Key Points
TORK/DNA-PK inhibition induces cytotoxicity and blocks signaling pathways important for CLL survival, proliferation, and drug resistance.
Preliminary clinical effects of TORK/DNA-PK inhibition show 7 of 8 CLL patients with decreased lymphadenopathy.
Abstract
Inhibition of B-cell receptor (BCR) signaling pathways in chronic lymphocytic leukemia (CLL) provides significant clinical benefit to patients, mainly by blocking adhesion of CLL cells in the lymph node microenvironment. The currently applied inhibitors ibrutinib and idelalisib have limited capacity however to induce cell death as monotherapy and are unlikely to eradicate the disease. Acquired resistance to therapy in CLL is often caused by mutations in the response network being targeted, both for DNA damage or BCR signaling pathways. Thus, drugs with dual targeting capacity could offer improved therapeutic value. Here, the potency of CC-115, a novel inhibitor of mammalian target of rapamycin kinase (TORK) and DNA-dependent protein kinase (DNA-PK), was evaluated in primary CLL cells in vitro and in CLL patients. Combined TORK and DNA-PK inhibition in vitro resulted in caspase-dependent cell killing irrespective of p53, ATM, NOTCH1, or SF3B1 status. Proliferation induced by CD40+ interleukin-21 stimulation was completely blocked by CC-115, and CD40-mediated resistance to fludarabine and venetoclax could be reverted by CC-115. BCR-mediated signaling was inhibited by CC-115 and also in CLL samples obtained from patients with acquired resistance to idelalisib treatment. Clinical efficacy of CC-115 was demonstrated in 8 patients with relapsed/refractory CLL/small lymphocytic lymphoma harboring ATM deletions/mutations; all but 1 patient had a decrease in lymphadenopathy, resulting in 1 IWCLL partial response (PR) and 3 PRs with lymphocytosis. In conclusion, these preclinical results, along with early promising clinical activity, suggest that CC-115 may be developed further for treatment of CLL. The trial was registered at www.clinicaltrials.gov as #NCT01353625.
Introduction
Chronic lymphocytic leukemia (CLL) cells highly depend on both B-cell receptor (BCR)-mediated signaling and stimuli received from the tumor microenvironment for their survival and proliferation.1-6 The importance of the microenvironment is substantiated by the recent success of novel drugs that target kinases involved in BCR signaling. Treatment with the Bruton tyrosine kinase (BTK) inhibitor ibrutinib or the phosphatidylinositol 3 kinase-δ (PI3Kδ) inhibitor idelalisib abolishes chemotaxis toward stroma and BCR-controlled integrin-mediated cell adhesion. This results in rapid reduction of lymph node (LN) size and is followed by prolonged lymphocytosis.7,8 Such prolonged lymphocytosis during kinase inhibitor treatment appears to pose no clinical disadvantage.9,10 However, prolonged lymphocytosis could enhance the chance of accumulating resistance-inducing mutations. Indeed, acquired resistance to ibrutinib was reported in patients due to mutations in BTK or in downstream kinases.11,12 Drugs that target both BCR signaling and critical survival pathways could provide an improved therapeutic strategy for CLL.
From this perspective, there is increased interest in compounds that target other kinases in the PI3K family.13-15 The PI3K-related protein kinase (PIKK) family includes mammalian target of rapamycin kinase (TORK), ataxia telangiectasia mutated (ATM), ataxia telangiectasia and Rad3 related (ATR), and DNA-dependent protein kinase (DNA-PK). TORK is the main downstream kinase of the PI3K/AKT pathway and exists in 2 protein complexes: mTORC1 and mTORC2.16 mTORC1 (Raptor) is activated by AKT and leads to phosphorylation of downstream effectors, which include 4EBP1 and S6,12,17 whereas mTORC2 (Rictor) phosphorylates AKT at the serine residue 473, leading to AKT activation.16,18 In healthy B cells, mTORC1 and mTORC2 are both critical for proliferation and differentiation through distinct mechanisms.19-22 Although inhibition of mTORC1 by rapamycin results in suppression of B-cell growth without induction of cell death,19 deletion of mTORC2 affects cell viability.23 Complete deletion of the TORK kinase (TORK) gene in mouse B cells resulted in impaired germinal center formation.24 Inhibition of mTORC1 by rapamycin in CLL cells results in increased fludarabine sensitivity25 and inhibition of cytosine guanine dinucleotide–induced proliferation of CLL cells.26
ATM, ATR, and DNA-PK are critical regulators of the DNA damage repair (DDR) pathway. DNA-PK is required for the repair of DNA double-strand breaks (DSBs) through the process of nonhomologous end joining (NHEJ).27 NHEJ is active throughout the cell cycle, whereas homologous recombination mediated by ATM/ATR is active in late S phase and in G2.28-30 As peripheral blood CLL cells are in cell cycle arrest,31 it is likely that DNA repair in CLL cells predominantly depends on NHEJ.
In this study, the potency of a dual TORK and DNA-PK inhibitor (CC-115) was analyzed in primary CLL samples of different prognostic subgroups with respect to induction of cytotoxicity and blocking of CD40-mediated chemo-resistance and proliferation. Furthermore, clinical efficacy of CC-115 was tested in 1 small lymphocytic lymphoma (SLL) patient and 7 CLL patients.
Materials and methods
Patient material
After written informed consent, patient material was obtained during diagnostic or follow-up procedures at the Department of Hematology of the Academic Medical Center Amsterdam and affiliated hospitals. This study was approved by the Academic Medical Center Ethical Review Board and the ethical board of the Dana Farber Medical Center (Boston, MA), and written informed consent was obtained in accordance with the Declaration of Helsinki. Blood mononuclear cells of patients with CLL (supplemental Table 1, available on the Blood Web site), obtained after Ficoll density gradient centrifugation (Pharmacia Biotech, Roosendaal, The Netherlands), were frozen and stored as previously described.32 Expression of CD5 and CD19 (both Beckton Dickinson [BD] Biosciences, San Jose, CA) on leukemic cells was assessed by flow cytometry (FACScanto; BD Biosciences). CLL samples included in this study contained 81% to 99% CD5+/CD19+ cells.
Peripheral blood mononuclear cells (PBMCs) were obtained from healthy blood donors derived buffy coats, aged between 18 and 64 years, from Sanquin Blood Supply (Amsterdam, The Netherlands). PBMCs were isolated and frozen and stored in liquid nitrogen until use.
Fluorescence in situ hybridization and gene mutational analyses
Deletions at the 11q22-q23 (ATM), 17p13 (TP53), and 13q14 loci and trisomy of chromosome 12 were detected by fluorescence in situ hybridization by using locus-specific probes (Abott Vysis). DNA was extracted by using the QiAamp DNA Blood Mini kit (Invitrogen) according to the manufacturer’s instructions. TP53 mutational analysis was either performed by a 454-based next generation sequencing approach (Junior 454 platform; Roche, Penzberg, Germany) or using Sanger sequencing (exons 4-10).33 Primer sequences are provided in supplemental Table 3. Mutation analysis of ATM (exons 1-62) was performed by Sanger sequencing as described previously.34,35
Reagents
CC-115, CC-214-1 [CC-214], and CC-292 were obtained from Celgene (Summit, NJ). The PI3Kδ inhibitor CAL-101/idelalisib and the DNA-PK inhibitor NU7441 were from Selleckchem (Housten, TX). Fludarabine, chlorambucil, bendamustine, and N-acetylcysteine (NAC) were purchased from Sigma Chemical (St. Louis, MO). The pan-caspase inhibitor Q-VD-OPH was purchased from R&D Systems (Minneapolis, MN). ABT-199 was purchased from Active Biochem (Bonn, Germany).
Cell culture and detection of apoptosis
Freshly isolated CLL cells were treated with different concentrations of CC-115 for 30 minutes. Subsequently, the cells were exposed to 5-Gy γ-radiation or treated with 10 µg/mL bleomycin (EMD Millipore, Billerica, MA) and incubated for 30 minutes, and cell lysates were made.
For the apoptosis assays, PBMCs from healthy donors or CLL patients were thawed and incubated with different concentrations of drugs for the indicated 48 hours. Samples were only used if the viability of untreated samples was ≥50% after 48 hours of culture. Where indicated, CLL cells were cocultured in the presence/absence of 20 μM of the pan-caspase inhibitor Q-VD or 5 mM NAC. Viability was measured by DiOC6/PI staining as previously described.32 Specific apoptosis was defined as ([% cell death in treated cells] – [% cell death in medium control])/(% viable cells medium control) × 100.
To mimic the antiapoptotic properties of microenvironmental stimulated CLL cells, CLL cells were stimulated by coculture with NIH3T3 fibroblasts stably transfected with human CD40L (3T40L) or negative control plasmid (3T3) as described32 and cocultured in the presence/absence of drugs at 1 μM or the indicated concentrations.
Western blot analysis
Cells were lysed in radioimmunoprecipitation sample buffer and sonificated.32 Samples were separated by 3% to 8% TA gel (Thermo Fisher Scientific, Grand Island, NY), 4% to 12% bris-tris protein gel (Thermo Fisher Scientific), or 13% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel electrophoresis. Membranes were probed with the following antibodies: anti-DNA-PK, pHSP90α, pS6, p4EBP1, pEIF4E, Mcl-1 (Cell Signaling, Boston, MA), pDNA-PK, ATM, HSP90α, γH2AX (Abcam, Cambridge, United Kingdom), Bcl-XL (BD Biosciences), Bim (Stressmarq, Victoria, Canada), pATM, vinculin (Sigma Chemical), actin (Santa Cruz Biotechnology, Dallas, TX), and anti–A1/Bfl-1 (kind gift of Prof Dr J. Borst [The Netherlands Cancer Institute, Amsterdam, The Netherlands]). Odyssey Imager (Li-Cor Biosciences) was used as a detection method according to the manufacturer’s protocol.
γH2AX FACS staining
CLL cells were thawed and incubated for 30 minutes either with or without 1 µM CC-115 or NU7441. Subsequently, the cells were exposed to 5-Gy γ-radiation and incubated for 2 hours. CLL cells were fixed and incubated with CD5-phycoerythrin (PE) (eBioscience), CD19-allophycocyanin (APC) (BD Biosciences), and intracellular staining was performed for isotype-AF488 or γH2AX-AF488 (Cell Signaling) for 30 minutes. Expression of γH2AX was determined within the CD5+CD19+ CLL cells using the FACSCalibur flow cytometer, and CellQuest software was used for data acquisition. Data were analyzed with FlowJo software (TreeStar, Ashland, OR).
Proliferation assay
CLL cells (1.0 × 107/mL) were labeled with 0.5 μM carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Life Technologies, Bleiswijk, The Netherlands) as described before.36 Cells were cultured on 3T40L cells, in the absence or presence of recombinant human interleukin 21 (IL-21; 25 ng/mL; Gibco, Invitrogen, Life Technologies), with or without 1 μM of drugs. After 4 days, proliferation was assessed in a FACSCanto (BD Biosciences) and analyzed with FlowJo software.
Activation of healthy PBMCs
PBMCs from healthy donors were stimulated with αCD3 (1xE, ascites) and αCD28 (15E8; 5 µg/mL) for 3 days. PBMCs were resuspended in phosphate-buffered saline, containing 0.5%(w/v) bovine serum albumin and 0.01% sodium azide. PBMCs were incubated with saturating concentrations of CD19-PerCP-Cy5.5, CD20-APC-H7, IgD-PE, CD27-APC, CD38-PE-Cy7, CD3-AF700, CD4-PE-Cy7, CD8- PerCP-Cy5.5, CD38-PE, and CD25-APC (BD Biosciences). Flow cytometry measurements were performed on a FACSCanto using FACSDiva Software (BD Biosciences).
Clinical study
Eight patients with refractory/relapsed CLL/SLL were enrolled into the dose expansion part of a larger phase 1A/1B multicenter study of CC-115 entitled a phase 1A/1B, multicenter open label, dose-finding study to assess the safety, tolerability, pharmacokinetics, and preliminary efficacy of the dual DNA-PK and TOR kinase inhibitor CC-115, administered orally to subjects with advanced solid tumors and hematologic malignancies. SLL/CLL patients with 11q22 (ATM) deletions were eligible. The second, nondeleted ATM allele was sequenced to determine heterozygosity at this locus. The study was approved by relevant Ethical Review Boards and regulatory authorities. Written informed consent was given, and the study was performed according to Good Clinical Practice. The clinical trial was sponsored by Celgene. Patients had received ≥1 prior line of systemic therapy and symptomatic progression or another indication for treatment was required. Patients received the earlier established recommended dose of 10 mg twice daily of CC-115 continuously in 28-day cycles, and no other concomitant antileukemia treatment. Steroids were only allowed in physiologic doses or for treatment of toxicities. The efficacy end point was response rate assessed by computed tomography scan and laboratory parameters at 8, 16, and 24 weeks and then every 12 weeks thereafter using the International Workshop on Chronic Lymphocytic Leukemia (IWCLL) 2008 guidelines. Patients meeting the IWCLL criteria for response, but with persistent lymphocytosis, were categorized as relevant response with lymphocytosis. Three patients are ongoing and have already received 13, 15, and 23 cycles of therapy.
Statistics
The 1-way analysis of variance (ANOVA) was used to analyze differences between groups.
Results
CC-115 is a dual DNA-PK and TORK inhibitor
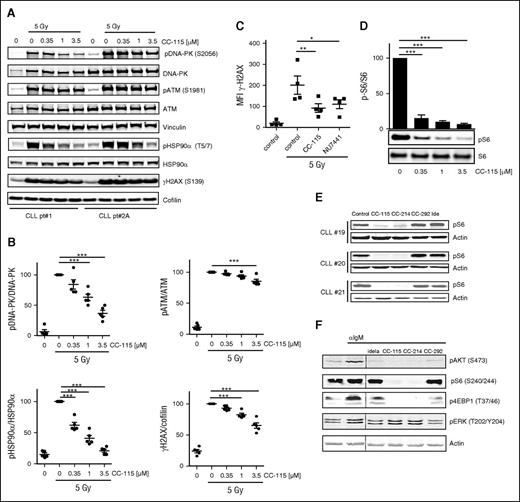
On induction of DNA DSBs, DNA-PK is recruited to the site of DNA damage and activated, which leads to phosphorylation of downstream targets H2AX and the heat shock protein 90α (HSP90α).37-40 Inhibition of DNA-PK and the immediate DNA damage response by CC-115 was measured on irradiation or treatment with the chemotherapeutic agent bleomycin, a potent inducer of DNA strand breaks, in primary CLL cells. Phosphorylation of DNA-PK, ATM, HSP90α, and H2AX was reduced to a variable degree (Figure 1A-B). CC-115 showed dose-dependent repression of Ser-2056 phosphorylation on DNA-PK at clinically achievable doses (0.1-0.35 µM) and reduced baseline and bleomycin-induced Ser-1981 phosphorylation on ATM and Thr-5/7 phosphorylation on HSP90α and γH2AX. These effects were comparable in wild type, as well as ATM/11q mutated CLL cells (supplemental Figure 1A-B). Dependence of ATM/11q mutated CLL cells on DNA-PK activity with respect to DNA repair was demonstrated by measuring γH2AX by a flow cytometry-based assay as described before.33 Treatment with the DNA-PK inhibitor NU7441 (1 µM) or CC-115 (1 µM) inhibited irradiation-induced γH2AX levels in ATM/11q mutated CLL cells (Figure 1C).
CC-115 inhibits the DNA damage repair pathway and TORK in CLL cells. (A-B) Freshly isolated CLL cells were incubated with the indicated concentration CC-115 for 30 minutes and irradiated (5 Gy), and lysates were made after 30 minutes. Protein lysates were probed for pDNA-PK (S2056), DNA-PK, pATM (S1981), ATM, pHSP90α (T5/7), HSP90α, yH2AX, and vinculin and cofilin for loading control. (A) Blots from 2 representative CLL samples are shown of 5 analyzed (supplemental Table 1; CLL patients 1, 2A, 3A, 4A, and 5). (B) Densitometric analysis of pDNA-PK, pATM, pHSP90α, and yH2AX is shown. ***P < .001 (1-way ANOVA). (C) CLL cells of ATM mutated patients (n = 4, patients 12-15) were treated with or without 1 µM NU7441 or CC-115 followed by irradiation (5 Gy), and yH2AX expression was measured at 2 hours using flow cytometry. *P < .05, **P < .01 (1-way ANOVA). (D) CLL cells were incubated with the indicated concentration of CC-115 for 30 minutes, and lysates were made after 30 minutes. Protein lysates were probed for pS6 (S240/244) and S6. Blot from patient 85 and densitometric analysis are shown (n = 3, patients 85, 87, and 88). Bars represent the mean ± standard error of the mean (SEM), ***P < .001 (1way ANOVA). (E) CLL cells were cultured in the presence or absence of 1 µM CC-115, CC-214, CC-292, or idelalisib for 1 hour. Protein lysates were probed for pS6 (S240/244) and actin for loading control. Blots from 3 representative CLL samples are shown (supplemental Table 1; CLL patients 19-21). (F) CLL cells (patient 20) pretreated with 1 µM idelalisib, CC-115, CC-214, and CC-292 were stimulated with αIgM for 20 minutes. Protein lysates were probed for pAKT(S473), pS6 (S240/244), p4EBP1 (T37/46), and pERK (T202/204) and actin as loading control.
CC-115 inhibits the DNA damage repair pathway and TORK in CLL cells. (A-B) Freshly isolated CLL cells were incubated with the indicated concentration CC-115 for 30 minutes and irradiated (5 Gy), and lysates were made after 30 minutes. Protein lysates were probed for pDNA-PK (S2056), DNA-PK, pATM (S1981), ATM, pHSP90α (T5/7), HSP90α, yH2AX, and vinculin and cofilin for loading control. (A) Blots from 2 representative CLL samples are shown of 5 analyzed (supplemental Table 1; CLL patients 1, 2A, 3A, 4A, and 5). (B) Densitometric analysis of pDNA-PK, pATM, pHSP90α, and yH2AX is shown. ***P < .001 (1-way ANOVA). (C) CLL cells of ATM mutated patients (n = 4, patients 12-15) were treated with or without 1 µM NU7441 or CC-115 followed by irradiation (5 Gy), and yH2AX expression was measured at 2 hours using flow cytometry. *P < .05, **P < .01 (1-way ANOVA). (D) CLL cells were incubated with the indicated concentration of CC-115 for 30 minutes, and lysates were made after 30 minutes. Protein lysates were probed for pS6 (S240/244) and S6. Blot from patient 85 and densitometric analysis are shown (n = 3, patients 85, 87, and 88). Bars represent the mean ± standard error of the mean (SEM), ***P < .001 (1way ANOVA). (E) CLL cells were cultured in the presence or absence of 1 µM CC-115, CC-214, CC-292, or idelalisib for 1 hour. Protein lysates were probed for pS6 (S240/244) and actin for loading control. Blots from 3 representative CLL samples are shown (supplemental Table 1; CLL patients 19-21). (F) CLL cells (patient 20) pretreated with 1 µM idelalisib, CC-115, CC-214, and CC-292 were stimulated with αIgM for 20 minutes. Protein lysates were probed for pAKT(S473), pS6 (S240/244), p4EBP1 (T37/46), and pERK (T202/204) and actin as loading control.
Next, TORK inhibitory activity of CC-115 was determined. Constitutive phosphorylation of S6, a marker for mTORC1 activity, was detected in all CLL samples, and this was inhibited by both CC-115 at low doses (Figure 1D) and the specific TORK inhibitor CC-214 at 1 µM (Figure 1E). Inhibitors of kinases more upstream in the BCR pathway, the BTK inhibitor CC-292, or the PI3Kδ inhibitor idelalisib, did not decrease levels of pS6 (S240/244) (Figure 1E). In BCR-stimulated CLL cells, phosphorylation of the mTORC2 target, AKT (S473), and the mTORC1 targets, S6 and 4EBP1, were blocked completely by CC-115 and CC-214, whereas phosphorylation of ERK was not affected by the TORK inhibitors (Figure 1F). These data demonstrate robust phosphorylation of DNA-PK both in ATM-mutant and wild-type CLL cells on DNA damage and constitutive activation of TORK. Both TORK and DNAP-PK were inhibited by CC-115 in these cultured primary CLL cells.
CC-115 induces caspase-dependent cell death in resting CLL cells
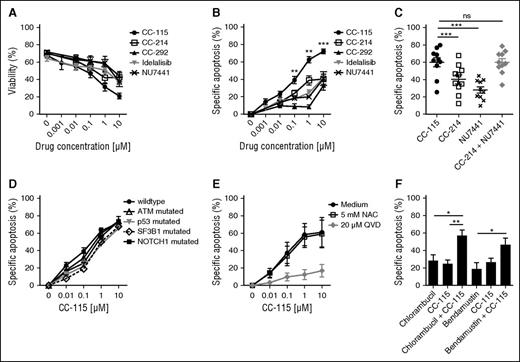
To determine whether combined inhibition of TORK and DNA-PK induces cell death, we compared CC-115 to more specific inhibitors (Figure 2A). To correct for variability in viability between thawed primary CLL samples, specific apoptosis was calculated (Figure 2B). CC-214 (TORKi), CC-292 (BTKi), idelalisib (PI3Kδi), and NU7441 (DNAPKi) induced modest cell death (IC50 > 10 µM and maximum induction of apoptosis at 10 µM of 30-40%; Figure 2B), whereas CC-115 induced cell death with an IC50 of 0.51 µM (Figure 2B). Cell death was due to on-target inhibition of TORK and DNA-PK, because the combination of the TORK inhibitor CC-214 and the DNA-PK inhibitor NU7441 resulted in cell death comparable to CC-115 (Figure 2C). CC-115 induced cell death in clinically relevant prognostic CLL subgroups. All subgroups, including those with high-risk features, were equally sensitive to ≥1 µM CC-115. CLL cells harboring TP53 and SF3B1 mutations appeared to be less sensitive to CC-115 at lower concentrations, but this was not statistically significant in this smaller patient cohort (TP53, n = 6; SF3B1, n = 4; Figure 2D).
CC-115 induces apoptosis in CLL cells from distinct prognostic groups. (A-B) CLL cells were incubated with 0.001 to 10 μM CC-115, CC-214, CC-292, idelalisib, or NU7441 for 48 hours. (A) Viability was assessed by DiOC6/PI staining, and (B) specific apoptosis was calculated (“Materials and methods”). Results are shown as mean ± SEM (n = 23; patients 6, 7, 8A, 21-31, 32A,B, 33A,B, 34A, 35A, and 36-38). (C) CLL cells were incubated with 1 µM CC-115, CC-214, NU7441, or CC-214 + NU7441 for 48 hours. Results are shown as mean ± SEM (n = 11; patients 8B, 23, and 39-46). (D) CLL cells of patients of distinct prognostic CLL groups, wild type (n = 23; patients 6, 7, 8A, 21-31, 32A,B, 33A,B, 34A, 35A, and 36-38), ATM mutated (n = 10; patients 2C and 47-55), p53 mutated (n = 6; patients 56-61), SF3B1 mutated (n = 4; patients 62-65), and NOTCH1 mutated (n = 3; patients 66-68) were incubated with CC-115 for 48 hours. Results are shown as mean ± SEM. (E) CLL cells were cultured with 20 µM Q-VD or 5 mM NAC and with increasing concentrations of CC-115 for 48 hours. Results are shown as mean ± SEM (n = 3; patients 21, 33B, and 31). (F) CLL cells were cultured with 6.25 µM chlorambucil, 6.25 µM bendamustine, 0.1 µM CC-115, or the combination of 6.25 µM chlorambucil/bendamustine and 0.1 µM CC-115 for 48 hours. Results are shown as mean ± SEM (n = 5; patients 2C, 19B, 29, 37, and 69). *P < .05, **P < .01, ***P < .001 (1-way ANOVA).
CC-115 induces apoptosis in CLL cells from distinct prognostic groups. (A-B) CLL cells were incubated with 0.001 to 10 μM CC-115, CC-214, CC-292, idelalisib, or NU7441 for 48 hours. (A) Viability was assessed by DiOC6/PI staining, and (B) specific apoptosis was calculated (“Materials and methods”). Results are shown as mean ± SEM (n = 23; patients 6, 7, 8A, 21-31, 32A,B, 33A,B, 34A, 35A, and 36-38). (C) CLL cells were incubated with 1 µM CC-115, CC-214, NU7441, or CC-214 + NU7441 for 48 hours. Results are shown as mean ± SEM (n = 11; patients 8B, 23, and 39-46). (D) CLL cells of patients of distinct prognostic CLL groups, wild type (n = 23; patients 6, 7, 8A, 21-31, 32A,B, 33A,B, 34A, 35A, and 36-38), ATM mutated (n = 10; patients 2C and 47-55), p53 mutated (n = 6; patients 56-61), SF3B1 mutated (n = 4; patients 62-65), and NOTCH1 mutated (n = 3; patients 66-68) were incubated with CC-115 for 48 hours. Results are shown as mean ± SEM. (E) CLL cells were cultured with 20 µM Q-VD or 5 mM NAC and with increasing concentrations of CC-115 for 48 hours. Results are shown as mean ± SEM (n = 3; patients 21, 33B, and 31). (F) CLL cells were cultured with 6.25 µM chlorambucil, 6.25 µM bendamustine, 0.1 µM CC-115, or the combination of 6.25 µM chlorambucil/bendamustine and 0.1 µM CC-115 for 48 hours. Results are shown as mean ± SEM (n = 5; patients 2C, 19B, 29, 37, and 69). *P < .05, **P < .01, ***P < .001 (1-way ANOVA).
The pan-caspase inhibitor Q-VD completely blocked CC-115–induced cytotoxicity, demonstrating that CC-115–induced cell death is caspase dependent (Figure 2E). A variety of chemotherapeutic drugs induce apoptosis in CLL cells by the generation of reactive oxygen species, as we have shown for platinum-based compounds.41 Cotreatment with NAC, a reactive oxygen species inhibitor, was able to rescue CCCP-induced cell death (data not shown) but not CC-115–induced cell death (Figure 2E).
Next, we assessed whether inhibition of the DNA repair pathway by CC-115 enhanced the sensitivity to the DNA damage-inducing agents chlorambucil and bendamustine. Combination of a low dose of the DNA-PK/TORK inhibitor with chlorambucil (6.25 µM) or bendamustine (6.25 µM) had a significant additive effect on the induced apoptosis (Figure 2F). Thus, combined inhibition of DNA-PK and TORK results in caspase-dependent cell death, irrespective of the p53 status, and enhances sensitivity to chemotherapy.
CC-115 reverts CD40-mediated resistance to chemotherapy or venetoclax
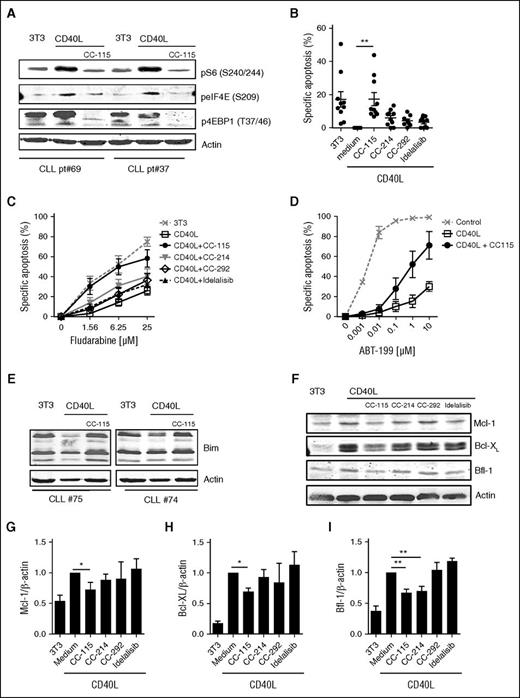
In the LN microenvironment, CLL cells receive prosurvival signals from surrounding cells.1,2,6,42 This can largely be mimicked by in vitro CD40 stimulation,6,42 which resulted in increased mTORC1 markers (pS6, peIF4E, and p4EBP1), which was inhibited by CC-115 (Figure 3A; supplemental Figure 2). CC-115 and the TORK inhibitor CC-214 inhibited CD40-mediated activation of CLL cells, as measured by induction of immune accessory molecules, such as death receptor (CD95) and adhesion receptors (CD54, CD58, CD44)42,43 (supplemental Figure 3). In contrast, BTK and PI3Kδ inhibitors modestly inhibited CD40-induced upregulation of CD58 and did not affect upregulation of other immune accessory molecules (supplemental Figure 3). CD40 stimulation inhibits spontaneous cell death, as previously reported.2,44 Of the 4 inhibitors tested, only CC-115 reverted CD40-induced survival (Figure 3B). This was also observed by combining the TORK and DNA-PK inhibitor (supplemental Figure 4). CD40 stimulation induces resistance to cytotoxic agents, including fludarabine and the specific Bcl-2 inhibitor ABT-199 (venetoclax).42,45 Inhibitors of BTK and PI3Kδ had no impact on CD40-induced chemoresistance. The TORK inhibitor CC-214 partly and the dual TORK/DNA-PK inhibitor CC-115 completely abolished CD40-induced fludarabine resistance (Figure 3C). Moreover, CC-115 also reduced the extensive ABT-199 resistance conferred by CD40 stimulation (Figure 3D).
CC-115 reverts CD40-induced chemoresistance. (A-I) CLL cells were cultured on 3T3 fibroblasts or CD40L-expressing fibroblasts (CD40L) in the presence or absence of 1 µM CC-115, CC-214, CC-292, or idelalisib for 3 days. (A) Protein lysates were probed for pS6 (S240/244), peIF4E (S209), p4EBP1 (T37/46), and actin as loading control. Blots from 2 representative CLL samples are shown (patients 70 and 37). (B) Survival was analyzed by DiOC6 staining. Results are shown as mean ± SEM. *P < .05, **P < .01 (1-way ANOVA; n = 10; patients 6, 25, 30, 33B, 34A, 43B, 72-74, and 75). (C) After 3 days, fludarabine sensitivity assay was performed. Apoptosis was assessed by DiOC6/PI staining, and specific apoptosis is shown as mean ± SEM (n = 9; patients 6, 25, 30, 33B, 34A, 43B, and 72-74). (D) After 3 days, an ABT-199 sensitivity assay was performed. Results are shown as mean ± SEM (n = 3; patients 33B, 35B, and 45). (E) Protein lysates were probed for Bim and actin as loading control. Blots of 2 representative CLL samples are shown of 7 analyzed. (F) Protein lysates were probed for Mcl-1, Bcl-XL, Bfl-1, and actin as loading control. Blot from 1 representative CLL sample is shown of 9 analyzed. (G-I) Densitometric analysis of (G) Mcl-1/actin, (H) Bcl-XL/actin, and (I) Bfl-1/actin level is shown. Bars represent mean ± SEM. *P < .05, ** P < .01 (1-way ANOVA).
CC-115 reverts CD40-induced chemoresistance. (A-I) CLL cells were cultured on 3T3 fibroblasts or CD40L-expressing fibroblasts (CD40L) in the presence or absence of 1 µM CC-115, CC-214, CC-292, or idelalisib for 3 days. (A) Protein lysates were probed for pS6 (S240/244), peIF4E (S209), p4EBP1 (T37/46), and actin as loading control. Blots from 2 representative CLL samples are shown (patients 70 and 37). (B) Survival was analyzed by DiOC6 staining. Results are shown as mean ± SEM. *P < .05, **P < .01 (1-way ANOVA; n = 10; patients 6, 25, 30, 33B, 34A, 43B, 72-74, and 75). (C) After 3 days, fludarabine sensitivity assay was performed. Apoptosis was assessed by DiOC6/PI staining, and specific apoptosis is shown as mean ± SEM (n = 9; patients 6, 25, 30, 33B, 34A, 43B, and 72-74). (D) After 3 days, an ABT-199 sensitivity assay was performed. Results are shown as mean ± SEM (n = 3; patients 33B, 35B, and 45). (E) Protein lysates were probed for Bim and actin as loading control. Blots of 2 representative CLL samples are shown of 7 analyzed. (F) Protein lysates were probed for Mcl-1, Bcl-XL, Bfl-1, and actin as loading control. Blot from 1 representative CLL sample is shown of 9 analyzed. (G-I) Densitometric analysis of (G) Mcl-1/actin, (H) Bcl-XL/actin, and (I) Bfl-1/actin level is shown. Bars represent mean ± SEM. *P < .05, ** P < .01 (1-way ANOVA).
CLL cells originating from LNs show an altered expression of anti- and proapoptotic proteins including increased expression of Bcl-XL, Bfl-1, and Mcl-1 and downregulation of Bim.32,45,46 CD40-mediated decreased expression of Bim was abolished by coculture with CC-115 (Figure 3E). In accordance, CC-115 treatment significantly reduced induction of expression Mcl-1, Bfl-1, and Bcl-XL on CD40 stimulation (Figure 3F-I).
Thus, CD40-mediated expression of immune accessory molecules and drug resistance could be reverted by CC-115, which correlated with suppressed induction of the antiapoptotic proteins Bcl-XL, Bfl-1, and Mcl-1 and repression of the CD40-mediated reduction of Bim expression.
CC-115 blocks downstream signaling pathways and induces cell death in idelalisib-resistant CLL cells
Targeting PI3Kδ downstream of the BCR by idelalisib showed significant clinical activity in CLL patients47 ; however, a proportion of CLL patients develop resistance to idelalisib. We assessed CC-115 activity in vitro in CLL cells from patients who had become resistant to idelalisib treatment. Following BCR triggering, phosphorylation of ERK was inhibited by idelalisib in CLL samples obtained from responding patients, but this no longer occurred following acquired idelalisib resistance (Figure 4A). CC-115 inhibited both BCR-mediated phosphorylation of S6 and CD40-mediated S6 and AKT (S473) phosphorylation (Figure 4A-B). Both idelalisib responsive and resistant samples were sensitive to CC-115–induced cell death (Figure 4C).
Idelalisib-resistant CLL cells are sensitive to CC-115 treatment. (A) CLL cells obtained from a patient clinically sensitive to idelalisib (−) and CLL cells obtained after the patient became resistant to idelalisib (idelalisib-resistant) were pretreated with 1 µM CC-115 or 1 µM idelalisib and stimulated with αIgM for 20 minutes. Protein lysates were probed for pS6 (S240/244), pERK (T202/204), and actin as loading control. (B) CLL cells obtained from a patient clinically sensitive to idelalisib (−) and CLL cells obtained after the patient became resistant to idelalisib (idelalisib-resistant) were cultured on CD40L-expressing fibroblast in the presence or absence of 1 µM CC-115 or idelalisib for 24 hours. Protein lysates were probed for pAKT (S473), pERK (T202/204), pS6 (S240/244), and actin as loading control. (C) CLL cells from 2 patients that became resistant to idelalisib (idelalisib-resistant) and samples that response to idelalisib treatment (−) were incubated with 0. 1 to 10 μM CC-115 for 48 hours. Viability was assessed by DiOC6/PI staining, and specific apoptosis was calculated.
Idelalisib-resistant CLL cells are sensitive to CC-115 treatment. (A) CLL cells obtained from a patient clinically sensitive to idelalisib (−) and CLL cells obtained after the patient became resistant to idelalisib (idelalisib-resistant) were pretreated with 1 µM CC-115 or 1 µM idelalisib and stimulated with αIgM for 20 minutes. Protein lysates were probed for pS6 (S240/244), pERK (T202/204), and actin as loading control. (B) CLL cells obtained from a patient clinically sensitive to idelalisib (−) and CLL cells obtained after the patient became resistant to idelalisib (idelalisib-resistant) were cultured on CD40L-expressing fibroblast in the presence or absence of 1 µM CC-115 or idelalisib for 24 hours. Protein lysates were probed for pAKT (S473), pERK (T202/204), pS6 (S240/244), and actin as loading control. (C) CLL cells from 2 patients that became resistant to idelalisib (idelalisib-resistant) and samples that response to idelalisib treatment (−) were incubated with 0. 1 to 10 μM CC-115 for 48 hours. Viability was assessed by DiOC6/PI staining, and specific apoptosis was calculated.
CC-115 blocks proliferation of CLL cells
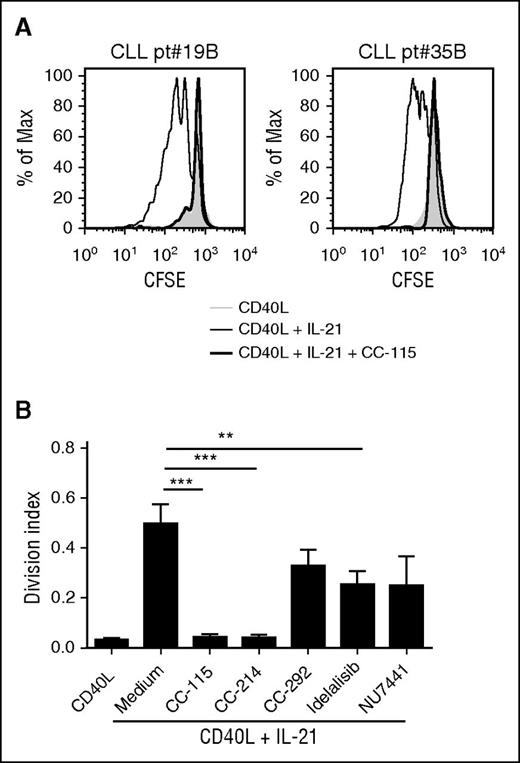
Activated T cells and follicular helper T cells present in the CLL LNs express membrane-bound CD40L and can secrete cytokines, such as IL-21. We have shown previously that, in vitro, combination of CD40 and IL-21 signals induces proliferation.36 This BCR-independent proliferation was inhibited partially by CC-292, idelalisib, or NU7441 and fully by CC-115 or CC-214 (Figure 5A-B).
CC-115 and mTOR inhibitors block proliferation of CLL cells. (A) CFSE-labeled CLL cells were cultured on fibroblast expressing CD40L with (black line) or without (gray) IL-21 and cotreated with 1 µM CC-115 (thick black line). After 4 days, CFSE was measured by FACS. Results are shown as representative histograms for 2 patients. (B) Division index was calculated with the FlowJo program. Results are shown as mean ± SEM (n = 11; patients 2C, 19B, 20, 35B, 71, and 78-82). *P < .05, **P < .01, ***P < .001 (1-way ANOVA).
CC-115 and mTOR inhibitors block proliferation of CLL cells. (A) CFSE-labeled CLL cells were cultured on fibroblast expressing CD40L with (black line) or without (gray) IL-21 and cotreated with 1 µM CC-115 (thick black line). After 4 days, CFSE was measured by FACS. Results are shown as representative histograms for 2 patients. (B) Division index was calculated with the FlowJo program. Results are shown as mean ± SEM (n = 11; patients 2C, 19B, 20, 35B, 71, and 78-82). *P < .05, **P < .01, ***P < .001 (1-way ANOVA).
Effects of CC-115 on B and T cells
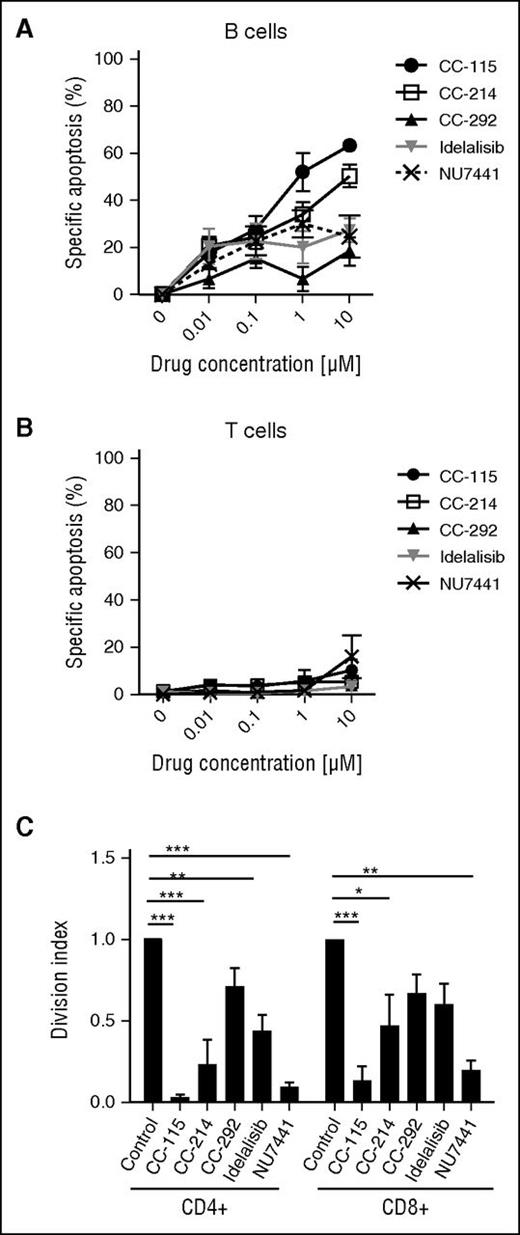
We next studied the impact of the different kinase inhibitors on cell death and function of healthy B and T cells. In healthy B cells, CC-115 induced cell death with an IC50 of 0.93 µM (Figure 6A), whereas the TORK, BTK, PI3Kδ, and DNA-PK inhibitors were not cytotoxic at doses up to 10 µM. None of the kinase inhibitors induced cell death in T cells from healthy donors or T cells from CLL patients (Figure 6B; supplemental Figure 4A). Furthermore, none of the kinase inhibitors significantly altered CD3/CD28-induced upregulation of the activation markers CD25 and CD38 (supplemental Figure 4B-C). CC-115 and NU7441 completely blocked the proliferation of CD4+ and CD8+ T cells (Figure 6C). Inhibitors of TORK, BTK, or PI3Kδ partially inhibited CD3/CD28-induced proliferation (Figure 6C).
CC-115 inhibits proliferation of healthy T cells. (A-B) PBMCs from healthy donors were incubated with 0.001 to 10 μM CC-115, CC-214, CC-292, idelalisib, or NU7441 for 48 hours. Specific apoptosis was analyzed in (A) CD19+ B cells or (B) CD3+ T cells. Results are shown as mean ± SEM, n = 3, not significant (1-way ANOVA). (C) PBMCs from healthy donors were stimulated with αCD3/αCD28 in the presence or absence of 1 μM CC-115, CC-214, CC-292, idelalisib, or NU7441 for 72 hours (n = 3). CFSE was measured by FACS in CD8+ T or CD4+ T cells. Bars represent mean ± SEM. *P < .05, **P < .01, ***P < .001 (1-way ANOVA).
CC-115 inhibits proliferation of healthy T cells. (A-B) PBMCs from healthy donors were incubated with 0.001 to 10 μM CC-115, CC-214, CC-292, idelalisib, or NU7441 for 48 hours. Specific apoptosis was analyzed in (A) CD19+ B cells or (B) CD3+ T cells. Results are shown as mean ± SEM, n = 3, not significant (1-way ANOVA). (C) PBMCs from healthy donors were stimulated with αCD3/αCD28 in the presence or absence of 1 μM CC-115, CC-214, CC-292, idelalisib, or NU7441 for 72 hours (n = 3). CFSE was measured by FACS in CD8+ T or CD4+ T cells. Bars represent mean ± SEM. *P < .05, **P < .01, ***P < .001 (1-way ANOVA).
Early clinical effect of CC-115 treatment
Seven CLL patients and 1 SLL patient were enrolled in a larger phase 1 clinical study, including 110 additional patients with solid tumors. Median age was 56 years (supplemental Table 2). Median number of prior therapies was 2 (range, 1-8), with all patients receiving prior chemotherapy with alkylating agents and/or fludarabine, anti-CD20 (rituximab), or anti-CD52 (alemtuzumab) therapy. All patients had a del(11q) clone and thus were devoid of ≥1 copy of ATM. Sequencing the second allele showed that 3 patients had mutations, which may be deleterious to ATM function, consistent with the possibility of biallelic ATM loss (supplemental Table 2). Three patients are still ongoing, and the median number of cycles was 13.5 (range, 1-23). All but 1 patient had a decrease in lymphadenopathy, with 4 subjects having decreases of ≥50% (Figure 7A). One of these patients, who received 21 days of therapy, had a >50% decrease in lymphadenopathy, but had Richter’s syndrome diagnosed simultaneously. Three patients experienced a ≥50% decrease in lymphocytes (Figure 7B). Overall, there was 1 partial response according to IWCLL criteria, and 3 partial responses with lymphocytosis.
CC-115 decreases lymphadenopathy in CLL patients. (A) Best percentage change in sum of product of diameters for target lesions for patients with single ATM deletion (black) and those with likely or potentially deleterious mutation in remaining ATM allele (gray). *Patient had Richter’s syndrome diagnosed at the same time. (B) Changes in lymphocyte counts of patients with single ATM deletion (black) and those with likely or potentially deleterious mutation in remaining ATM allele (gray) (supplemental Table 2) during CC-115 treatment over time. (C) Western blot analysis of phospho- and total DNA-PK in PBMCs from CC-115-ST-001 patient 2011007. PBMCs in plasma were collected at screening, cycle 1 day 1/baseline (before and 1.5 hours after CC-115 dose), and cycle 1 day 15 (before and 1.5 hours after CC-115 dose). PBMCs in plasma were incubated with or without 10 µg/mL bleomycin for 60 minutes. Total and phospho-DNA-PK was detected by western blot analysis of protein lysates. (D) Quantification of the western blots in C.
CC-115 decreases lymphadenopathy in CLL patients. (A) Best percentage change in sum of product of diameters for target lesions for patients with single ATM deletion (black) and those with likely or potentially deleterious mutation in remaining ATM allele (gray). *Patient had Richter’s syndrome diagnosed at the same time. (B) Changes in lymphocyte counts of patients with single ATM deletion (black) and those with likely or potentially deleterious mutation in remaining ATM allele (gray) (supplemental Table 2) during CC-115 treatment over time. (C) Western blot analysis of phospho- and total DNA-PK in PBMCs from CC-115-ST-001 patient 2011007. PBMCs in plasma were collected at screening, cycle 1 day 1/baseline (before and 1.5 hours after CC-115 dose), and cycle 1 day 15 (before and 1.5 hours after CC-115 dose). PBMCs in plasma were incubated with or without 10 µg/mL bleomycin for 60 minutes. Total and phospho-DNA-PK was detected by western blot analysis of protein lysates. (D) Quantification of the western blots in C.
Analysis of 1 full set of pharmacodynamics blood samples from 1 of 7 patients treated with CC-115 showed >50% inhibition of DNA-PK in circulating CLL cells (Figure 7C-D).
Discussion
DNA-damaging chemotherapeutic agents cause the formation of toxic DNA DSBs, leading to cell cycle arrest and cell death. Cells have multiple mechanisms for repairing DSBs, which include homologous recombination and NHEJ.48 Homologous recombination, an error-free repair process, depends on the availability of a homologous DNA template and, as such, functions primarily in late S-phase and G2. NHEJ, although error prone, is able to function at all stages of the cell cycle and is thought to be the main pathway for DSB repair.28 As such, it has been postulated that targeting the molecular machinery driving the DDR, particularly NHEJ and DSB repair, with small molecule inhibitors, will effectively enhance the efficacy of current cancer treatments that generate DNA damage.49 Indeed, DNA-PK inhibition using small molecule inhibitors showed promising preclinical results in both solid malignancies and in lymphoma and CLL.50-52 In fact, a previous study showed that DNA-PK activity is highly increased in resistant CLL cells,53 and high concentrations of the DNA-PK inhibitor NU7026 or NU7441 could restore irradiation-induced or chlorambucil-induced apoptosis sensitivity.52-55 Clinical development of DNA-PK inhibitors has been hampered thus far due to poor pharmacokinetics.49
CLL cells harboring a 17p or 11q deletion have been reported to be protected from chemotherapy by DNA-PK overexpression,54,56-58 and inhibition of DNA-PK restores sensitivity to chemotherapeutic drugs in these CLL cells.52-54 Inhibitors of DNA-PK might therefore be of clinical interest in CLL, especially in patients with high-risk disease. Indeed, our findings confirm that treatment with a DNA-PK inhibitor, CC-115 or NU7441, inhibited the DDR pathway in ATM mutated CLL cells, as demonstrated by the inhibition of irradiation-induced γH2AX. We found high expression of pDNA-PK and pHSP90α in irradiated CLL cells, which was blocked by clinically achievable doses of CC-115. Pharmacodynamic analysis of blood samples after CC-115 treatment showed inhibition of pDNA-PK in PBMCs. Inhibition of the DDR pathway by CC-115 resulted in increased sensitivity for chlorambucil and bendamustine in CLL cells. Treatment with the mTORC1 inhibitor everolimus has been reported to decrease lymphadenopathy due to mobilization of CLL cells in some patients.59 Treatment with the TORK inhibitor CC-214 blocked proliferation and partially inhibited CD40-induced drug resistance, with minimal direct cytotoxicity. In contrast, combined inhibition of TORK and DNA-PK by CC-115 induced caspase-dependent cell death in CLL cells. As CC-115–induced cytotoxicity was observed in p53-, ATM-, SF3B1-, or NOTCH1-deficient samples, this points to potential clinical activity of CC-115 in CLL, irrespective of mutation/prognostic status. In accordance, murine studies with MYC-driven lymphomas revealed that combined inhibition of TORK and DNA- PK results in strong induction of p53-independent cell death and in tumor regression and prolonged survival.13
CC-115 treatment diminished CD40-mediated suppression of Bim protein level. Dual PI3K/TORK inhibition results in decreased microenvironment-induced chemoresistance by increased levels of Bim.60 Likewise, rapamycin-induced apoptosis has been reported to be mediated by Bim.61 Combined with increased levels of Bim, the levels of the antiapoptotic proteins Bcl-XL, Mcl-1, and Bfl-1 following CD40 activation were lower in CC-115–treated cells, which might contribute to decreased resistance to fludarabine and venetoclax.
Despite the significant clinical activity of inhibitors of BTK or PI3Kδ, they induce little/no direct cell death as evidenced by prolonged presence of leukemia cells under treatment.9-11 The in vitro activities of CC-115 regarding cytotoxicity, reversal of CD40-induced drug resistance, and inhibition of non–BCR-mediated proliferation suggest a favorable profile to prevent emergence or outgrowth of resistant clones. We demonstrated that CC-115 induces cytotoxicity and reduces downstream BCR and CD40L signaling as evidenced by inhibition of pS6, also including in CLL cells obtained from idelalisib-resistant patients. These results provide a rationale for clinical testing of CC-115 in patients resistant to idelalisib, who currently have very poor prognosis.
CC-115 did not induce cytotoxicity in healthy T cells. Activated T cells upregulate the PI3K/mTOR pathway, which leads to activation of metabolism, proliferation, and survival.16,62 Accordingly, activated T cells treated with CC-115 showed inhibition of proliferation, which could play an adverse role in protection against infections in vivo, especially on long-term treatment. On the other hand, it has been reported that PI3Kδ inhibition reduces regulatory T cell–mediated suppression of cancer immune surveillance.63
Preliminary clinical testing of CC-115 in patients harboring ATM alterations revealed reduction in LN sizes in almost all patients, whereas effects on lymphocytosis were more variable. Interestingly, although preclinical data suggest cytotoxic effects of CC-115 to be independent of functional p53 or ATM, the clinical data might point to enhanced effects on lymphocytosis in patients with biallelic ATM deletions/mutations, compared with monoallelic deletions. This observation suggests increased dependency on DNA-PK for DNA damage repair in cells with biallelic ATM deletions and is in line with in vivo studies using an ATM-defective Eµ:Myc-driven lymphoma model.64 However, patients with a monoallelic ATM alteration also showed a decrease in lymphadenopathy. Moreover, this clinical study included only patients with an ATM mutation, and it would therefore be interesting to clinically evaluate CC-115 in patients with various prognostic subgroups.
Taken together, our study reveals that dual TORK/DNA-PK inhibition by CC-115 induces direct cytotoxicity and can block signaling pathways that are important for CLL survival, chemo-resistance and proliferation in the in the LN microenvironment. Preliminary data indeed indicate clinical activity of CC-115. Further clinical evaluation, especially combination therapy with agents that induce DNA damage including fludarabine or combination with venetoclax, seems warranted. Clinical results suggest that CC-115 can be useful for the treatment of CLL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the CLL patients for blood donations and Marjolein Spiering and Dieuwertje Luijks for database management and CLL cell phenotyping. A.P.K. is a recipient of a Dutch Cancer Society clinical fellowship. H.C.R. received funding through the German Research Foundation (KFO-286-RP2).
Authorship
Contribution: R.T. performed experiments, interpreted data, and wrote the paper; J.t.B., B.G., G.G.W.v.B., and I.A.M.D. performed and interpreted experiments; R.J.W.v.K. provided patient samples and edited the paper; K.H. and M.J.K. suggested and interpreted experiments and edited the paper; M.S.R., J.-M.M., M.H., B.E., H.C.R., and J.B. contributed with the inclusion of patients and served as principal investigators; J.R.B. and S.M.F. provided patient samples (idelalisib); T.T. and E.H.F. performed analyses of CLL samples, interpreted the data, and supplied related text and figures; and E.E. and A.P.K. supervised the study and wrote the paper.
Conflict-of-interest disclosure: This work was sponsored via a research agreement between AMC and Celgene. The clinical study was sponsored by Celgene. B.G., K.H., T.T., and E.H.F. are employees of Celgene. M.J.K. and A.P.K. have received research funding and compensation for advisory board meetings from Celgene. All other authors declare no competing financial interests.
Correspondence: Arnon P. Kater, Department of Hematology, Academic Medical Center, 1105AZ, Amsterdam, The Netherlands; e-mail: a.p.kater@amc.uva.nl.
References
Author notes
E.E. and A.P.K. contributed equally to this work.