenotyping at diagnosis. (A) PFS stratified by monotypic PCs cutoff at 2.5%. (B) Overall survival stratified by monotypic PCs cutoff at 2.5%. (C) PFS stratified by pPCs/BMPCs ratio cutoff at 5%. (D) Overall survival stratified by pPCs/BMPCs ratio cutoff at 5%.
Survival analysis was stratified by ASCT intervention. In patients who did not undergo ASCT (n = 108), PFS and OS were significantly shorter in patients with ≥2.5% monotypic PCs compared with those with monotypic PCs below this threshold (2-year PFS 27% vs 43%, P = .02; 2-year OS 36% vs 53% months; P = .01). Similarly, in patients who did not undergo ASCT, PFS and OS were significantly shorter for patients with pPCs/BMPCs ratio ≤5% compared with those with a ratio >5% (2-year PFS 30% vs 40%, P = .02; 2-year OS 37% vs 51%; P = .04). However, in patients who underwent ASCT (n = 65), no difference was seen for PFS and OS when assessing monotypic PCs at a 2.5% cutoff (2-year PFS 66% vs 72%, P = .4; 2-year OS 92% vs 92%, P = .98) or pPCs/BMPCs ratio at a 5% cutoff (2-year PFS 66% vs 73%, P = .28; 2-year OS 91% vs 93%, P = .82), suggests the ability of intensive therapy to neutralize the adverse impact of MFC monotypic PC and/or polytypic PC compartments.
In multivariate models for PFS and OS, monotypic PCs at 2.5% cutoff retained independent impact on PFS and OS, whereas the pPCs/BMPCs did so only for PFS (Table 3).
Univariate and multivariate Cox proportional hazard regression analysis for PFS and OS
| . | Univariate analysis . | Multivariate analysis . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictors for PFS . | . | Model 1 . | Model 2 . | ||||||
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Age ≥65 | 1.4 | 0.9-2.1 | .09 | ||||||
| No. of involved organ >2 | 2 | 1.2-3.2 | .005 | 1.8 | 1.1-3 | .02 | 1.6 | 0.99-2.6 | .02 |
| ≥2.5% monotypic PCs | 1.7 | 1.2-2.6 | .007 | 1.8 | 1.2-2.7 | .006 | Not included | ||
| pPC/BMPC ratio ≤5% | 1.6 | 1.1-2.5 | .02 | Not included | 1.6 | 1.1-2.5 | .01 | ||
| Mayo stage 2004 | |||||||||
| 1 | Reference | 1.1-4.1 | .01 | Reference | 0.9-3.2 | .13 | Reference | 0.8-3.1 | .18 |
| 2 | 2.1 | 2.1-7.1 | <.001 | 1.6 | 1.4-5.1 | .002 | 1.5 | 1.3-5 | .003 |
| 3 | 3.8 | 2.6 | 2.5 | ||||||
| dFLC ≥18 mg/dL | 1.4 | 0.9-2.1 | .15 | ||||||
| ASCT at first line | 0.4 | 0.3-0.6 | <.0001 | 0.6 | 0.3-0.9 | .01 | 0.5 | 0.3-0.9 | .01 |
| . | Univariate analysis . | Multivariate analysis . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictors for PFS . | . | Model 1 . | Model 2 . | ||||||
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Age ≥65 | 1.4 | 0.9-2.1 | .09 | ||||||
| No. of involved organ >2 | 2 | 1.2-3.2 | .005 | 1.8 | 1.1-3 | .02 | 1.6 | 0.99-2.6 | .02 |
| ≥2.5% monotypic PCs | 1.7 | 1.2-2.6 | .007 | 1.8 | 1.2-2.7 | .006 | Not included | ||
| pPC/BMPC ratio ≤5% | 1.6 | 1.1-2.5 | .02 | Not included | 1.6 | 1.1-2.5 | .01 | ||
| Mayo stage 2004 | |||||||||
| 1 | Reference | 1.1-4.1 | .01 | Reference | 0.9-3.2 | .13 | Reference | 0.8-3.1 | .18 |
| 2 | 2.1 | 2.1-7.1 | <.001 | 1.6 | 1.4-5.1 | .002 | 1.5 | 1.3-5 | .003 |
| 3 | 3.8 | 2.6 | 2.5 | ||||||
| dFLC ≥18 mg/dL | 1.4 | 0.9-2.1 | .15 | ||||||
| ASCT at first line | 0.4 | 0.3-0.6 | <.0001 | 0.6 | 0.3-0.9 | .01 | 0.5 | 0.3-0.9 | .01 |
| Predictors for OS . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | . | . | . |
|---|---|---|---|---|---|---|---|---|---|
| Age ≥65 | 1.8 | 1.1-3.1 | .01 | 1.4 | 0.8-2.4 | .19 | |||
| No. of involved organ > 2 | 2.1 | 1.2-3.5 | .008 | 1.7 | 1-3 | .05 | |||
| ≥2.5% monotypic PCs | 1.9 | 1.2-3.1 | .01 | 1.9 | 1.1-3.1 | .01 | |||
| pPC/BMPC ratio ≤5% | 1.4 | 0.8-2.3 | .19 | ||||||
| Mayo stage 2004 | |||||||||
| 1 | Reference | 1.6-4.8 | .002 | Reference | 1-12.6 | .05 | |||
| 2 | 2.8 | 4.8-55 | <.0001 | 2.9 | 2.3-28 | .0001 | |||
| 3 | 13.3 | 6.6 | |||||||
| dFLC ≥18 mg/dL | 1.5 | 0.9-2.6 | .11 | ||||||
| ASCT at first line | 0.1 | 0.05-0.3 | <.0001 | 0.3 | 0.1-0.6 | .0005 |
| Predictors for OS . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | . | . | . |
|---|---|---|---|---|---|---|---|---|---|
| Age ≥65 | 1.8 | 1.1-3.1 | .01 | 1.4 | 0.8-2.4 | .19 | |||
| No. of involved organ > 2 | 2.1 | 1.2-3.5 | .008 | 1.7 | 1-3 | .05 | |||
| ≥2.5% monotypic PCs | 1.9 | 1.2-3.1 | .01 | 1.9 | 1.1-3.1 | .01 | |||
| pPC/BMPC ratio ≤5% | 1.4 | 0.8-2.3 | .19 | ||||||
| Mayo stage 2004 | |||||||||
| 1 | Reference | 1.6-4.8 | .002 | Reference | 1-12.6 | .05 | |||
| 2 | 2.8 | 4.8-55 | <.0001 | 2.9 | 2.3-28 | .0001 | |||
| 3 | 13.3 | 6.6 | |||||||
| dFLC ≥18 mg/dL | 1.5 | 0.9-2.6 | .11 | ||||||
| ASCT at first line | 0.1 | 0.05-0.3 | <.0001 | 0.3 | 0.1-0.6 | .0005 |
CI, confidence interval; HR, hazard ratio.
MFC immunophenotyping at the end of first-line treatment
Of the 82 examinations performed at the end of first-line treatment, 69 (84%) were performed after ASCT and 13 (16%) were performed at the end of standard induction treatment. The median time from diagnosis to EOT MFC study was 6 months (range 3.5-18), longer in patients who received standard induction compared with ASCT (median 11 vs 6 months, P = .002).
The median percentage of monotypic PCs at EOT was 0.1% (range 0%-6.4%) and the median monotypic PCs S-phase was 0.6% (range 0%-5.2%). In comparison, the median enumeration of plasma cells by aspiration and/or bone marrow biopsy was 1% (range 0%-10%). The median percentage of polytypic PCs at EOT was 0.09% (range 0.002%-0.74%) and the median pPCs/BMPCs ratio was 53%. This reflects the immediate efficacy of the antiplasma cell therapy on clonal plasma cells, but not on the polytypic PCs. The fraction of patients by their monotypic PCs (using a 0.1% cutoff) and by the pPCs/BMPCs ratio (using a 50% cutoff) can be viewed in Table 4. The concordance between the 2 variables was 89%, reflecting the dominance of the monotypic PCs on the pPCs/BMPCs ratio at EOT. Therefore, to avoid duplication we evaluated only the monotypic PC compartment at EOT.
Distribution and concordance between the monotypic PCs and the pPCs/BMPCs at EOT
| . | pPCs/BMPCs ratio ≤50% (n = 38) . | pPCs/BMPCs ratio >50% (n = 44) . | Total . |
|---|---|---|---|
| ≥0.1% monotypic PCs (n = 39) | 41% | 6% | 47% |
| <0.1 monotypic PCs (n = 43) | 5% | 48% | 53% |
| Total | 46% | 54% | 100% |
| . | pPCs/BMPCs ratio ≤50% (n = 38) . | pPCs/BMPCs ratio >50% (n = 44) . | Total . |
|---|---|---|---|
| ≥0.1% monotypic PCs (n = 39) | 41% | 6% | 47% |
| <0.1 monotypic PCs (n = 43) | 5% | 48% | 53% |
| Total | 46% | 54% | 100% |
Correlation between MFC at diagnosis and at EOT
Data for both examinations were available for 64 of the patients (78% of patients with EOT MFC). Patients with ≥2.5% monotypic PCs at diagnosis were marginally less likely to achieve <0.1% monotypic PCs at EOT (41% vs 65%; P = .05) compared with patients with <2.5% monotypic PCs at diagnosis. However, there was no correlation between the polytypic PCs compartment at diagnosis and at EOT using the 5% and 50% cutoffs, respectively (P = .19). Also there was no association between pPCs/BMPCs ratio ≤5% at diagnosis and the EOT monotypic PCs at the 0.1% cutoff (P = .98).
Correlation between response and survival and the monotypic bone marrow plasma cells at EOT
Patients with ≥0.1% monotypic PCs (n = 39) were less likely to attain VGPR or better to first-line treatment compared with patients with a monotypic PCs percentage <0.1% (n = 43) (36% vs 95%; P < .0001).
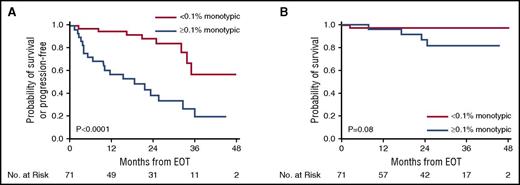
Patients with ≥0.1% monotypic PCs at EOT had a shorter PFS compared with patients with <0.1% monotypic PCs (2-year PFS 31% vs 87%, P < .0001), with a significant difference seen in OS (2-year OS 87% vs 98%, P = .02). When analysis was restricted to 71 patients without evidence for progression by standard criteria at EOT, PFS was shorter in patients with ≥0.1% monotypic PCs compared with patients with <0.1% monotypic PCs (2-year PFS 39% vs 89%, P < .0001), with a trend toward a shorter OS (2-year OS 87% vs 98%, P = .08) (Figure 2).
Survival curves from EOT for patients without evidence for progression at EOT. (A) PFS stratified by monotypic PCs at 0.1% cutoff. (B) Overall survival stratified by monotypic PCs at 0.1% cutoff.
Survival curves from EOT for patients without evidence for progression at EOT. (A) PFS stratified by monotypic PCs at 0.1% cutoff. (B) Overall survival stratified by monotypic PCs at 0.1% cutoff.
Additive predictive value of MFC in deep responders
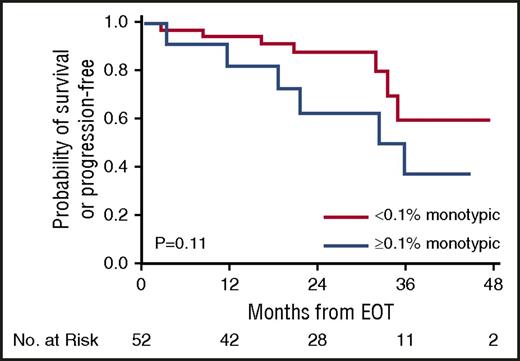
The 2-year PFS and OS of patients who attained greater than or equal to VGPR at EOT (n = 54) were 82% and 98%, respectively (VGPR 77% and 97%; CR 93% and 100%, respectively). We then assessed MFC at EOT of relapse and survival in those who attained greater than or equal to VGPR at EOT, stratifying the deep responders by the monotypic PCs at 0.1% cutoff. Patients who attained greater than or equal to VGPR but had ≥0.1% monotypic PCs by MFC had a higher rate of progression compared with those who achieved greater than or equal to VGPR and had <0.1% monotypic PCs by MFC (54% vs 17%, P=.01), a trend toward a shorter PFS (2-year PFS 63% vs 88%, respectively; P = .11) (Figure 3) but without an OS difference (2-year OS 100% vs 98%, respectively; P = .57).
PFS for patients at EOT who achieved at least very good partial response stratified by the monotypic PCs at 0.1% cutoff.
PFS for patients at EOT who achieved at least very good partial response stratified by the monotypic PCs at 0.1% cutoff.
Discussion
The routine use of flow cytometry in plasma cell proliferative disorders is limited. It is not surprising that studies focusing on flow cytometry in AL amyloidosis are few, given the rarity of the disease. This study describes the predictive value of MFC on response and survival in AL amyloidosis. We were able to demonstrate a correlation between the monotypic and the polytypic plasma cell compartments before therapy and the likelihood of achieving deep response (greater than or equal to VGPR) as well as their correlation with PFS and OS. After treatment, MFC immunophenotyping correlated with response depth by the traditional serologic criteria and survival, but also segregated patients with deep hematologic response (ie, greater than or equal to VGPR) into 2 groups with different risks of progression. Therefore, MFC at completion of therapy adds information on outcome that is not available by using the standard response criteria defined by serum biomarkers alone. This finding emphasizes an important role of MFC as a sensitive marker of response and should be validated by other groups.
Paiva et al reported a survival predictive value at diagnosis for the monotypic PCs at a lower cutoff (1%) compared with ours (2.5%). That study reported also a lower enumeration of bone marrow plasma cells by morphology (median 4% compared with 10% in our cohort). This difference is a magnitude of 2.5, identical to the difference in magnitude between the MFC enumerations in the 2 studies. Therefore, this difference corresponds to more advanced disease in our patients, as reflected by our higher difference between involved and uninvolved light chains (24.8 mg/dL) compared with the Spanish study (15.9 mg/dL, given as the involved light chain). This difference may reflect a referral bias in our study population, but reports by others should clarify the best MFC parameters. In contrast to Paiva et al, we did not find that the pPCs/BMPCs ratio ≤5% at diagnosis correlates with OS, although we found it to correlate with PFS, and longer follow-up might result in a difference also in OS.
In patients who underwent ASCT, MFC at diagnosis was not correlated with survival, whereas in patients who were treated with conventional chemotherapy the survival discrimination based on MFC parameters was maintained. This difference between groups might represent the ability of ASCT to overcome poor prognosis features. Nevertheless, patients who underwent ASCT are characterized by more favorable disease, and this might be a confounder. Multivariate analysis, however, demonstrated independent PFS/OS prediction for the monotypic PCs compartment when ASCT was one of the variables included in this analysis. Because our results are novel, this requires confirmation from other groups.
At EOT, reduction of the monotypic PC compartment to <0.1% was associated with better outcomes. A similar observation has been made in patients with high-risk smoldering myeloma treated with lenalidomide-dexamethasone,16 which suggests that other factors besides tumor burden are responsible for long-term disease control. One potential factor that has been proposed is regulatory immunosurveillance.17 Immunosurveillance might be represented in the MFC by the pPCs/BMPCs ratio, although we cannot address this directly. Moreover, in the EOT, the pPCs/BMPCs ratio was mainly governed by the reduction in the monotypic PCs rather than a change in the polytypic PC compartment. This might be a result of the short interval from end of treatment to MFC exam, which prevented adequate time for immune reconstitution to take place. Therefore, MFC exams should also be performed remote from EOT, to allow more time for polyclonal PCs recovery after chemotherapy completion.
Our study also suggests that MFC might be of value for response evaluation. Specifically, patients who achieved VGPR or better by the standard response criteria were more likely to progress and had a trend toward a shorter PFS if they had ≥0.1% monotypic PCs in their bone marrow compared with those with a lower monotypic PC percentage. This difference may reflect an important difference in light-chain amyloidogenic production capacity. So although the level of the amyloidogenic light chain had been significantly reduced, the marrow tumor burden may remain elevated and serve as a source for relapse. This finding is of clinical importance and should be validated by other groups.
This study has several limitations. First, it is retrospective. The median follow-up of this study is <2 years and survival analysis may be immature, especially because many patients underwent ASCT, which is associated with a prolonged survival.18 In the EOT cohort, 84% of patients underwent ASCT with more favorable clinical features. This cohort is biased by the fact that bone marrow assessment is performed in our routine practice in those who undergo ASCT or are planned for collection of stem cells. Therefore, our EOT MFC data are selective and is limited primarily to the transplant/transplant-eligible setting. Finally, our current panel does not include additional antigens, such as CD56, which may increase the sensitivity of clonal PC detection, particularly at the lower limit of the assay sensitivity.
In conclusion, this study is the largest report on MFC immunophenotyping in AL amyloidosis. Its strength relies on the large number of patients as well as assessment of MFC in 2 settings, newly diagnosed patients, and at EOT. We have demonstrated that the percentage of monotypic PCs as well as the pPCs/BMPCs ratio are important prognostic determinants at diagnosis, and are superior in survival discrimination compared with the traditional morphologic assessment of BMPCs. Moreover, at EOT, the monotypic PC compartment maintained a predictive role. MFC might have a role in refining response evaluation. Future studies should focus on using MFC as a clinical tool in guiding treatment. Furthermore, MFC immunophenotyping should be used in exploration of mechanisms responsible for impaired immunosurveillance and how this connects with a poorer outcome.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: E.M. designed the study, analyzed the data, wrote the first draft, and approved the final version of the manuscript; D.J. designed the study, revised the manuscript critically, and approved its final version; A.D., D.D., F.K.B., M.Q.L., W.G., S.R.H., P.K., N.L., S.R., J.A.L., Y.L., R.S.G., S.Z., S.K.K., and S.V.K. performed patient management, revised the manuscript critically, and approved the final version of the manuscript; R.C. provided critical review of the manuscript and approved the last version of the manuscript; R.A.K. Performed patients’ follow-up, revised the manuscript critically, and participated in final data analysis and approval of the final version of the manuscript; and M.A.G. performed patient management, designed the study, analyzed the data, wrote the first draft, and approved the final version of the manuscript.
Conflict-of-interest disclosure: A.D. received research funding from Celgene, Millennium, Pfizer, and Janssen; and a travel grant from Pfizer. M.Q.L. received research funding from Celgene. P.K. received research funding from Millennium (Takeda), Celgene, and Onyx (Amgen). S.K. was a consultant for and received research funding from Celgene, Millennium, Onyx, Janssen, and BMS; and received research funding from Novartis and AbbVie. M.A.G. received honoraria from Celgene, Onyx, Novartis, SmithKline, Prothena, and Ionis; and was a consultant to and received honoraria from Millennium. The remaining authors declare no competing financial interests.
Correspondence: Morie A. Gertz, Division of Hematology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: gertz.morie@mayo.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal