Abstract
Background: BETs are evolutionarily conserved proteins that specifically bind to acetyl-lysines on histones and other proteins to recruit transcriptional machinery and influence gene expression (Shi and Vakoc. 2014 Mol Cell 54, 728-736). Small molecules that target BETs, such as JQ1 and I-BET, can effectively inhibit the growth of midline carcinoma cells that have a BRD4-NUT gene fusionas well as leukemia cells, such as AML, which have a diverse genetic background and do not have BRD4 rearrangements. DNA methyltransferase (DNMT) inhibitors, such as azacitidine (5-AZA) and decitabine, have been proven effective in some patients with hematologic malignancies including AML and MDS. However, the detailed mechanisms underlying the selectivity and resistance of these epigenetic-modifying drugs remain elusive. Our previous genome-wide epigenetic profiling data have demonstrated epigenetic inactivation of the SPI1/PU.1 pathway in cytogenetically normal MDS with erythroid predominance (Cheng et al. 2013 Leukemia 27, 1291-1300). This study aims to elucidate the role of chromatin structure in the regulation of response to epigenetic drugs in MDS and AML.
Methods: Co-immunoprecipitation (co-IP) coupled with RNase/DNase digestion, super-resolution two-photon stimulated-emission depletion (STED) confocal microscopy, high-throughput sequencing and chromatin conformation capture (3C) were used.
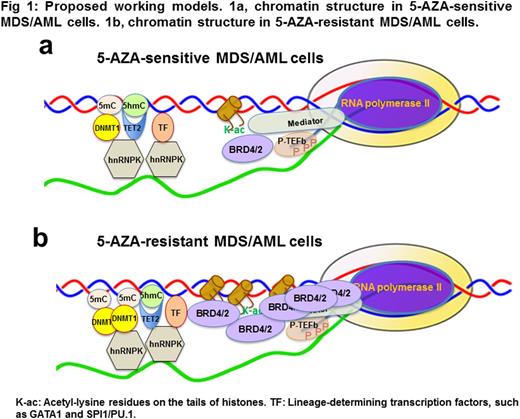
Results: Our data demonstrate that GATA1 and SPI1/PU.1, erythroid- vs. myeloid-determining transcription factors (TFs), selectively interact with specific sets of DNA/histone modifiers, such as TET2, DNMTs and EZH2, to form distinct lineage-specific, drug-responsive chromatin structures at specific gene loci. The interactions between the TFs, DNA/histone modifiers and chromatin structures are highly sensitive to RNase and DNase digestions, i.e., dependent on native chromatin environment. Our experiments show that hnRNPK, a conserved factor in the heterogeneous nuclear RNA-binding protein (hnRNP) complexes, directly interact with the lineage-determining TFs and DNA/histone modifiers as well as RNAs/DNAs to form distinct chromatin-structures in MDS/AML cells. Epigenetic drugs, such as 5-AZA, efficiently disrupt the hnRNPK-mediated chromatin structures in 5-AZA-sensitive MDS/AML cells, but not in 5-AZA-resistant MDS/AML cells. Our data demonstrate a marked (~4-6 fold) increase in BRD4, BRD2 and DNMT1 in the 5-AZA-resistant MDS/AML cell lines compared to the 5-AZA-sensitive MDS/AML cell lines. Strikingly, a massive amount of BRD4-associated RNA polymerase II (pol-II) is observed in the 5-AZA-resistant MDS/AML cells, but not in the 5-AZA-senstive MDS/AML, and there is approximately 70-80 fold increase in BRD4-associated pol-II, but not in BRD4-associated GATA1, in the 5-AZA-resistant cells compared to the 5-AZA-sensitive cells. Such BRD4-associated pol-II is almost exclusively in a processive pol-II complex that is associated with the phosphorylated C-terminal domain (S2p/S5p-CTD) of pol-II, and sensitive to JQ1. Furthermore, JQ1 significantly decreases the interactions between BRD4 and lineage-determining TFs GATA1 and SPI1/PU.1, in the 5-AZA-sensitive MDS/AML cells, but not in the 5-AZA-resistant MDS/AML cells. A very low concentration (0.01 uM) of JQ1 can re-sensitize the 5-AZA-resistant MDS/AML cell lines and completely reverse the drug resistance to 5-AZA, suggesting a BET-mediated 5-AZA-resistance in those cells. Genome-wide chromatin immunoprecipitation coupled with high-throughput sequencing has been carried out to map the BRD4-associated RNA pol-II at the genome-wide level in the 5-AZA resistant and sensitive MDS/AML cells. Our experiments using clinical specimens demonstrate a positive correlation between MDS/AML progression and increase in expression of hnRNPK, BRD2 and BRD4, confirming their importance and clinical relevance.
Conclusion: Our study has revealed a novel drug resistance mechanism that operates through BET-mediated recruitment of an active pol-II complex and induction of drug-responsive chromatin structural changes in MDS/AML cells (Fig 1). Such BET-mediated changes in pol-II complexes and chromatin structure could become useful biomarkers to predict drug responses, leading to rational designs and actionable targets to reprogram therapeutic drug susceptibility for more effective therapy in MDS and AML.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal