In this issue of Blood, Muchtar and colleagues report on the prognostic value of multiparametric flow cytometry (MFC) to measure clonal plasma cell burden at diagnosis and at end of treatment in patients with amyloid light-chain (AL) amyloidosis.1
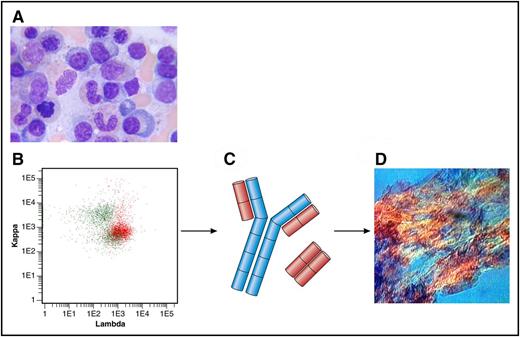
(A) Bone marrow aspirate with few plasma cells (original magnification ×100, Giemsa stain). (B) Flow cytometry plot of bone marrow showing λ-positive plasma cells (in red) and polyclonal B cells (in green). Anti-κ (TB 28-2) allophycocyanin stain (APC), Becton, Dickinson (BD), catalogue number: 341108; Anti-Λ (1-155-2) APC-H7, BD, catalogue number: 656648. (C) Heavy chain in blue; Λ light chains in red. (D) Amyloid deposits in a fat aspirate (original magnification ×10, congo red staining, polarized light). Professional illustration by Patrick Lane, ScEYEnce Studios.
(A) Bone marrow aspirate with few plasma cells (original magnification ×100, Giemsa stain). (B) Flow cytometry plot of bone marrow showing λ-positive plasma cells (in red) and polyclonal B cells (in green). Anti-κ (TB 28-2) allophycocyanin stain (APC), Becton, Dickinson (BD), catalogue number: 341108; Anti-Λ (1-155-2) APC-H7, BD, catalogue number: 656648. (C) Heavy chain in blue; Λ light chains in red. (D) Amyloid deposits in a fat aspirate (original magnification ×10, congo red staining, polarized light). Professional illustration by Patrick Lane, ScEYEnce Studios.
Evaluation of a newly diagnosed patient with AL amyloidosis is a rather elaborate issue. The treating hematologist has to characterize and stage the underlying clonal bone marrow disorder, mostly a plasma cell dyscrasia. In addition, multiple organ systems have to be screened to judge how severely they are affected from the secreted toxic light chains and the deposited amyloid fibrils (see figure). The latter is most easily done with the validated staging systems using cardiac and renal biomarkers.2,3 The most commonly used hematologic prognostic factor is the free light-chain level (calculated as the difference between the involved and uninvolved light chain [dFLC]) with a cutoff of 180 mg/L at diagnosis.2 The primary goal of chemotherapy is achieving at least a very good partial response (VGPR) with a dFLC of <40 mg/L, which is associated with organ function and survival improvement.4 Using bone marrow aspirates and biopsies, the amyloidosis team of Mayo Clinic has previously reported a 10% cutoff for plasma cells percentage as another prognostic factor5 ; however, it is difficult to reliably quantify cells on smears and slides. To more accurately measure clonal burden, they have now developed a MFC assay to quantify monotypic plasma cells at diagnosis and at the end of first-line treatment.
In a previous work, Paiva et al6 analyzed 35 patients with AL amyloidosis and found that a 1% cutoff of bone marrow plasma cells for MFC was of prognostic value for overall survival (OS) at 2 years (44% vs 90%). In the Mayo Clinic study, <2.5% of monotypic plasma cells at diagnosis (analyzed in 173 patients) was significantly associated with an improved progression-free survival (PFS) and OS at 2 years of 56% and 70%, respectively. These results were also confirmed in a multivariate analysis, which included cardiac biomarkers. Importantly, in the current work, the 10% cutoff using the morphological assessment did not separate the groups.
In the second and more important part of the paper, the authors used MFC at the end of first-line treatment of minimal residual disease analysis in 82 patients. Patients achieving <0.1% of monotypic plasma cells had a better response (VGPR or better in 96% vs 36%) and consequently a longer PFS and OS at 2 years of 87% and 98%, respectively, than patients not achieving that level of reduction in plasma cells. However, probably due to the rather small sample size and short follow-up, there was only a trend toward improved PFS and no OS difference in patients with VGPR or better using the 0.1% MFC cutoff.
This study also found that prognostic (and especially plasma cell derived) parameters can depend on the administered therapy as MFC at diagnosis predicted for better outcome in the cohort of patients not receiving autologous stem cell transplantation (ASCT), but not in those treated with ASCT. This result underlines the need to evaluate prognostic factors in homogeneously treated cohorts and is reminiscent of previous studies from our group wherein the most prevalent cytogenetic aberration t(11;14) was a favorable prognostic factor in patients treated with ASCT7 but adverse in patients treated with bortezomib.8
What should be the next steps? The new and interesting findings of the study of Muchtar et al have to be validated by other groups to confirm the prognostic value of MFC for OS. It would be optimal to validate the findings in prospective clinical trials using different treatment modalities. Most importantly, it must be determined if MFC after chemotherapy is prognostic in conjunction with serological response assessment and can predict the risk of early progression in patients with VGPR which could help the treating hematologist decide when to stop chemotherapy. Finally, within the amyloidosis community, a consensus (like in multiple myeloma)9 on how to use flow cytometry in AL amyloidosis (eg, amount of cells, which markers and gating strategies)10 should be determined.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal